Abstract
Background
Community interventions to promote condom use are considered to be a valuable tool to reduce the transmission of human immunodeficiency virus (HIV) and other sexually transmitted infections (STIs). In particular, special emphasis has been placed on implementing such interventions through structural changes, a concept that implies public health actions that aim to improve society's health through modifications in the context wherein health‐related risk behavior takes place. This strategy attempts to increase condom use and in turn lower the transmission of HIV and other STIs.
Objectives
To assess the effects of structural and community‐level interventions for increasing condom use in both general and high‐risk populations to reduce the incidence of HIV and STI transmission by comparing alternative strategies, or by assessing the effects of a strategy compared with a control.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library, from 2007, Issue 1), as well as MEDLINE, EMBASE, AEGIS and ClinicalTrials.gov, from January 1980 to April 2014. We also handsearched proceedings of international acquired immunodeficiency syndrome (AIDS) conferences, as well as major behavioral studies conferences focusing on HIV/AIDS and STIs.
Selection criteria
Randomized control trials (RCTs) featuring all of the following.
1. Community interventions ('community' defined as a geographical entity, such as cities, counties, villages).
2. One or more structural interventions whose objective was to promote condom use. These type of interventions can be defined as those actions improving accessibility, availability and acceptability of any given health program/technology.
3. Trials that confirmed biological outcomes using laboratory testing.
Data collection and analysis
Two authors independently screened and selected relevant studies, and conducted further risk of bias assessment. We assessed the effect of treatment by pooling trials with comparable characteristics and quantified its effect size using risk ratio. The effect of clustering at the community level was addressed through intra‐cluster correlation coefficients (ICCs), and sensitivity analysis was carried out with different design effect values.
Main results
We included nine trials (plus one study that was a subanalysis) for quantitative assessment. The studies were conducted in Tanzania, Zimbabwe, South Africa, Uganda, Kenya, Peru, China, India and Russia, comprising 75,891 participants, mostly including the general population (not the high‐risk population). The main intervention was condom promotion, or distribution, or both. In general, control groups did not receive any active intervention. The main risk of bias was incomplete outcome data.
In the meta‐analysis, there was no clear evidence that the intervention had an effect on either HIV seroprevalence or HIV seroincidence when compared to controls: HIV incidence (risk ratio (RR) 0.90, 95% confidence interval (CI) 0.69 to 1.19) and HIV prevalence (RR 1.02, 95% CI 0.79 to 1.32). The estimated effect of the intervention on other outcomes was similarly uncertain: Herpes simplex virus 2 (HSV‐2) incidence (RR 0.76, 95% CI 0.55 to 1.04); HSV‐2 prevalence (RR 1.01, 95% CI 0.85 to 1.20); syphilis prevalence (RR 0.91, 95% CI 0.71 to 1.17); gonorrhoea prevalence (RR 1.16, 95% CI 0.67 to 2.02); chlamydia prevalence (RR 0.94, 95% CI 0.75 to 1.18); and trichomonas prevalence (RR 1.00, 95% CI 0.77 to 1.30). Reported condom use increased in the experimental arm (RR 1.20, 95% CI 1.03 to 1.40). In the intervention groups, the number of people reporting two or more sexual partners in the past year did not show a clear decrease when compared with control groups (RR 0.90, 95% CI 0.78 to 1.04), but knowledge about HIV and other STIs improved (RR 1.15, 95% CI 1.04 to 1.28, and RR 1.23, 95% CI 1.07 to 1.41, respectively). The quality of the evidence was deemed to be moderate for nearly all key outcomes.
Authors' conclusions
There is no clear evidence that structural interventions at the community level to increase condom use prevent the transmission of HIV and other STIs. However, this conclusion should be interpreted with caution since our results have wide confidence intervals and the results for prevalence may be affected by attrition bias. In addition, it was not possible to find RCTs in which extended changes to policies were conducted and the results only apply to general populations in developing nations, particularly to Sub‐Saharan Africa, a region which in turn is widely diverse.
Keywords: Adult; Humans; Chlamydia Infections; Chlamydia Infections/epidemiology; Chlamydia Infections/prevention & control; Condoms; Condoms/statistics & numerical data; Gonorrhea; Gonorrhea/epidemiology; Gonorrhea/prevention & control; HIV Infections; HIV Infections/epidemiology; HIV Infections/prevention & control; Health Promotion; Health Promotion/organization & administration; Herpes Genitalis; Herpes Genitalis/epidemiology; Herpes Genitalis/prevention & control; Herpesvirus 2, Human; Incidence; Prevalence; Randomized Controlled Trials as Topic; Sexually Transmitted Diseases; Sexually Transmitted Diseases/epidemiology; Sexually Transmitted Diseases/prevention & control; Syphilis; Syphilis/epidemiology; Syphilis/prevention & control; Trichomonas Infections; Trichomonas Infections/epidemiology; Trichomonas Infections/prevention & control
Plain language summary
The effectiveness of community interventions to promote condom use in the prevention of HIV and sexually transmitted infections
Bakground
Since the advent of the HIV/AIDS epidemic in the 1980s, condom promotion has become one of the most widely used interventions to prevent transmission of HIV and sexually transmitted infections (STIs). However, despite widespread promotion of condom use globally, new cases of HIV and other STIs either remain high or continue to rise in some particular regions and settings across the world. It is believed that by modifying the environment in which people live, it is possible to improve access and use of condoms on a large scale so that the transmission of HIV and other STIs decreases. This review aimed to assess if this theory was correct.
Methods
We screened all relevant literature from January 1980 to April 2014. Two independent authors selected and assessed the trials.
Results
We obtained nine studies, involving 75,891 participants and with a duration raging from one to nine years. Seven of these studies were conducted in Sub‐Saharan Africa, one in Peru, and one in a multi‐country location. Condom promotion was implemented in all the studies. Our results did not provide clear evidence that condom promotion in these specific contexts led to a decrease in the transmission of HIV and other STIs. However, knowledge about HIV and other STIs increased, as did reported condom use. A likely reason for the negative results in this review is that sexual behaviors are difficult to change. For example, we found no difference in the number of sexual partners after the intervention was implemented. Also, if there is not consistent condom use the risk of transmission remains for HIV and other STIs. The quality of the evidence was deemed to be moderate.
Conclusions
Our findings should be interpreted with caution since most of the studies in the present review were carried out in Sub‐Saharan Africa, region that is very diverse, and whose social and cultural characteristics are different from those in other developing nations. Thus, our results present a limited generalizability.
Summary of findings
Summary of findings for the main comparison. Structural and community‐level interventions for increasing condom use versus standard care to prevent the transmission of HIV and other sexually transmitted infections.
| Structural and community‐level interventions for increasing condom use versus standard care to prevent the transmission of HIV and other sexually transmitted infections | ||||||
| Patient or population: low and high‐risk populations Settings: community‐based Intervention: structural and community‐level interventions to increase condom use Comparison: standard care | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Standard care | Structural and community‐level interventions to increase condom use | |||||
| HIV incidence (person/years) Follow‐up: 2 to 4 years | Study population | RR 0.90 (0.69 to 1.19) | 14279 (4 studies) | ⊕⊕⊕⊝ moderate1,2 | ||
| 14 per 1000 | 13 per 1000 (10 to 17) | |||||
| Moderate | ||||||
| 11 per 1000 | 10 per 1000 (8 to 13) | |||||
| HIV prevalence Follow‐up: 3 to 9 years | Study population | RR 1.02 (0.79 to 1.32) | 9042 (3 studies) | ⊕⊕⊝⊝ low3,4 | ||
| 24 per 1000 | 25 per 1000 (19 to 32) | |||||
| Moderate | ||||||
| 29 per 1000 | 30 per 1000 (23 to 38) | |||||
| Herpes Simplex Virus 2 (HSV‐2) incidence (person/years) Follow‐up: 2 to 4 years | Study population | RR 0.76 (0.55 to 1.04) | 3877 (2 studies) | ⊕⊕⊕⊝ moderate5 | ||
| 44 per 1000 | 33 per 1000 (24 to 45) | |||||
| Moderate | ||||||
| 41 per 1000 | 31 per 1000 (23 to 43) | |||||
| HSV‐2 prevalence Follow‐up: 4 to 9 years | Study population | RR 1.01 (0.85 to 1.20) | 2974 (2 studies) | ⊕⊕⊕⊝ moderate6 | ||
| 130 per 1000 | 131 per 1000 (110 to 156) | |||||
| Moderate | ||||||
| 202 per 1000 | 204 per 1000 (172 to 242) | |||||
| Two or more sexual partners in last year Follow‐up: 3 to 9 years | Study population | RR 0.90 (0.78 to 1.04) | 4008 (5 studies) | ⊕⊕⊕⊝ moderate7 | ||
| 156 per 1000 | 140 per 1000 (121 to 162) | |||||
| Moderate | ||||||
| 173 per 1000 | 156 per 1000 (135 to 180) | |||||
| Use of condom during last sexual intercourse Follow‐up: 3 to 9 years | Study population | RR 1.20 (1.03 to 1.4) | 1898 (3 studies) | ⊕⊕⊕⊝ moderate8 | ||
| 221 per 1000 | 265 per 1000 (228 to 309) | |||||
| Moderate | ||||||
| 217 per 1000 | 260 per 1000 (224 to 304) | |||||
| Knowledge: condom can prevent STIs Follow‐up: 3 to 9 years | Study population | RR 1.23 (1.07 to 1.41) | 1231 (3 studies) | ⊕⊕⊕⊝ moderate8 | ||
| 368 per 1000 | 452 per 1000 (393 to 518) | |||||
| Moderate | ||||||
| 372 per 1000 | 458 per 1000 (398 to 525) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; HIV: human immunodeficiency virus; RR: Risk ratio; STI: sexually transmitted infection | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Although there was attrition bias higher than 30% in one study, it was the only one classified as high risk among a total of four studies assessing this outcome. 2 Wide confidence intervals (null effect with a confidence interval that goes beyond 0.75) and a total number of events inferior to 300 with a small risk ratio reduction (< 20%). 3 High risk of incomplete outcome data in two studies, and unknown in 1 study. 4 Wide confidence intervals (null effect with a confidence interval that goes beyond 1.25) and a total number of events inferior to 300 with no risk ratio reduction. 5 Wide confidence intervals, few number of events (< 100). 6 Both studies with high risk of incomplete outcome data. 7 Three studies with high risk of incomplete outcome data and two studies with unknown technique for randomisation. 8 Two studies with high risk of incomplete outcome data and two studies with unknown technique of randomisation.
Background
Description of the condition
HIV is a virus that attacks the immune system, weakening it progressively and leading some years later to the onset of opportunistic infections, a stage that is known as acquired immunodeficiency syndrome (AIDS). If not treated, the natural progression of this syndrome winds up in death (Weiss 1993). HIV's routes of transmission are sexual intercourse through contact of mucosae with semen or vaginal fluids, exchange of intravenous syringes, blood transfusion, hemodialysis, and mother‐to‐child transmission via blood transfer and breastfeeding.
In 2012 the World Health Organization (WHO) and the Joint United Nations Programme on HIV/AIDS (UNAIDS) estimated that globally 2.3 million people were newly infected with HIV, 35.3 million people were already living with the virus, and 1.6 million people died from AIDS‐related causes (UNAIDS 2013). Moreover, the HIV/AIDS pandemic affects disproportionally low‐ and middle‐income countries (LMICs), with several regions in Sub‐Saharan Africa facing the highest burden of the disease (Ortblad 2013).
Since the onset of the pandemic, condoms have been considered one of the most effective biological mechanisms for reducing the transmission of HIV (Holmes 2004). Laboratory and epidemiological studies have demonstrated that condoms are effective against a number of sexually transmitted infections (STIs), such as gonorrhoea, non‐gonococcal urethritis, trichomoniasis, genital herpes (da Silveira 2005), and HIV (Weller 2002). Of the many strategies to reduce infection, promotion of condom use continues to be the keystone of HIV and STI prevention worldwide (Matson 2010). However, the positive impact of condoms may be limited by their inconsistent or low use (Hearst 2004). Several factors are known to be associated with condom non‐use during sexual intercourse, including perception of reduced pleasure (Sarkar 2008; Sunmola 2005), discomfort (Crosby 2005), partner trust (Abdullah 2002; Jadack 1997), use of other contraceptives (Abdullah 2002), fear of revealing HIV status to partners (Medley 2004), and religious beliefs (Thomsen 2004). It has also been suggested that the spread of information about highly active antiretroviral therapy (HAART) reducing the infectiousness of HIV may be a potential hindrance to condom use (Akinyemi 2010); nonetheless, evidence supporting this theory is mixed (Berhan 2012; Crepaz 2004).
The availability of and accessibility to condoms is often a major obstacle to condom use. In most low‐ and middle‐income countries cost is a barrier to purchase condoms among the poor (Essien 2005). Even when cost is not an issue, non‐availability of condoms often results in unprotected sexual practices (Kumar 2006). This problem affects not only LMICs, but also high‐income countries such as the United States, where many African‐Americans are unable to purchase condoms due to financial barriers (Essien 2005). Other authors have also mentioned that in certain societies, the setting in which condoms are provided, commercialized and purchased may hinder their acquisition from possible buyers/consumers (e.g. buying them in a public place (Roth 2001)). Also, in many societies moral values and ethnic and religious factors play an important role in health behaviors and health promotion policies (Koenig 2012). For example, the Catholic Church considers any contraceptive method as a transgression of divine law (HUMANAE VITAE 1968), a mandate that some devout Catholics observe (Lefkowitz 2004).
Moreover, lack of condom usage is also a consequence of limited knowledge about HIV prevention and a low level of education (Dandona 2005). Other social determinants such as women's lack of empowerment, scarce dialogue within couples, domestic violence, and prejudice related to condom use could increase the risk of unsafe sexual practices (Sarkar 2008).
Among female sex workers (FSW), limited knowledge about HIV transmission, a client's refusal to use condoms, or coercion and control by pimps or brothel owners act as barriers to condom use (Xia 2005). For men who have sex with men (MSM), negative community attitudes towards homosexuality combined with legal restrictions on their sexual behavior and official relationships contribute to the marginalization and stigmatization of this community and drive their relationships underground, hindering an easy acquisition of condoms and resulting in risky sexual behavior (Adimora 2010).
It is also important to mention that depending on the local context, prevalence of condom use tends to differ between different sex‐risk‐behavior groups. Several studies have described a tendency of high‐risk groups such as individuals with multiple sexual partners and frequent casual sexual encounters (Maticka‐Tyndale 2012), MSM (Adam 2009; Lau 2008; Reece 2010) and FSWs (Maticka‐Tyndale 2012; Rojanapithayakorn 1996; Suleman 2012) to report higher condom use figures than non‐high‐risk groups (Abdullah 2002; Jones 2012; Reece 2010). The general population tends to present a lower condom use prevalence for reasons such as use of other contraceptive methods (Abdullah 2002; Jones 2012) and a negative perception of condoms, which symbolize promiscuity and unfaithfulness (Maticka‐Tyndale 2012). Consequently, condom promotion interventions may also have a different impact on each of these populations.
Description of the intervention
It is widely understood in the field of public health that social determinants of health, or the circumstances in which individuals are born, live, work and age, play an important role in their health (WHO 2012). Those contexts are shaped by structural determinants such as physical, social, cultural, legal, and organizational factors, and several other aspects of the environment that hinder or promote HIV prevention (Gupta 2008; Sumartojo 2000).
A structural intervention is defined as any public health intervention that aims to improve society's health status by modifying the context in which health‐related risk behavior takes place (Blankenship 2000). When addressing condom use through structural interventions, there are three key concepts to consider (Blankenship 2000). The first one is availability, which focuses on the attitudes, materials, or settings that are essential to reduce exposure to a specific health problem (Blankenship 2000). For example, community campaigns may install condom machines in public places, or implement a policy of 100% condom use in brothels (Blankenship 2000; Charania 2011). The second factor is acceptability, which sets out to modify the preconceptions in a society, allowing people to consider and accept new ideas (Blankenship 2000) mainly through social learning theory (Bandura 1986). Programs to prevent the stigmatization of prostitution, sexual education, and marketing of condoms are examples (Blankenship 2000; Charania 2011). The last factor is accessibility, which aims to improve access to interventions, services and goods in health for those who face unfavourable social or economic conditions (Blankenship 2000). Examples of this concept include distribution of free or low‐cost condoms and the creation of female controlled prevention strategies, such as female condom use (Blankenship 2000; Charania 2011). Therefore, structural interventions usually include changes to policies, environment, or social beliefs, and in many cases also involve the empowerment of minorities and other disenfranchised groups (Adimora 2010).
Structural interventions can be implemented at three levels: individual, group, and community (Charania 2011). Individual and group level interventions target knowledge, attitudes, and behaviors associated with the condom use of the participant in one‐on‐one settings and in existing (e.g. a couple) or recently created groups, respectively (Charania 2011). Community‐level interventions have typically been conceptualized as geographic units (e.g. cities, counties, villages), although alternative conceptualizations of smaller units also exist (e.g. workplaces and schools) (Atienza 2002); the present review was developed based on the former concept. Interventions at a community level directly and indirectly concentrate on knowledge, attitudes and behaviors in relation to the entire community and strongly address the alteration of social norms (Charania 2011). This strategy might have the capacity to modify social determinants of health at all levels in which it is implemented (Swerissen 2004).
How the intervention might work
Previous research on HIV and STI prevention strategies shows that structural interventions have the capacity to initiate significant change in behaviors and attitudes.
For example, Thailand's renowned 100% Condom Program modified availability and accessibility of condoms by empowering sex workers to consistently engage in safe sexual practices via government policies that enforced mandatory condom use (Rojanapithayakorn 1996). Likewise, in the Dominican Republic two non‐governmental organizations (NGOs) investigated in 1995 the adoption of Thailand's initiative into the Dominican setting (Kerrigan 2006). This intervention addressed the three different components of structural interventions at a group and community level. Assessment of this program revealed that condom use did increase after the introduction of this intervention (Kerrigan 2006). Another program, the Sonagachi project in India, focused on community mobilization, aiming to reduce discrimination and stigma against FSWs (Basu 2004).
Dealing with inequitable gender norms, especially those that define masculinity, was an important component of HIV prevention strategies in Brazil (Pulerwitz 2006). In this intervention, the aim was to modify acceptability at a community level. In South Africa an HIV prevention strategy was implemented to modify both accessibility through a local micro‐finance program addressed at women and acceptability by initiating community activism in opposition to women mistreatment (Gupta 2008). Such interventions aim to empower women, decrease domestic violence and enable women to negotiate safe sex (Gupta 2008).
Finally, media campaigns promoting condom use also have the potential to influence norms and change values, expectations, and behaviors by modifying acceptability at a community level (Cohen 2006).
In spite of the cost, financial analyses demonstrate that structural interventions may have the most impact in HIV prevention, and may also generate other social advantages, such as stimulating the economy and making gains in human rights (Hecht 2009). Given the large amount of resources required for individual‐based interventions to be successfully implemented on a broader scale, it has been hypothesized that structural interventions might be an adequate solution since they are more ample in their scope (Cohen 2006).
Why it is important to do this review
Despite the implementation of extensive HIV prevention programs over the past decades, the number of people newly infected with HIV continues to rise in some regions across the globe (UNAIDS 2013). Some studies argue that one of the reasons for this phenomenon is the excessive attention to individual behaviors at the expense of changes to the context in which those behaviors occur (Barnett 2006).
The consensus in recent years that social factors affect HIV transmission as well as risky sexual behaviors has prompted an increasing attention on structural interventions for HIV and other STIs (Gupta 2008). However, few strategies to prevent HIV transmission have implemented structural interventions, mainly due to a lack of clear definition, a poor understanding of technical implementation, and insufficient data to support the effectiveness of such interventions in the context of people living with HIV (Gupta 2008).
Behavioral interventions that aim to encourage a change in individual attitudes toward safer sexual practices such as condom use or reduction of sexual partners play an important role in overall HIV prevention efforts. However, these interventions alone may be inadequate to produce substantial and long‐term reductions in HIV transmission (Coates 2008). As a result, taking into account the limitations of these strategies, structural interventions offer a potentially powerful shift in the manner HIV prevention is implemented (Blankenship 2010).
Despite a growing interest in the topic, there is little evidence on structural interventions at a community level that aim to increase condom use to prevent HIV and other STIs. Policy makers require high quality quantitative information in order to develop and implement prevention programes, as well as to set priorities in research and funding (Johnson 2008). Determining if structural interventions for promoting condom use are effective in halting HIV transmission at a community level is central to the current strategies against HIV, given the number of people whose lives may be improved by this policy. In addition,deciding what the most effective approaches to reducing HIV transmission are will allow wise investment in public health resources.
Objectives
The objective of this review is to assess the effects of structural and community‐level interventions for increasing condom use in both general and high‐risk populations to reduce the incidence of HIV and STI transmission by comparing alternative strategies, or by assessing the effects of a strategy compared with a control.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials (RCTs) including cluster‐RCTs that compared either two or more alternative condom promotion strategies, or one condom promotion strategy with a control (i.e. no condom promotion strategy). We decided to search only for RCTs since they are the gold standard in scientific experimentation, reducing the likelihood of bias and misleading causality, thus providing the highest quality of primary data.
We only included studies conducted at a community level in this review. We defined community as a geographical area with delimited boundaries such as a village, city or county (Atienza 2002).
Types of participants
We assessed studies that included the following types of participants and satisfied this review's definition of 'community'.
General population (adolescents and adults).
Patients with STIs, including those living with HIV, or serodiscordant couples, or both.
Drug users, including injecting drug users (IDUs) as well as other drug‐using populations.
Sex workers (male and female).
Men who have sex with men (MSM).
Types of interventions
A. Experimental group
We attempted to include all the following types of interventions at a community level.
Interventions focusing on increasing the acceptability of condom use behaviors by altering social norms, such as the social‐cognitive theory (Bandura 1986), mass media campaigns, use of public opinion, and social marketing strategies.
Interventions which improve condom accessibility, or change or improve distribution, whether through the public sector, the private sector, or NGOs via condom subsidization (either total or partial), as well as strategies focusing on altering economic situations which impact condom use, such as condom taxes or tariffs removal. Female empowerment was also included as an intervention to improve their access to condoms.
Interventions aimed to extend the availability of condoms such as condom machines in clubs, brothels, or other public spaces.
Combinations of the above.
B. Control group
Standard intervention or standard care. These terms refer to the usual HIV and STIs knowledge to which communities are exposed in daily life, such as regular STIs/HIV education programs in school, television advertisements, medical advice, and the like.
Delayed intervention.
Partial or shortened version of the intervention.
Types of outcome measures
Primary outcomes
HIV incidence/prevalence.
STI incidence/prevalence.
Secondary outcomes
Self reported condom use (male or female condoms).
Self reported number of sexual partners in the last year.
Knowledge about HIV.
Knowledge about STIs.
Knowledge about condom use (both self reported and tested).
Self efficacy (assessing self confidence related to condom use and sexual practices after receiving the intervention through standard questionnaires).
Since the primary objective of our revision was to measure the impact of condom promotion on the transmission of HIV and other STIs, it was decided to set up as inclusion criteria the reporting of any of the primary outcomes proposed for this review (i.e. HIV and/or other STIs), as long as they were confirmed through laboratory markers.
Search methods for identification of studies
Electronic searches
We used the Cochrane search strategy for RCTs with the assistance of the Cochrane HIV/AIDS Review Group to identify appropriate studies within electronic databases (Higgins 2011). We did not impose any restrictions on language or publication status. We searched the Cochrane Central Register of Controlled Trials (CENTRAL) from 2007 (issue 1) to April 2014, as well as MEDLINE, EMBASE, Psychological Abstracts and Sociological Abstracts using a strategy similar to MEDLINE, from January 1980 to April 2014 (see Appendix 1). The sensitivity of the search strategies was improved when possible by including text and key words from relevant trials accessed by the authors that were not detected by earlier searches. In order to maximize this sensitivity, searches were performed both with and without keywords related to the study design.
Searching other resources
We handsearched the following sources.
Proceedings of the International AIDS Conferences.
Proceedings of the International Society of STD Research (ISSTDR).
Other proceedings, including those of major behavioral studies conferences focusing on people living with HIV/AIDS, STIs, or other reproductive health issues.
We examined bibliographies of studies and previous reviews to find other relevant evaluation studies. We contacted professionals in the field (e.g. authors of previous reviews or investigators of previous primary research) (via email) to identify continuing studies. We also contacted relevant organizations possibly involved in condom promotion interventions, including international agencies (e.g. UNAIDS, WHO, United Nations Population Fund (UNFPA), national public health agencies with projects in developing countries, major NGOs and major academic centers).
Data collection and analysis
Selection of studies
The search for trials was performed with the assistance of the Cochrane HIV/AIDS Group. Two authors (HN and RM) critically appraised all identified citations independently, in order to establish their relevance for inclusion into the review. Studies were reviewed for relevance based on study design, types of participants, interventions and outcome measures. Any disagreement was resolved by discussion or by contacting a third author (EO). We provided reasons for excluding potentially relevant trials in the Characteristics of excluded studies table.
Data extraction and management
We used a standardized form for data extraction. Two reviewers (HN and RM) independently extracted study characteristics and outcomes including information on:
contexts of study setting;
study populations (including sociocultural and economic characteristics, and possible previous exposure to similar interventions);
duration of exposure (or 'dose') involved in the intervention, such as measurements of total time period over which the intervention took place;
type of intervention;
outcome measures; and
findings.
Assessment of risk of bias in included studies
Two review authors (HN and RM) independently assessed the quality of all selected studies using the Cochrane 'Risk of bias' assessment tool (Higgins 2011).
1. Random sequence generation (selection bias)
Low risk: the process of randomization was properly described
Unclear risk: the information provided is not enough to judge if the sequence generation was at random or not
High risk: a non‐random approach was used
2. Allocation concealment (selection bias)
Low risk: participants and researchers were unaware of participants' future allocation to the condition until after decisions about eligibility were made and informed consent was obtained
Unclear risk: allocation concealment measures were not described in detail
High risk: allocation was not concealed from either participants before informed consent or from researchers before decisions about inclusion were made
3. Blinding of participants, assessors and providers of care (performance bias)
Low risk: assessor blind to condition
Unclear risk: blinding of assessor not reported and information not available from researchers
High risk: assessor not blind to condition
4. Blinding of outcome assessment (detection bias)
Low risk: blinding of outcome assessment was ensured, or the outcome measurement is unlikely to be biased by lack of blinding
Unclear risk: Outcome assessment was not described
High risk: no blinding of outcome assessment and the results are likely to be biased if blinding did not take place
5. Incomplete outcome data (attrition bias)
Low risk: losses to follow up were equally distributed between treatment and comparison groups
Unclear risk: information about losses to follow up unavailable
High risk: losses to follow up in excess of 30% or unevenly distributed between treatment and comparison groups. It was decided to set up the threshold for attrition bias above the usual 20% since all studies were community based, thus large loss to follow‐up was expected due to migration and death
6. Selective reporting (reporting bias)
Low risk: the outcomes of interest are the same in the protocol and in the published paper
High risk: the outcomes pre‐specified in the protocol are not the same than those presented in the paper
7. Other bias
Low risk: other bias such as different individual recruitment, cluster imbalance, loss of cluster and secular trend are unlikely to have taken place
Unclear risk: it s not possible to judge if the abovementioned bias took place
High risk: it is likely that any of the abovementioned bias took place
For each trial, two reviewers (HN and RM, see Contributions of authors) categorised the risk of bias as 'high', 'unclear', or 'low'. When there was any disagreement between them regarding the quality of a particular study, a third reviewer (EO) reconciled the disagreement.
Measures of treatment effect
We measured the size of the intervention effect using risk ratio (RR), with either the number of participants as the denominator for estimated prevalence or person‐years for estimated incidence. We adjusted event rates and sample sizes using methods outlined under Sensitivity analysis.
Unit of analysis issues
Studies with similar units of analysis (e.g. incidence, prevalence, type of outcome) were grouped together for the purposes of analysis, while studies with different units of analysis were not. In case of multiple‐arms trials, we combined groups to generate a single‐pair wise comparison.
Dealing with missing data
In cases of missing or inadequate data, we made three attempts to obtain the data by contacting authors via email.
Assessment of heterogeneity
We anticipated substantial heterogeneity across studies, and thus we undertook meta‐analysis of these studies with caution. For studies that were homogenous with respect to types of study populations, interventions and outcome measures, overall risk ratios were calculated and translated into risk ratio reductions. When there was no statistically significant heterogeneity (P < 0.10) in the results, we used a fixed‐effect model (Mantel‐Haenszel). When there was statistically significant heterogeneity we identified possible explanations and used a random‐effects model (DerSimonian and Laird method); this was compared with the corresponding results of the fixed‐effect model. The following factors were considered as possible explanations for significant heterogeneity: study quality, patient gender or age, or both, study setting (low‐ and middle‐income or high‐income countries), and type of intervention. To test for robustness of results, we performed sensitivity analyses.
Given the likelihood of substantial heterogeneity across studies, and the subsequent insufficient data for meta‐analysis, we included in this review a qualitative overview of the studies and their findings. The main aim of this analysis was to identify interventions that have been shown to be effective, the circumstances under which they have been shown to be effective, and the specific effects that have been measured. We also identified patterns of intervention strategies that appeared ineffective, as well as important gaps in existing knowledge.
Assessment of reporting biases
We evaluated possible reporting bias through asymmetry in funnel plots. We screened meta‐analyses containing five or more studies with this technique.
Data synthesis
Two authors (HN and RM) extracted the data and entered all eligible studies into RevMan. All the entries were rechecked by both authors. Disagreements were resolved by discussion. When no consensus was reached, the HIV/AIDS mentor for this review (EO) was contacted.
Subgroup analysis and investigation of heterogeneity
When possible, we performed stratified analysis among subgroups of participants to assess the origin of heterogeneity. Subgroups included age of target population (e.g. adolescent versus adult), gender, motivation for condom use (contraception and prophylaxis versus prophylaxis only), and combinations of different interventions. We performed additional stratified analyses by methodological quality, when possible. Only those analyses in which a particular subgroup explained heterogeneity were shown in this review.
Sensitivity analysis
Correction for cluster effect in RCTs: intra‐cluster correlation coefficient (ICC)
As community‐level interventions were the objective of the present analysis, we only included cluster‐RCTs in this review. Therefore, calculation of design effects (DEs) was necessary to estimate effective sample sizes. In order to calculate these figures (DE), intra‐cluster correlation coefficients (ICCs) were required (Donner 2000). Since the majority of studies did not report on ICCs, we attempted to contact the authors (via email) to obtain those figures. Unfortunately, we often failed to receive any response, or the answer did not provide the information we needed (e.g. the study was conducted a long time ago, and those data were not available). We therefore decided to screen other trials with similar populations in order to infer the ICCs. It proved difficult to obtain studies in which the cluster size matched studies in this review. Furthermore, when correcting for ICCs it is preferable to assign an individual ICC value to each outcome instead of using one single ICC for all outcomes in one trial (Taljaard 2008). Given these obstacles, we decided to employ a regression model to generate an estimated ICC value for every single outcome in each trial. We ran a model with natural logarithm of ICC as the dependent variable, and both average cluster size and outcome prevalence as the independent variables. We hypothesized that this specific set of variables would predict the value of any given ICC. The required data to build the model was obtained from studies that reported both the covariates and an individual ICC value for each outcome. Five studies were included in the final model (D'Amico 2012; Feldblum 2001; Garcia 2012; Majoko 2007; Taljaard 2008). After running the linear regression model, we found that the hypothesized independent variables (average cluster size and outcome prevalence) were significant and in agreement with other studies (Killip 2004; Taljaard 2008). The model explained 45% of variation in ICCs (Figure 1). Residuals were calculated and displayed no evident pattern, indicating ordinary least squares regression was appropriate for this data. Finally, the model was used to estimate the ICC for a given prevalence and average cluster size for each outcome.
1.
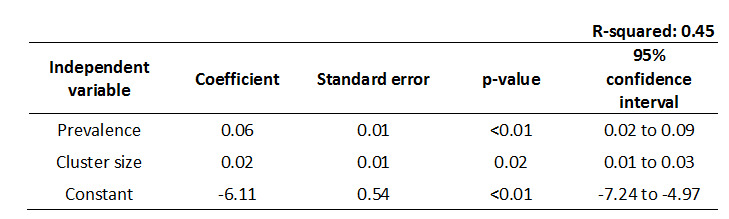
ICC Linear Regression Model.
Then the DE was calculated using the standard formula: 1+ ((average cluster size ‐1) * ICC) (Higgins 2011, Section 16.3.4). Once the DE was estimated, effective sample sizes for each individual outcome were calculated by dividing the original sample size by the DE. Finally, these effective sample sizes were the figures used in the meta‐analysis. We set an upper limit of 40 to the design effect, which corresponds to ICCs higher than 0.1 for the cluster sizes in the selected studies. This limit was chosen based on the trend reported by Taljaard and Donner (Taljaard 2008) in which design effects rarely surpass this value.
Since outcomes may vary based on ICC fluctuation, sensitivity analysis was carried out with an ICC of 0 (i.e. original sample size with no correction), 0.001, 0.005, 0.01, 0.05 and 0.1 for every outcome in which a meta‐analysis was possible.
Results
Description of studies
Results of the search
See Characteristics of included studies; Characteristics of excluded studies.
We retrieved a total of 6,667 citations, ranging from those published between January 1980 to April 2014. After thorough screening, we selected 48 full‐text studies and assessed for eligibility, with an inter‐rater agreement (kappa) score of 0.34. In the subsequent analysis, we excluded 38 studies for different reasons (Characteristics of excluded studies), leaving nine studies for risk of bias assessment (Cowan 2010; Doyle 2010; Feldblum 2001; Garcia 2012, Gregson 2007; Jewkes 2008, Kamali 2003, NIMH 2010; Ross 2007), and one study that was a subanalysis from Feldblum 2001, in which condom use was reported (see Welsh 2001, under included studies, Feldblum 2001). The kappa score for this second screening process was of 0.46. Out of these trials, we selected eight for meta‐analysis, excluding NIMH 2010 since it only reported one pooled result (see Figure 2). The nine studies included 75,891 participants.
2.
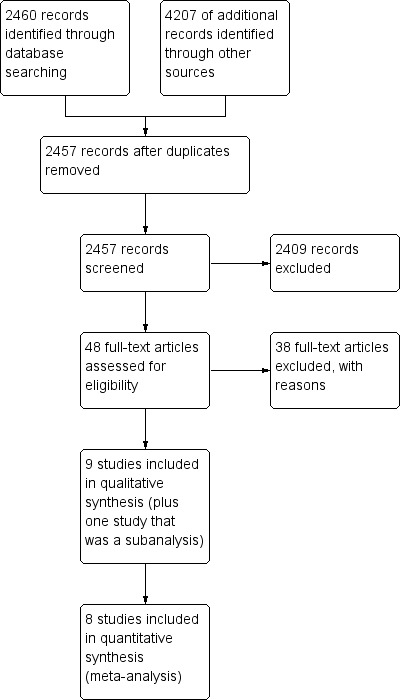
Study flow diagram.
Included studies
Seven studies were conducted in Sub‐Saharan Africa: Zimbabwe (Cowan 2010; Gregson 2007), South Africa (Jewkes 2008), Uganda (Kamali 2003), Kenya (Feldblum 2001) and Tanzania (Doyle 2010; Ross 2007); one study was conducted in Peru (Garcia 2012), and one was a multi‐country study conducted in China, India, Peru, Russia and Zimbabwe (NIMH 2010). The multi‐country study only provided a composite biological result, including HIV, Herpes simplex virus 2 (HSV‐2), syphilis, gonorrhea, chlamydia, and trichomoniasis in women, which was not comparable to other studies. Therefore, it was not possible to include it in the meta‐analysis; nonetheless, risk of bias assessment was carried out. Ross 2007 and Doyle 2010 are studies that developed the same intervention but with differences in methodology (Ross 2007 was a cohort study and Doyle 2010 was a cross‐sectional study) and follow‐up times (three and nine years after the initial intervention, respectively).
Given the definition of 'community' we used in this review, all studies were conducted in the general population, rather than in high‐risk groups. All studies were obtained through database searching.
All nine studies were cluster‐RCTs. Eight trials were originally designed as cohort studies (Cowan 2010; Doyle 2010, Feldblum 2001; Gregson 2007; Jewkes 2008; Kamali 2003; NIMH 2010; Ross 2007), and one as a repeated cross‐sectional study (Garcia 2012); however, due to methodological issues such as attrition bias during the trials’ development, Cowan 2010 and Doyle 2010 switched to a repeated cross‐sectional approach. Incomplete outcome data ranged from 18% in NIMH 2010, to 60% in Doyle 2010.
All studies included primary outcomes (Cowan 2010; Doyle 2010; Feldblum 2001; Garcia 2012; Gregson 2007; Jewkes 2008; Kamali 2003; NIMH 2010; Ross 2007), but NIMH 2010 only reported a unique biological‐composite score. Four studies included HIV incidence (Gregson 2007; Jewkes 2008; Kamali 2003; Ross 2007), and three included HIV prevalence (Cowan 2010; Doyle 2010; Garcia 2012). Two studies included incidence of HSV‐2 (Jewkes 2008; Kamali 2003), and six studies included prevalence of HSV‐2, or any other STI, or both (Cowan 2010; Doyle 2010; Feldblum 2001; Garcia 2012; Gregson 2007; Ross 2007). Three studies included composite biological scores (Feldblum 2001; Garcia 2012; NIMH 2010).
Secondary outcomes were identified in all included studies; however, for the purpose of comparison and in order to carry out meta‐analyses, only those primary and secondary results that were both common across studies and reported in at least two trials were included in the quantitative analysis.
The follow‐up period varied substantially between trials: one year in Feldblum 2001 (although an interim assessment was conducted at six months), two years in Jewkes 2008 and NIMH 2010, three years in Garcia 2012, Gregson 2007 and Ross 2007, four years in Cowan 2010 and Kamali 2003, and nine years in Doyle 2010.
All trials in this review were sponsored by local or foreign governments, education institutions or international NGOs. Thus, all studies declared no conflict of interest. The included studies did not report any adverse effects after the interventions were implemented.
Interventions performed in the included studies
The structural and community‐level interventions that aimed to promote condom use were acceptability and accessibility.
Acceptability, through specific programs such as:
sexual health education in schools and communities, provided by teachers and professional peer educators (Cowan 2010; Doyle 2010; Garcia 2012; Kamali 2003; Ross 2007);
improving reproductive knowledge in the community (Feldblum 2001), promoting communication between parents and teenagers and enhancing community support for the reproductive health of adolescents (Cowan 2010);
participatory learning approaches such as dramas and role playing (Jewkes 2008; Kamali 2003);
training of medical staff with the purpose of creating a clinical environment suitable to the care of adolescents' reproductive health (Cowan 2010; Doyle 2010; Ross 2007);
social marketing of condoms (Doyle 2010; Garcia 2012; Gregson 2007; Ross 2007); and
task‐shifting through community popular opinion leaders (C‐POLs), who are identified as natural leaders among their communities. These people then imparted educational messages about HIV and other STIs (NIMH 2010).
Accessibility, through specific programs such as:
distribution of free condoms (Doyle 2010; Feldblum 2001; Gregson 2007; Ross 2007); and
income‐generating projects (Gregson 2007).
All the selected interventions incorporated or promoted condoms as an important mechanism to prevent HIV and STI transmission.
Risk of bias in included studies
Risk of bias assessment is illustrated in Figure 3 and Figure 4.
3.
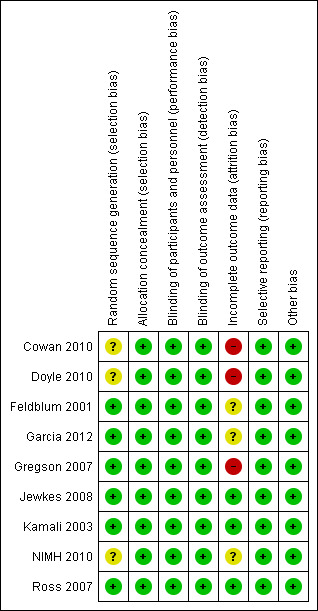
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
4.
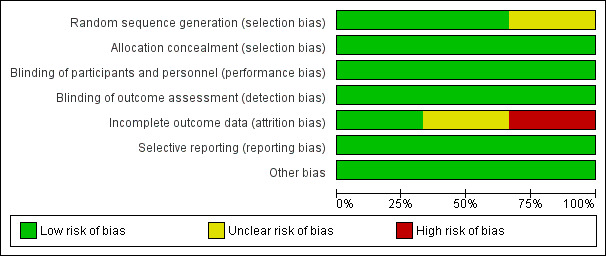
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Random sequence generation
Random sequence generation: six studies were considered to be at low risk of bias (Feldblum 2001; Garcia 2012; Gregson 2007; Jewkes 2008; Kamali 2003; Ross 2007). The other three studies were deemed to be at unclear risk of bias; although all three implemented random sequence generation, the method was not specified (Cowan 2010; Doyle 2010; NIMH 2010).
Allocation concealment
All studies were deemed to be at low risk of bias, since allocation concealment does not influence the results in cluster‐RCTs (Higgins 2011).
Blinding
Due to the nature of the intervention, blinding was not feasible. Since the primary outcomes were biological, it is unlikely that lack of blinding influenced those results. Therefore, all studies were considered to be at low risk of bias for the primary outcomes. It is also unlikely that lack of blinding biased the measurement of knowledge since this outcome was measured through standardized questionnaires. On the other hand, results including self reported behaviors may be prone to bias when individuals know they have taken part in an intervention. Thus, significant changes for this type of outcome (i.e. condom use during last sexual intercourse and two or more sexual partners in the last year) should be interpreted with caution.
Incomplete outcome data
Three studies were considered to be at low risk of attrition bias (Jewkes 2008; Kamali 2003; Ross 2007). Three studies were considered to be at unclear risk of bias: Garcia 2012 was cross‐sectional, therefore there was no mention of attrition bias; Feldblum 2001 and NIMH 2010 had attrition bias below 30% (the threshold point chosen for this systematic review), but the specific reasons for loss to follow‐up were not clearly stated. Three studies were deemed to be at high risk of bias (Cowan 2010; Doyle 2010; Gregson 2007): although all three trials stated that the loss to follow‐up was balanced between the intervention and control groups, and clearly stated the reasons for attrition (i.e. migration, death and absence), the loss to follow‐up was higher than 30%. Two of these studies (Cowan 2010; Doyle 2010) tried to compensate for this deficiency by changing the methodological approach from cohort to cross‐sectional.
Selective reporting
All trials were judged to be at low risk of selective reporting bias since they reported the outcomes originally proposed in each individual study's protocol.
Other potential sources of bias
Different individual recruitment, loss of clusters, early trial termination and inadequate statistical analysis were not detected. Although only four studies conducted pair‐matched randomisation/block randomisation (Feldblum 2001; Garcia 2012; Jewkes 2008; Ross 2007), no study was found to present baseline characteristic imbalance. Thus, all nine studies were deemed as at low risk of bias from these other sources.
Since all studies were community trials, control groups might have been exposed to external sources of information (i.e. national media advertisement, sex education programs in schools) related to condom use and HIV prevention, thus decreasing the relative effect of educational programs in the experimental group. This is known as secular trend, and it is considered as a possible source of bias in any trial conducted at community level (Atienza 2002). In this review, this possible source of bias was judged as unclear since it was not feasible to either confirm it or rule it out.
Funnel plots were possible for three outcomes: gonorrhea prevalence, chlamydia prevalence and two or more sexual partners in last year. Publication bias was not detected for gonorrhea prevalence (Figure 5). Although the results for chlamydia prevalence suggested possible publication bias, this is unlikely since the direction of the effect indicates a higher risk of infection in the treatment group (i.e. four out of five studies reported a risk ratio above one; Figure 6). The same trend was displayed by the outcome two or more sexual partners in the last year, in which four out of five trials reported a higher risk ratio in the treatment group when compared to controls (Figure 7).
5.
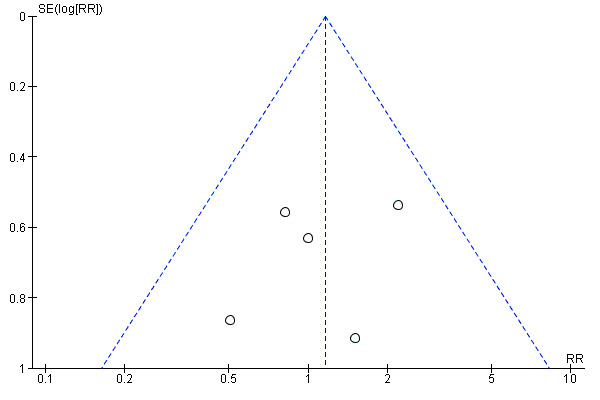
Funnel plot of comparison: Interventions to increase condom use versus standard care. Outcome: 1.7 Gonorrhoea prevalence.
6.
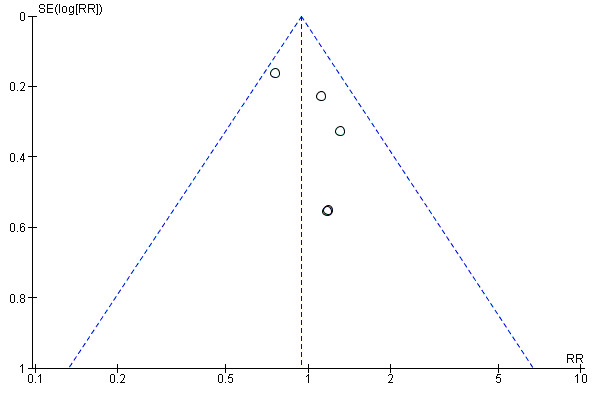
Funnel plot of comparison: Interventions to increase condom use versus standard care. Outcome: 1.8 Chlamydia prevalence.
7.

Funnel plot of comparison: Interventions to increase condom use versus standard care. Outcome: 1.14 Two or more sexual partners in last year.
Effects of interventions
See: Table 1
Primary outcomes
1. HIV incidence / prevalence
2. Other STI’s incidence / prevalence
Four studies reported HIV incidence (Gregson 2007; Jewkes 2008; Kamali 2003; Ross 2007), and all showed no statistical difference between intervention and control groups (RR 0.90, 95% CI 0.69 to 1.19; Analysis 1.1)
1.1. Analysis.
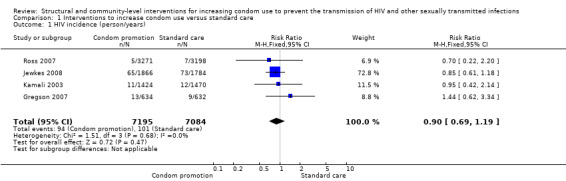
Comparison 1 Interventions to increase condom use versus standard care, Outcome 1 HIV incidence (person/years).
Three studies reported HIV prevalence (Cowan 2010; Doyle 2010; Garcia 2012), demonstrating non‐significant effect after the intervention (RR 1.02, 95% CI 0.79 to 1.32; Analysis 1.2)
1.2. Analysis.
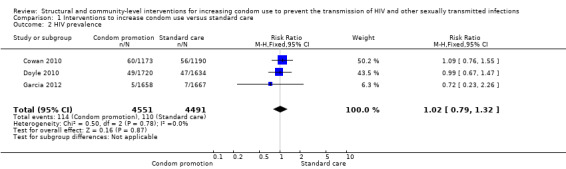
Comparison 1 Interventions to increase condom use versus standard care, Outcome 2 HIV prevalence.
Other individual STIs were also reported in seven (Cowan 2010; Doyle 2010; Feldblum 2001; Garcia 2012; Jewkes 2008; Kamali 2003; Ross 2007) out of the eight studies included in the quantitative analysis (Gregson 2007 only reported HIV incidence).
Two studies (Jewkes 2008; Kamali 2003) yielded no difference in incidence of HSV‐2 (RR 0.76, 95% CI 0.55 to 1.04; Analysis 1.3); two studies (Cowan 2010; Doyle 2010) found no difference in prevalence of HSV‐2 (RR 1.01, 95% CI 0.85 to 1.20; Analysis 1.4).
1.3. Analysis.
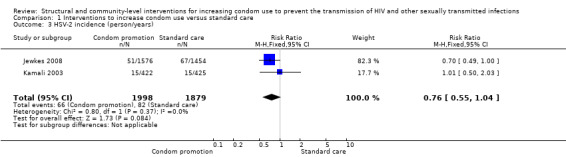
Comparison 1 Interventions to increase condom use versus standard care, Outcome 3 HSV‐2 incidence (person/years).
1.4. Analysis.
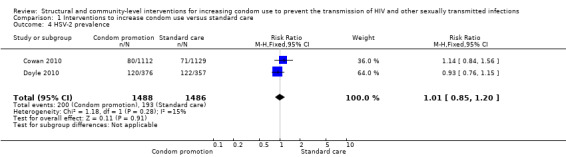
Comparison 1 Interventions to increase condom use versus standard care, Outcome 4 HSV‐2 prevalence.
Only one study reported syphilis incidence (Kamali 2003), finding no change in this result (RR 0.59, 95% CI 0.18 to 1.95; Analysis 1.5). Three trials (Doyle 2010; Garcia 2012; Ross 2007) addressed syphilis prevalence, finding no change after the intervention (RR 0.91, 95% CI 0.71 to 1.17; Analysis 1.6).
1.5. Analysis.
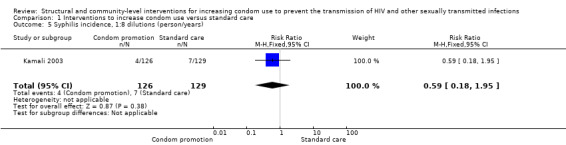
Comparison 1 Interventions to increase condom use versus standard care, Outcome 5 Syphilis incidence, 1:8 dilutions (person/years).
1.6. Analysis.
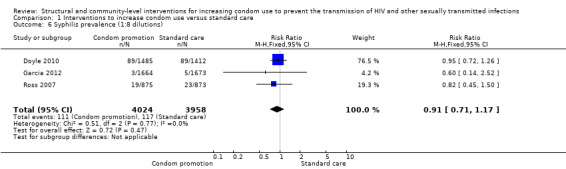
Comparison 1 Interventions to increase condom use versus standard care, Outcome 6 Syphilis prevalence (1:8 dilutions).
Gonorrhea prevalence was reported in five studies (Doyle 2010; Feldblum 2001; Garcia 2012; Kamali 2003; Ross 2007), finding no change after the intervention (RR 1.16, 95% CI 0.67 to 2.02); Analysis 1.7).
1.7. Analysis.
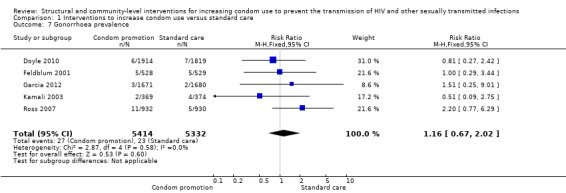
Comparison 1 Interventions to increase condom use versus standard care, Outcome 7 Gonorrhoea prevalence.
Five trials (Doyle 2010; Feldblum 2001; Garcia 2012; Kamali 2003; Ross 2007) reported chlamydia prevalence, finding the intervention non‐effective (RR 0.94, 95% CI 0.75 to 1.18; Analysis 1.8).
1.8. Analysis.
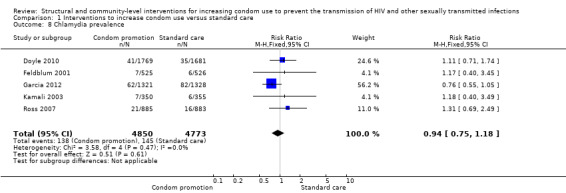
Comparison 1 Interventions to increase condom use versus standard care, Outcome 8 Chlamydia prevalence.
Trichomoniasis' prevalence was measured in different types of populations (i.e. both genders and only in women), thus differential analysis was conducted. Two trials (Feldblum 2001; Ross 2007) assessed this outcome in women, finding no difference between the intervention and control group (RR 1.00, 95% CI 0.77 to 1.30; Analysis 1.9). One trial (Garcia 2012) found a significant reduction in trichomoniasis in both genders after intervention (RR 0.07, 95% CI 0.02 to 0.18; Analysis 1.10).
1.9. Analysis.
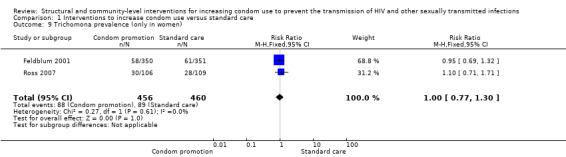
Comparison 1 Interventions to increase condom use versus standard care, Outcome 9 Trichomona prevalence (only in women).
1.10. Analysis.

Comparison 1 Interventions to increase condom use versus standard care, Outcome 10 Trichomona prevalence (both sexes).
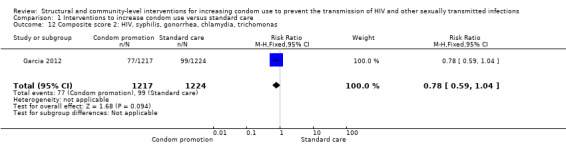
Three studies reported composite biological scores , (Feldblum 2001; Garcia 2012; NIMH 2010). Since the STIs included in each study were different, we decided not to conduct a meta‐analysis, but instead report individual results. We found the three studies to have non‐significant results (RR 0.94, 95% CI 0.84 to 1.06 (Analysis 1.11), RR 0.78, 95% CI 0.59 to 1.04 (Analysis 1.12) and RR 0.99, 95% CI 0.72 to 1.36 (Analysis 1.13), respectively).
1.11. Analysis.
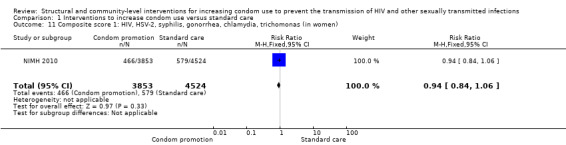
Comparison 1 Interventions to increase condom use versus standard care, Outcome 11 Composite score 1: HIV, HSV‐2, syphilis, gonorrhea, chlamydia, trichomonas (in women).
1.12. Analysis.
Comparison 1 Interventions to increase condom use versus standard care, Outcome 12 Composite score 2: HIV, syphilis, gonorrhea, chlamydia, trichomonas.
1.13. Analysis.
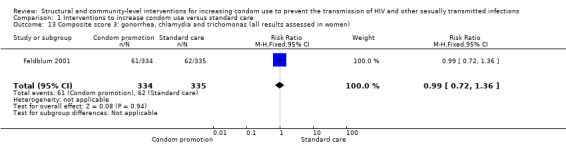
Comparison 1 Interventions to increase condom use versus standard care, Outcome 13 Composite score 3: gonorrhea, chlamydia and trichomonas (all results assessed in women).
Secondary outcomes
1. Self reported condom use (male or female condoms).
2. Self reported number of sexual partners in the last year.
3. Knowledge about HIV.
4. Knowledge about STIs.
5. Knowledge about condom use (both self reported and tested).
6. Self efficacy (assessing self confidence related to condom use and sexual practices after receiving the intervention through standard questionnaires).
Behaviors
Five trials (Cowan 2010; Doyle 2010; Gregson 2007; Kamali 2003; Ross 2007) reported prevalence of two or more sexual partners during the last year, finding no differences between intervention and control groups (RR 0.90, 95% CI 0.78 to 1.04; Analysis 1.14).
1.14. Analysis.
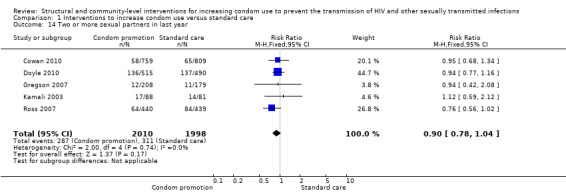
Comparison 1 Interventions to increase condom use versus standard care, Outcome 14 Two or more sexual partners in last year.
Three studies reported on the use of condom during last sexual intercourse without specifying whether sex was with a casual or regular partner (Cowan 2010; Doyle 2010; Ross 2007), finding a statistically significant change after intervention (RR 1.20, 95% CI 1.03 to 1.40; Analysis 1.15).
1.15. Analysis.
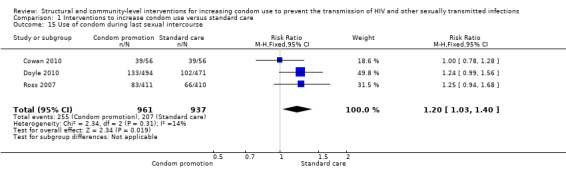
Comparison 1 Interventions to increase condom use versus standard care, Outcome 15 Use of condom during last sexual intercourse.
Doyle 2010 reported use of condom at last sexual intercourse with a non‐regular partner, yielding a non‐significant result (RR 1.16, 95% CI 0.78 to 1.72; Analysis 1.16).
1.16. Analysis.
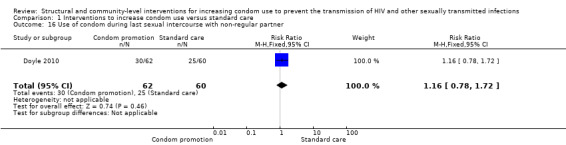
Comparison 1 Interventions to increase condom use versus standard care, Outcome 16 Use of condom during last sexual intercourse with non‐regular partner.
Gregson 2007 reported unprotected sex with a regular partner during the last three years, finding no significant results (RR 1.03, 95% CI 0.91 to 1.16; Analysis 1.17).
1.17. Analysis.
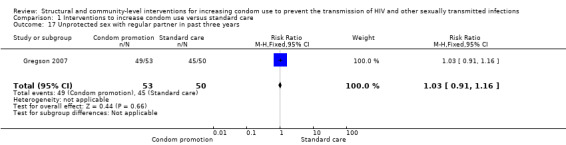
Comparison 1 Interventions to increase condom use versus standard care, Outcome 17 Unprotected sex with regular partner in past three years.
One cohort study reported correct condom use at last sexual intercourse (Jewkes 2008) at 12 and 24 months; the results showed no significant change (RR 0.98, 95% CI 0.85 to 1.12 (Analysis 1.18), and RR 0.97, 95% CI 0.84 to 1.12 (Analysis 1.19), respectively).
1.18. Analysis.
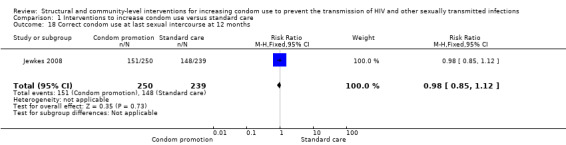
Comparison 1 Interventions to increase condom use versus standard care, Outcome 18 Correct condom use at last sexual intercourse at 12 months.
1.19. Analysis.
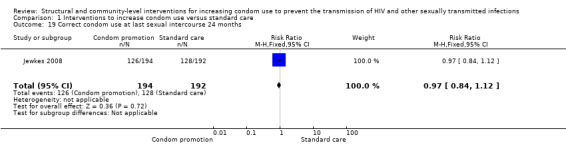
Comparison 1 Interventions to increase condom use versus standard care, Outcome 19 Correct condom use at last sexual intercourse 24 months.
One study (Feldblum 2001) assessed consistent condom use at 6 and 12 months; both results showed non‐significant difference between treatment and control groups (RR 0.63, 95% CI 0.38 to 1.05 (Analysis 1.20) and RR 0.92, 95% CI 0.53 to 1.60 (Analysis 1.21), respectively).
1.20. Analysis.
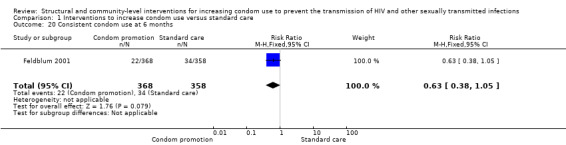
Comparison 1 Interventions to increase condom use versus standard care, Outcome 20 Consistent condom use at 6 months.
1.21. Analysis.
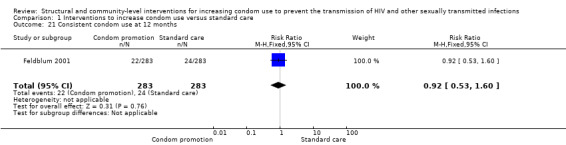
Comparison 1 Interventions to increase condom use versus standard care, Outcome 21 Consistent condom use at 12 months.
Knowledge
Four trials (Cowan 2010; Doyle 2010; Gregson 2007; Ross 2007) reported improvement in knowledge about HIV, finding a statistically significant change after intervention (RR 1.16, 95% CI 1.03 to 1.29; Analysis 1.22).
1.22. Analysis.
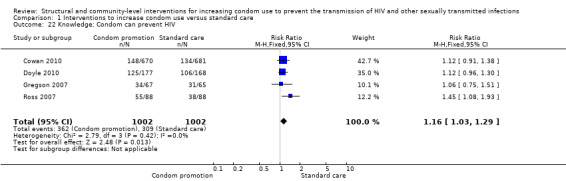
Comparison 1 Interventions to increase condom use versus standard care, Outcome 22 Knowledge: Condom can prevent HIV.
Three trials (Cowan 2010; Doyle 2010; Ross 2007) reported improvement in knowledge about other STIs, finding the intervention to be effective (RR 1.23, 95% CI 1.07 to 1.41; Analysis 1.23).
1.23. Analysis.
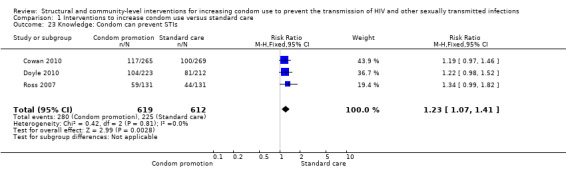
Comparison 1 Interventions to increase condom use versus standard care, Outcome 23 Knowledge: Condom can prevent STIs.
Self efficacy
Since all results under this classification assessed different and non‐comparable outcomes, a meta‐analysis was not feasible; instead, individual results are described below.
Cowan 2010 reported condom self efficacy and HIV self efficacy, yielding no significant results in both cases (RR 1.16, 95% CI 0.94 to 1.41 (Analysis 1.24), and RR 0.99, 95% CI 0.68 to 1.46 (Analysis 1.25) respectively).
1.24. Analysis.

Comparison 1 Interventions to increase condom use versus standard care, Outcome 24 Condom self efficacy.
1.25. Analysis.
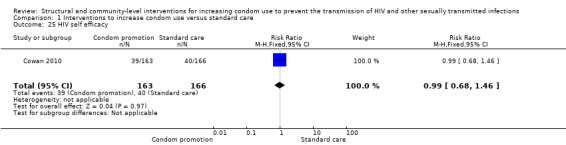
Comparison 1 Interventions to increase condom use versus standard care, Outcome 25 HIV self efficacy.
Gregson 2007 reported non‐specified self efficacy, finding no changes after intervention (RR 1.02, 95% CI 0.90 to 1.17; Analysis 1.26).
1.26. Analysis.
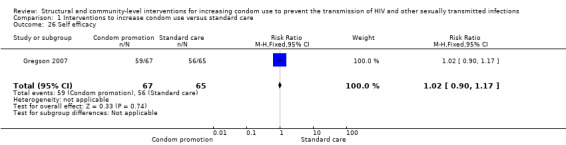
Comparison 1 Interventions to increase condom use versus standard care, Outcome 26 Self efficacy.
Sensitivity analysis
Primary outcomes
There was no significant change in any of the biological results at any of the six different ICC values (all results remained non‐significant: Figure 8, Figure 9, Figure 10, Figure 11, Figure 12, Figure 13, Figure 14, Figure 15).
8.
Sensitivity analysis ICCs; outcome: HIV incidence.
9.
Sensitivity analysis ICCs; outcome: HIV prevalence.
10.
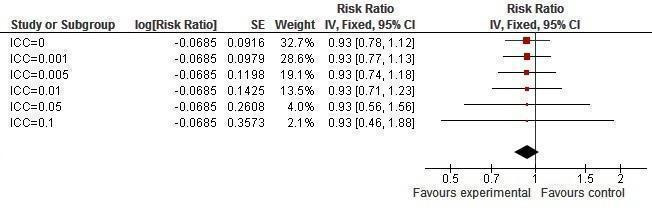
Sensitivity analysis ICCs; outcome: HSV‐2 incidence.
11.
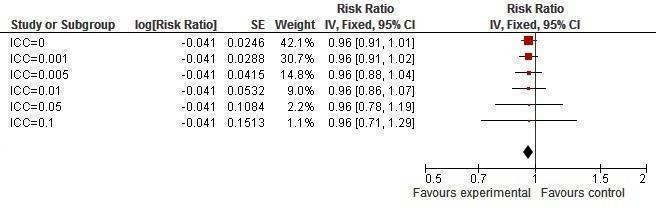
Sensitivity analysis ICCs; outcome: HSV‐2 prevalence.
12.
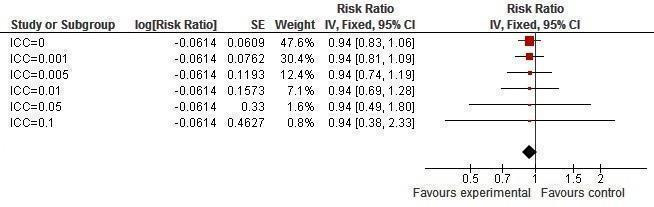
Sensitivity analysis ICCs; outcome: Syphilis prevalence.
13.
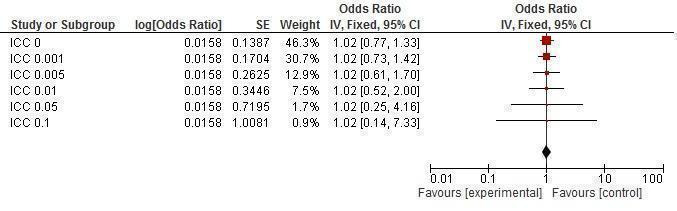
Sensitivity analysis ICCs; outcome: Gonorrhea prevalence.
14.
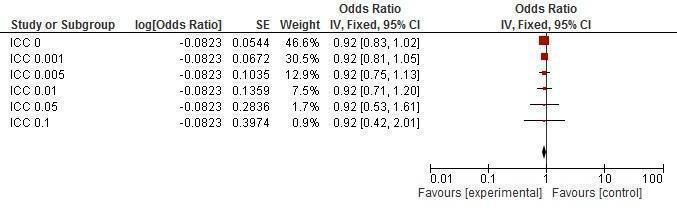
Sensitivity analysis ICCs; outcome: Chlamydia prevalence.
15.
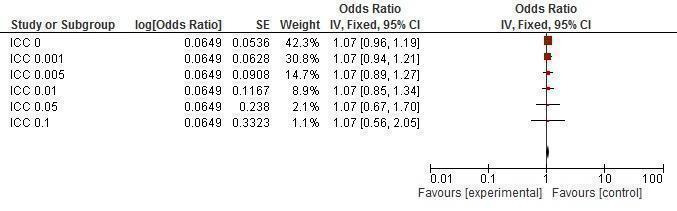
Sensitivity analysis ICCs; outcome: Trichomonas prevalence.
Secondary outcomes
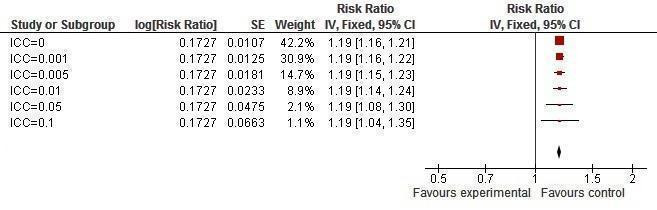
It was found that prevalence of two or more sexual partners became significant with an ICC value of 0.005 or less (Figure 16). Condom use at last sexual intercourse became non‐significant at an ICC value of 0.05 and above (Figure 17). Knowledge about HIV and other STIs remained significant, irrespective of the ICC value (Figure 18, Figure 19).
16.
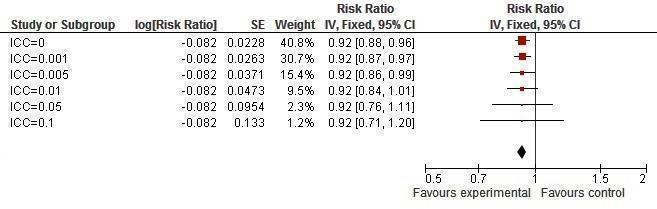
Sensitivity analysis ICCs; outcome: Two or more sexual partners in last year.
17.
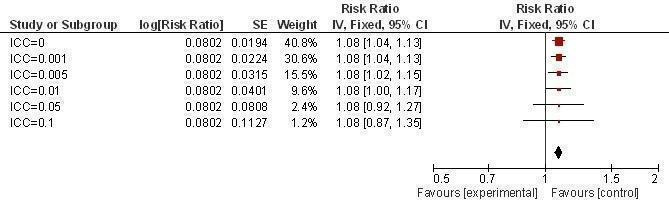
Sensitivity analysis ICCs; outcome: Condom use at last sexual intercourse.
18.
Sensitivity analysis ICCs; outcome: Knowledge, condoms can prevent HIV.
19.
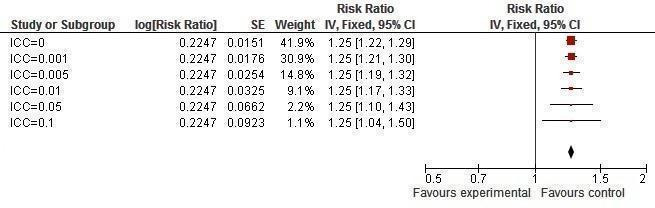
Sensitivity analysis ICCs; outcome: Knowledge, condoms can prevent STIs.
Discussion
Summary of main results
This review addressed the question of whether structural interventions to promote condom use at the community level reduced the incidence or prevalence of HIV and other STIs, assessed through laboratory test, as well as other secondary outcomes such as knowledge, self efficacy and sexual behaviors.
Nine studies meeting all inclusion criteria were obtained. Loss to follow‐up in three studies was the only relevant source of bias. The studies featured condom acceptability by imparting sexual education and promoting social marketing of condoms; accessibility was addressed through provision of free condoms and implementation of micro‐credits. After adjusting for clustering effects, the meta‐analysis demonstrated the intervention(s) to have a non‐significant effect in reducing incidence or prevalence of HIV, nor did they reduce prevalence or incidence of HSV‐2, syphilis, gonorrhea, chlamydia or trichomonas. One individual study (Garcia 2012) proved to be effective in reducing the prevalence of trichomoniasis in the general population when addressing both genders.
In each trial authors reported the possible reasons for the intervention(s) to have not had a significant effect on biological outcomes, including: a 'diluted' intervention in which the results were assessed in some people who were not directly exposed to the intervention (Cowan 2010); social ambivalence toward condoms, thus hindering their use (Cowan 2010; Doyle 2010; Feldblum 2001); attrition bias since some people who received the intervention then left the study, consequently reducing the effect of the intervention (Cowan 2010; Doyle 2010; Garcia 2012); 'secular trend', defined as the exposure of the control groups to other sources of HIV and condom awareness such as media condom campaigns or sex education programs, thus reducing the relative impact of the intervention(s) in the experimental group (Doyle 2010; Gregson 2007; Kamali 2003; NIMH 2010); difficulty in changing behaviors (Doyle 2010); insufficient statistical power (Gregson 2007; Jewkes 2008; Kamali 2003); insufficient follow‐up (Gregson 2007); and self reporting bias related to sexual behaviors (Jewkes 2008; Kamali 2003; Ross 2007).
The results varied substantially regarding secondary outcomes. There was no significant change in the prevalence of respondents with two or more sexual partners in the last year. Although individual trials did not demonstrate any statistically significant change in unprotected sex, the meta‐analysis proved the intervention(s) to be effective; individual trials exploring consistent condom use showed non‐significant results. All meta‐analyses regarding change in knowledge yielded statistically significant results in favor of the intervention(s). Meta‐analysis addressing self efficacy was not feasible, but all three individual studies reported no significant results.
After conducting sensitivity analysis with different ICC values, we found that biological and knowledge‐related outcomes were robust, whereas behavioral outcomes such as condom use and number of sexual partners tended to fluctuate between significant and non‐significant depending on the magnitude of the ICC.
Possible reasons for non‐significant results
One plausible explanation for the non‐significant results regarding biological outcomes is that not all studies widely distributed free or low‐cost condoms, limiting the effect of social acceptability and education regarding HIV and STIs due to financial barriers. However, this possible explanation was ruled out after conducting a stratified analysis which included studies featuring both education and free condom distribution, obtaining non‐significant results (Analysis 2.1, Analysis 2.2, Analysis 2.3, Analysis 2.4, Analysis 2.5, Analysis 2.6).
2.1. Analysis.
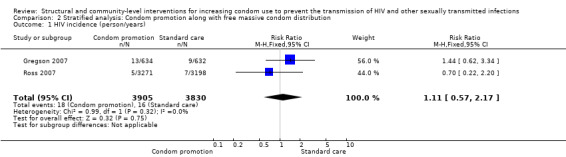
Comparison 2 Stratified analysis: Condom promotion along with free massive condom distribution, Outcome 1 HIV incidence (person/years).
2.2. Analysis.
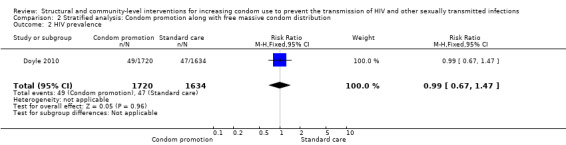
Comparison 2 Stratified analysis: Condom promotion along with free massive condom distribution, Outcome 2 HIV prevalence.
2.3. Analysis.
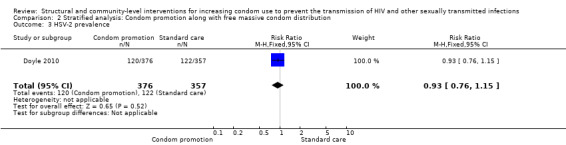
Comparison 2 Stratified analysis: Condom promotion along with free massive condom distribution, Outcome 3 HSV‐2 prevalence.
2.4. Analysis.
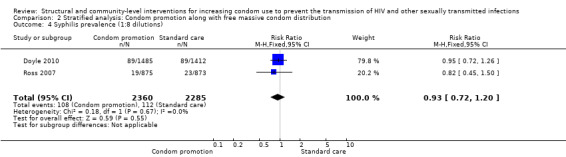
Comparison 2 Stratified analysis: Condom promotion along with free massive condom distribution, Outcome 4 Syphilis prevalence (1:8 dilutions).
2.5. Analysis.
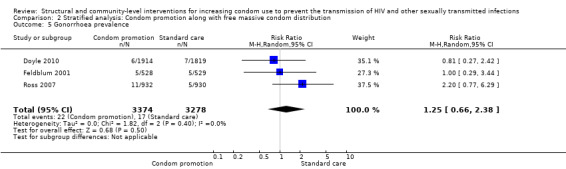
Comparison 2 Stratified analysis: Condom promotion along with free massive condom distribution, Outcome 5 Gonorrhoea prevalence.
2.6. Analysis.
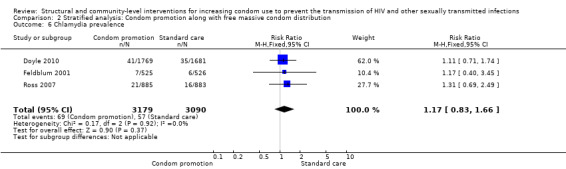
Comparison 2 Stratified analysis: Condom promotion along with free massive condom distribution, Outcome 6 Chlamydia prevalence.
There is a a gap between improvement in knowledge, behaviors and biological outcomes. This review showed that people in intervention groups indeed acquired more knowledge about HIV prevention, but HIV and other STIs' prevalence and incidence did not decrease. Results involving knowledge and biological markers were robust, presenting no heterogeneity and similar outcomes when sensitivity analysis was conducted. In contrast, the intermediate results for behaviors showed a more diverse pattern; condom use showed a statistically significant change, whereas number of sexual partners in the last year did not. However, both of these outcomes showed volatility when sensitivity analysis was conducted. To explain this, several authors have pointed out that modifying sexual behaviors is a difficult task, irrespective of the approach used (Foss 2007; Michielsen 2010; Ojo 2011; Ota 2011). Another study showed that levels of consistent condom use have been far from optimum in many communities, despite continuous and intensive promotion campaigns (Potts 2008). This in turn may be accounted for the fact that sex occurs in private settings, making these behaviors more difficult to change (Coates 2008), and because of the entrenched influence of cultural norms and religion (Lefkowitz 2004; Thomsen 2004).
It is also extremely difficult to confirm the validity of self reported behaviors (Coates 2008) given the tendency of people to over‐report practices that are perceived as positive, and similarly, to under‐report behaviors that have social negative connotations. This is called social desirability bias, and it is considered an undermining factor in self reported studies (Randall 1991). Likewise, lack of blinding could have introduced some bias in self reported condom use. Thus, the validity of the significant findings for these results is questionable, and may explain to some extent the poor impact on biological outcomes.
This review found no change in HIV/STI incidence and prevalence despite an apparent increase in condom use. This may be explained by inconsistent condom use (Hearst 2004). People may increase condom use as a result of campaigns, social acceptability and condom availability, but if condom use is not constant and concurrency takes place, particularly with multiple regular partners where condom use is more likely to be irregular (Foss 2007; Mah 2010), the probability of acquiring an HIV and other STI remains (Foss 2007; Mah 2010; Senn 2009; Subramanian 2008). Although this review did not present results regarding concurrency, and only included number of sexual partners, it is likely that both circumstances have played a role in the non‐significant biological results (Gorbach 2005). Most of the retrieved studies were obtained from Sub‐Saharan Africa, a region in which concurrency has been frequently reported (Mah 2010).
In addition, a methodological issue involving the nature of control groups may explain to some degree the non‐significant outcomes. Since constant campaigns on HIV prevention have been implemented in Sub‐Saharan Africa (Painter 2001), some communities (e.g. South Africa, Senegal and Uganda) already had some awareness about HIV (Shisana 2001; UNAIDS 1999; USAID 2002). As a consequence, control groups are likely to have been exposed to some type of 'intervention' by the time the reviewed strategies were implemented, partially offsetting the overall effect in the experimental group. This is a phenomenon known as a 'secular trend', and it has been regarded as a potential threat in any community trial (Atienza 2002).
Finally, this review attempted to be comprehensive by including all possible rigorous studies in which structural and community level interventions promoted condom use. However, there could be other structural and community‐level strategies that may yield positive results, but have not been conducted/published under rigorous methodologies to date. For example, media advertisements or placement of public condom machines using a RCT design coupled with biological outcomes. Thus, future studies may change the conclusions we are presenting in this revision.
Overall completeness and applicability of evidence
A potential limitation to the external validity of this review is that seven out of the nine trials were carried out in Sub‐Saharan Africa, a region that in turn is widely diverse. For example, male circumcision is practiced in most of West Africa, rather infrequent in several southern African countries, and variable in central and eastern Africa (UNAIDS 2007); Islam is the predominant religion in the countries located in the Sahel and the African horn, whereas Christianity is widely extended in central and southern Africa (Britannica 2003); also, concurrency has been reported as more prevalent in western and central Africa when compared to eastern and southern Africa (Reniers 2010). In addition, the multi‐country trial (NIMH 2010) did not provide individual figures for each disease, leaving only one study (Garcia 2012) with specific results conducted outside Sub‐Saharan Africa. Although the results from this review may not be applicable in other settings, most of the global HIV burden is concentrated in Sub‐Saharan Africa, which underlines the relevance of the results to the current global HIV prevention context.
Furthermore, given both our definition of community and the population to which the interventions were addressed, the participants included in each trial were mainly drawn from the general population rather than high‐risk populations such as men who have sex with men (MSM), injecting drug users (IDUs) or female sex workers (FSWs). Had the trials included and identified large segments of high‐risk populations, our meta‐analysis may have yielded different results.
Each individual trial proposed a specific set of STIs to be measured, thus making it unfeasible to assess the same STIs in all studies. This was also the case with the proposed secondary outcomes.
It is important to mention that although most studies reported on condom use, their type of assessment was not always comparable across trials; thus preventing a larger pooling of studies in the meta‐analysis.
Quality of the evidence
Although only nine trials were retrieved, the number of total participants was large at 75,891. To the best of our knowledge, this meta‐analysis covers the largest total sample size related to this topic. Moreover, all meta‐analyses were consistent and had low heterogeneity.
An important finding of this review is that none of the studies implemented promotion of condoms as a unique intervention. In fact, all trials promoted it through education, distribution, or both, in combination with one or several of the following: training of medical staff, providing accessible and open health services to adolescents, educating parents on sexuality and encouraging dialogue about sex with teenagers, task‐shifting through community members, income generation and strengthened treatment of STIs. Therefore, these results reflect the synergy of promoting condom use along with other strategies as opposed to an outcome derived solely from condom promotion. Nevertheless, conclusions can still be inferred based on these outcomes. Incidence or prevalence of HIV and most biological results did not change after the interventions, implying that condom use combined with other strategies enacted at a community level may not work effectively.
Potential biases in the review process
This review only included biological outcomes confirmed by laboratory tests as primary results and thus excluded the possibility of self reporting bias. Moreover, all studies were RCTs, reducing the likelihood of imbalance of known and unknown baseline characteristics and confounders.
In order to conduct meta‐analysis, each set of results should be comparable. Due to the limited number of studies that were retrieved and a large variation in methodology, performing meta‐analysis was not always possible. When meta‐analyses were feasible, only a few studies were included with an average of three trials per analysis.
Most studies reported blinding as unfeasible, or did not mention it at all, leading to possible bias in self reported outcomes. This may be the case with measures of condom use, in which a significant result was obtained. Bias arising from lack of blinding is unlikely in the other self reported outcome (two or more sexual partners in the last year) since it did not yield a significant result.
Attrition bias was an important finding in this review. Since the present study focused only on community interventions, entire populations were the subject of research. Under these circumstances, it was frequent that large numbers of participants left the study due to either migration or death, leading to incomplete data. This situation may have undermined to some extent the validity of our findings (Higgins 2011).
In addition, the secular trend phenomenon that tends to take place in community trials may have biased to some extent the results in this review by diminishing the apparent effect that education could have on HIV/STIs prevention.
Moreover, It is possible that HIV/STIs prevention programs have different levels of impact depending on the actor leading the initiative. For example, the success of the100% condom use program in Thailand's brothels is likely to have worked since there was government enforcement at a national level (Rojanapithayakorn 1996). In Uganda, the early commitment of the government coupled with intersectoral collaboration (i.e. civil organizations, NGOs, private sector) is thought to have hampered the HIV epidemic (USAID 2002). In most of the included studies for this revision, the interventions were led by an international organization/NGO, and some through a partnership between these institutions and local governments. Unfortunately, it was not possible to obtain studies in which a policy change led primarily by the local government took place under the experimental conditions proposed in this review.
Finally, the primary purpose of this review was to explore the effect of condom promotion on the transmission of HIV and other STIs; thus, only studies reporting biological outcomes were included. This approach, in consequence, may have limited the validity of the secondary outcomes reported in this revision. Depending on the scope and purpose of further updates of this document, the inclusion of studies might not be restricted by the type of outcomes so that all results are free of bias.
Agreements and disagreements with other studies or reviews
This review found that the intervention(s) had a significant impact in increasing both condom use at last sex and knowledge that condoms can prevent the transmission of HIV and other STIs. However, the incidence and prevalence for HIV and HSV‐2 as well as the prevalence of gonorrhea, chlamydia, syphilis and trichomonas did not yield a significant change after the interventions were implemented. Similarly, number of sexual partners did not show a significant decrease.
The present findings are in agreement with other systematic reviews that found the continued transmission of HIV despite increased condom use (Ng 2011; Ota 2011; Shahmanesh 2008) One systematic review featuring behavioral interventions (i.e acceptability) at group and community level in FSWs (Wariki 2012) found that HIV transmission declined when results were assessed within six months immediately after the intervention was conducted; however, a non‐significant effect was found at 12 months’ follow‐up. Sangani 2004 found that population‐based interventions implementing education for preventing STIs did not reduce HIV transmission. It should be also noted that few publications have directly assessed HIV sero‐prevalence as an outcome, so many reviews could not be compared.
Systematic reviews have found contradictory results regarding condom use along with behavioral interventions and their impact in transmission of other STIs. We found that the intervention(s) described in this paper do not reduce transmission of most STIs. Thus, our results were consistent with other systematic reviews on this topic: Medley 2009 and Ota 2011 found a non‐significant effect in STI reduction, Underhill 2008 did not find any statistical effect on self reported STIs, and Michielsen 2010 found only one study reporting a decrease in HSV‐2. In contrast, studies with pooled results of several STIs such as Charania 2011; Herbst 2007a; Lyles 2007; Shahmanesh 2008, and with individual STI outcomes such as Ng 2011 and Wariki 2012 found statistical differences between intervention and control groups after the interventions were conducted. Sangani 2004 found mixed results (individual STI results). It is, however, difficult to establish a direct comparison with several of those analyses, since many of them did not provide independent figures for each disease, but aggregated all STIs into a unique composite score.
This review yielded a non‐significant effect in changing the number of sexual partners. This was consistent with previous studies by Ojo 2011, Michielsen 2010 (who found heterogeneous results, highlighting an overall non‐significant result), and partially by Townsend 2013 who found mixed or little effect. The opposite result was reported by Paul‐Ebhohimhen 2008, Lyles 2007, Herbst 2007a, and Noar 2008. However, in the majority of systematic reviews we found, the number of sexual partners is usually either aggregated into a composite score (risky behaviors) or not assessed at all.
This review found that condom use increased after intervention. This was also reported by Charania 2011, Medley 2009, Herbst 2007a, Herbst 2007b, Lyles 2007, Shahmanesh 2008, Johnson 2008, Underhill 2008 and Wariki 2012; studies in which the target was mainly high risk populations (i.e. FSWs, MSM, IDUs). Wang 2011, Bertrand 2006 and Sangani 2004 found a small increase in condom use; with the exception of Wang 2011, they were conducted in the general population. Conversely, Michielsen 2010, Paul‐Ebhohimhen 2008 and Ota 2011 found no significant change; apart from Ota 2011 which targeted FSWs, these trials were carried out in general populations. Foss 2007 reported that interventions were not effective in increasing condom use in steady couples and evidence for casual partners was scarce, whereas the intervention worked for FSWs and their clients.
Findings in this review demonstrated increased knowledge about HIV and STIs. The same result was reported by Medley 2009, Paul‐Ebhohimhen 2008, Underhill 2008, Ota 2011 and Bertrand 2006. No systematic review was found stating that knowledge did not increase after intervention.
Among the previous reviews Charania 2011, Herbst 2007a, Johnson 2008, Underhill 2008, Wariki 2012, Ng 2011, Sangani 2004 and Bertrand 2006 included studies that were conducted at the community level.
Although several of these systematic reviews have directly or indirectly assessed the impact of condom promotion, this is the first review reporting on individual biological outcomes and including only community‐based interventions. For example, Charania 2011 focused on reported condom use and only provided a unique pooled figure for STIs incidence in which all three levels of interventions (i.e. individual, groups and community) were combined; moreover, it did not mention what particular STIs were included in the analysis. Herbst 2007a did not include the general population, and Ng 2011 focused on STI control and did not use structural approaches. Moreover, despite the different methodologies used in the trials included in this review, a quantitative estimate of the intervention's effect (i.e. meta‐analysis) was possible.
Authors' conclusions
Implications for practice.
We did not find clear evidence that condom promotion, when implemented at community and structural levels, reduces the transmission of HIV or other STIs. The quality of evidence for HIV transmission was moderate when analysed as incidence data due to imprecision, and low when analysed as prevalence data due to imprecision and risk of bias. Moreover, caution is advised regarding to what extent this conclusion reflects the full spectrum of structural interventions, as well as the population in which it is applicable. The selected studies were conducted primarily in Eastern and Southern Africa, and to a lesser extent, in other developing nations (i.e. Peru, China, India, Rusia). Therefore, our conclusions are mainly applicable to these settings and inferences may not be adequate in other contexts. Despite these limitations, it is important to note that the largest proportion of the global HIV burden takes place in these countries, with Sub‐Saharan Africa the most affected region worldwide; therefore, our conclusions are pertinent to the current context of the HIV epidemic.
Other considerations
Since the intervention assessed in this review did not have a clear impact in preventing the transmission of HIV and other STIs, efforts to scale up programs that have already proven useful in doing so, such as, but not limited to, circumcision for heterosexual men (Arora 2012; Siegfried 2009; Weiss 2000) and provision of antiretroviral treatment for people living with HIV (in general and high‐risk populations) should be evaluated (Anglemyer 2011; Cohen 2011). Consequently, governments, private sector stakeholders, NGOs and policy makers may need to consider reallocation of some resources from broad‐based condom use campaigns in the general population to, for example, HIV screening and treatment programs and male circumcision projects in those developing regions in which the latter interventions have been overlooked. This is of special relevance in those contexts wherein HIV is prevalent. These interventions could be enhanced with community engagement, awareness building and innovative ideas to promote condoms. Also, efforts from the scientific community and financial sponsors should be devoted to search and develop more effective and cost‐effective strategies to decrease HIV transmission, such as vaccination or identification of what kinds of voluntary testing and counseling programs are feasible and cost‐effective in specific settings. We suggest that these initiatives should be tested within community and structural frameworks to determine their effectiveness under such conditions.
Implications for research.
To evaluate the full extent of the role that structural interventions play in condom promotion, we recommend applying strategies that engage different mechanisms to those described in this review. Such interventions should be methodologically strict (i.e. RCTs, providing biological data) and may include changes to policies, installing condom machines, use of public health media campaigns and women empowerment. It is possible that these strategies may have the potential to prevent the transmission of HIV and other STIs.
A significant finding in our review is the inconsistent reporting of ICCs in the literature, suggesting that most cluster trials do not provide ICCs when presenting their results. When conducting systematic reviews that include cluster‐RCTs, it is essential to take into account and report design effects and ICCs in order to adjust for the heterogeneity within and between groups. Therefore, we call on researchers to report individual ICCs for each included outcome when conducting cluster trials. In the case of systematic reviews that include cluster studies where ICCs were not reported, it is important to implement a standard procedure to estimate those figures accurately.
Future research that explores structural interventions should both state which of its three main components ‐ acceptability, accessibility or availability – was conducted and identify the underlying factors influencing these components. If more than one of these interventions were carried out, the review should implement stratified analysis, since each subintervention tackles different obstacles. For example, if a community changes its perception about certain technology (e.g. condoms) but faces economic barriers to purchase it, it is unlikely the intervention produces the expected changes. Using this approach would allow authors to pinpoint which substrategy or combination of substrategies yielded results and which did not.
Finally, since the present review treated the term 'community' as a whole geographical area (i.e. towns, cities), the participants were mainly general and not high‐risk populations. Thus, those definitions in which a community implies a smaller group of people were excluded. These 'smaller communities' include brothels, MSM groups, and STI clinics. Therefore, further research should be undertaken to evaluate if high‐risk community interventions, at a structural level, and promoting condom use yield better results than those obtained in this review.
History
Protocol first published: Issue 4, 2001 Review first published: Issue 7, 2014
| Date | Event | Description |
|---|---|---|
| 5 October 2011 | New citation required but conclusions have not changed | New author team, and complete revision of protocol |
| 6 January 2011 | Amended | "Clean slate" for new author team |
| 12 November 2008 | Amended | Converted to RevMan 5 and re‐published without new citation |
Acknowledgements
We thank the developers of the original protocol: Landon Myer, Chelsea Morroni, Catherine Mathews, and Maya Tholandi. We would also like to thank Ai Koyanagi and Tobe‐Gai for their collaboration in the development of the last published protocol. Finally, thanks to Emma Barber for her valuable contribution to the editing of this paper.
Appendices
Appendix 1. Search strategy
Title: Structural and community level intervention for increasing condom use to prevent the transmission of HIV and others STI
Database: WHO International Clinical Trials Registry Platform Date: 1 April 2014 Search terms: HIV AND condom
Number of records retrieved: 29 records for 27 trials
Database: Clinicaltrials.gov
Date: 1 April 2014
Search strategy: HIV and CONDOM | Interventional studies | received from 01/01/1980 to 01/04/2014
Number of studies retrieved: 164 studies
Database: CLIB 2014, Issue 4 of 4 (1980 – 2014)
ID Search
Date: 1 April 2014
#1 MeSH descriptor HIV Infections explode all trees
#2 MeSH descriptor HIV explode all trees
#3 hiv OR hiv‐1* OR hiv‐2* OR hiv1 OR hiv2 OR HIV INFECT* OR HUMAN
IMMUNODEFICIENCY VIRUS OR HUMAN IMMUNEDEFICIENCY VIRUS OR HUMAN
IMMUNE‐DEFICIENCY VIRUS OR HUMAN IMMUNO‐DEFICIENCY VIRUS OR
HUMAN IMMUN* DEFICIENCY VIRUS OR ACQUIRED IMMUNODEFICIENCY
SYNDROME OR ACQUIRED IMMUNEDEFICIENCY SYNDROME OR ACQUIRED
IMMUNO‐DEFICIENCY SYNDROME OR ACQUIRED IMMUNE‐DEFICIENCY
SYNDROME OR ACQUIRED IMMUN* DEFICIENCY SYNDROME
#4 MeSH descriptor Lymphoma, AIDS‐Related, this term only
#5 MeSH descriptor Sexually Transmitted Diseases, Viral, this term only
#6 (#1 OR #2 OR #3 OR #4 OR #5)
#7sexually transmitted disease*:ti,ab,kw OR sexually transmissible disease*:ti,ab,kw OR
sexually transmitted infection*:ti,ab,kw OR sexually transmissible infection*:ti,ab,kw OR
sexually transmitted infectious disease*:ti,ab,kw OR sexually transmissible infectious
disease*:ti,ab,kw OR sexually transmitted disorder*:ti,ab,kw OR sexually transmissible
disorder*:ti,ab,kw OR STI:ti,ab,kw OR STIs:ti,ab,kw OR STD:ti,ab,kw OR STDs:ti,ab,kw
#8 MeSH descriptor Sexually Transmitted Diseases explode all trees
#9 (#7 OR #8)
#10 (#6 OR #9)
#11 MeSH descriptor Community Networks, this term only
#12 (structural OR community OR communities OR school OR schools):ti,ab,kw
#13 (#11 OR #12)
#14 (#13 AND ( intervention OR interventions ))
#15 MeSH descriptor Health Knowledge, Attitudes, Practice, this term only
#16 MeSH descriptor Risk Reduction Behavior explode all trees
#17 (risk reduction OR risk reducing):ti,ab,kw
#18 (#15 OR #16 OR #17)
#19 MeSH descriptor Socioeconomic Factors explode all trees
#20 (socioeconomic OR socio economic OR social or economic*):ti,ab,kw
#21 MeSH descriptor Social Marketing explode all trees
#22 (#19 OR #20 OR #21)
#23 MeSH descriptor Public Opinion, this term only
#24 MeSH descriptor Women's Rights, this term only
#25 public opinion OR ((woman OR female OR women*) AND empower*)
#26 (#23 OR #24 OR #25)
#27 MeSH descriptor Mass Media explode all trees
#28 (media OR mass communication OR mass communications OR campaign OR
campaigns):ti,ab,kw
#29 (#27 OR #28)
#30 MeSH descriptor Health Policy, this term only
#31(legisation OR political OR policy OR legal OR subsidy OR subsidisation OR
subsidization OR subsidies OR environment OR environmental OR access OR
accessibility OR availability OR distribution):ti,ab,kw
#32 (#30 OR #31)
#33 (#14 OR #18 OR #22 OR #26 OR #29)
#34 (condom OR condoms):ti,ab,kw
#35 (#10 AND #33 AND #34)
#36 (#10 AND #33 AND #34), from 1980 to 2011
Database: PubMed (1980 – 2014) Date: 1 April 2014
Search Most Recent Queries Time
#15 Search #11 AND #12 AND #13 Limits: Publication Date from
1980/01/01 to 2011/12/05
03:27:20
#14 Search #11 AND #12 AND #13 03:25:38
#13 Search (randomized controlled trial [pt] OR controlled clinical trial
[pt] OR randomized [tiab] OR placebo [tiab] OR "clinical trials as
topic"[mesh: noexp] OR randomly [tiab] OR trial [ti]) NOT (animals
[mh] NOT humans [mh])
03:25:25
#12 Search Condoms[MH] OR condom*[tiab] 03:25:03
#11 Search #3 AND #10 03:24:49
#10 Search #4 OR #5 OR #6 OR #7 OR #8 OR #9 03:24:34
#9 Search legislation[tiab] OR health policy[mh] OR political[tiab] OR
policy[tiab] OR legal[tiab] OR subsidy[tiab] OR subsidies[tiab] OR
environment[tiab] OR environmental[tiab] OR subsidization[tiab] OR
subsidisation[tiab] OR access[tiab] OR accessibility[tiab] OR
availability[tiab] OR distribution[tiab]
03:23:49
#8 Search mass media[mh] OR media[tiab] OR mass
communication[tiab] OR mass communications[tiab] OR
campaign[tiab] OR campaigns[tiab]
03:23:34
#7 Search public opinion[mh:noexp] OR public opinion[tiab] OR
women’s rights[mh] OR ((woman[tiab] OR female[tiab] OR
women*[tiab]) AND empower*[tiab])
03:23:22
#6 Search socioeconomic factors[mh] OR socioeconomic[tiab] OR
socio economic[tiab] OR social marketing[mh] OR social[tiab] OR
economic*[tiab]
03:22:59
#5 Search health knowledge, attitudes, practice[mh] OR risk reduction
behavior[mh] OR risk reduction[tiab] OR risk reducing[tiab]
03:22:45
#4 Search (structural[tiab] OR community[tiab] OR communities[tiab]
OR school[tiab] OR schools[tiab] OR community networks[mh]) AND
(intervention[tiab] OR interventions[tiab])
03:22:38
#3 Search #1 OR #2 03:22:13
#2 Search sexually transmitted diseases[mh] OR sexually transmitted
disease*[tiab] OR sexually transmissible disease*[tiab] OR sexually
transmitted infection*[tiab] OR sexually transmissible infection*[tiab]
OR sexually transmitted infectious disease*[tiab] OR sexually
transmissible infectious disease*[tiab] OR sexually transmitted
disorder*[tiab] OR sexually transmissible disorder*[tiab] OR STI[tiab]
OR STIs[tiab] OR STD[tiab] OR STDs[tiab]
03:21:55
#1 Search HIV Infections[MeSH] OR HIV[MeSH] OR hiv[tw] OR hiv‐
1*[tw] OR hiv‐2*[tw] OR hiv1[tw] OR hiv2[tw] OR hiv infect*[tw] OR
human immunodeficiency virus[tw] OR human immunedeficiency
virus[tw] OR human immuno‐deficiency virus[tw] OR human
03:21:36
immune‐deficiency virus[tw] OR ((human immun*) AND (deficiency
virus[tw])) OR acquired immunodeficiency syndrome[tw] OR
acquired immunedeficiency syndrome[tw] OR acquired immunodeficiency
syndrome[tw] OR acquired immune‐deficiency
syndrome[tw] OR ((acquired immun*) AND (deficiency
syndrome[tw]))
Database: EMBASE (1980 – 2014) Date: 1 April 2014
No. Query
#16 #12 AND #13 AND #14 AND [humans]/lim AND [embase]/lim AND [1980‐2011]/py
#15 #12 AND #13 AND #14
#14 random*:ti OR random*:ab OR factorial*:ti OR factorial*:ab OR cross?over*:ti OR
cross?over:ab OR crossover*:ti OR crossover*:ab OR placebo*:ti OR placebo*:ab
OR (doubl*:ti AND blind*:ti) OR (doubl*:ab AND blind*:ab) OR (singl*:ti AND blind*:ti)
OR (singl*:ab AND blind*:ab) OR assign*:ti OR assign*:ab OR volunteer*:ti OR
volunteer*:ab OR 'crossover procedure'/de OR 'crossover procedure' OR 'doubleblind
procedure'/de OR 'double‐blind procedure' OR 'single‐blind procedure'/de OR
'single‐blind procedure' OR 'randomized controlled trial'/de OR 'randomized
controlled trial' OR allocat*:ti OR allocat*:ab
#13 'condom'/exp OR condom*:ab,ti
#12 #3 AND #11
#11 #4 OR #5 OR #6 OR #7 OR #9 OR #10
#10 'health care policy'/de OR legislation:ab,ti OR political:ab,ti OR policy:ab,ti OR
legal:ab,ti OR subsidy:ab,ti OR subsidies:ab,ti OR subsidisation:ab,ti OR
subsidization:ab,ti OR environment:ab,ti OR environmental:ab,ti OR access:ab,ti OR
accessibility:ab,ti OR availability:ab,ti OR distribution:ab,ti
#9 'mass media'/de OR media:ab,ti OR 'mass communication':ab,ti OR 'mass
communications':ab,ti OR campaign:ab,ti OR campaigns:ab,ti
#8 'public opinion'/de OR 'public opinion':ab,ti OR 'womens rights'/de
#7 empower* NEAR/3 (women OR female OR women*)
#6 'socioeconomics'/de OR socioeconomic*:ab,ti OR 'social marketing'/de OR
social:ab,ti OR economic*:ab,ti
#5 'attitude to health'/de OR 'risk reduction'/de OR 'risk reduction':ab,ti OR 'risk
reducing':ab,ti
#4 structural:ab,ti OR community:ab,ti OR communities:ab,ti OR school:ab,ti OR
schools:ab,ti OR 'community program'/de AND (intervention:ab,ti OR
interventions:ab,ti)
#3 #1 OR #2
#2 'sexually transmitted diseases'/exp OR 'sexually transmitted diseases' OR 'sexually
transmitted diseases, bacterial'/exp OR 'sexually transmitted diseases, bacterial'
OR 'sexually transmitted diseases, viral'/exp OR 'sexually transmitted diseases,
viral' OR (sexually AND transmitted AND disease*:ti OR sexually AND transmitted
AND disease*:ab) OR (sexually AND transmissible AND disease*:ti OR sexually AND
transmissible AND disease*:ab) OR (sexually AND transmitted AND infection*:ti OR
sexually AND transmitted AND infection*:ab) OR (sexually AND transmissible AND
infection*:ti OR sexually AND transmissible AND infection*:ab) OR (sexually AND
transmitted AND infectious AND disease*:ti OR sexually AND transmitted AND
infectious AND disease*:ab) OR (sexually AND transmissible AND infectious AND
disease*:ti OR sexually AND transmissible AND infectious AND disease*:ab) OR
(sexually AND transmitted AND disorder*:ti OR sexually AND transmitted AND
disorder*:ab) OR (sexually AND transmissible AND disorder*:ti OR sexually AND
transmissible AND disorder*:ab) OR sti:ti OR sti:ab OR std:ti OR std:ab
#1 'human immunodeficiency virus infection'/exp OR 'human immunodeficiency virus
infection' OR 'human immunodeficiency virus'/exp OR 'human immunodeficiency
virus' OR 'b cell lymphoma'/de OR 'b cell lymphoma' OR hiv:ti OR hiv:ab OR 'hiv‐1':ti
OR 'hiv‐1':ab OR 'hiv‐2':ti OR 'hiv‐2':ab OR 'human immunodeficiency virus':ti OR
'human immunodeficiency virus':ab OR 'human immunedeficiency virus':ti OR
'human immunedeficiency virus':ab OR 'human immune‐deficiency virus':ti OR
'human immune‐deficiency virus':ab OR 'human immuno‐deficiency virus':ti OR
'human immuno‐deficiency virus':ab OR 'acquired immunodeficiency syndrome':ti
OR 'acquired immunodeficiency syndrome':ab OR 'acquired immuno‐deficiency
syndrome':ti OR 'acquired immuno‐deficiency syndrome':ab OR 'acquired immunedeficiency
syndrome':ti OR 'acquired immune‐deficiency syndrome':ab OR
'acquired immunedeficiency syndrome':ti OR 'acquired immunedeficiency
syndrome':ab
Data and analyses
Comparison 1. Interventions to increase condom use versus standard care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 HIV incidence (person/years) | 4 | 14279 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.69, 1.19] |
| 2 HIV prevalence | 3 | 9042 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.79, 1.32] |
| 3 HSV‐2 incidence (person/years) | 2 | 3877 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.55, 1.04] |
| 4 HSV‐2 prevalence | 2 | 2974 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.85, 1.20] |
| 5 Syphilis incidence, 1:8 dilutions (person/years) | 1 | 255 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.18, 1.95] |
| 6 Syphilis prevalence (1:8 dilutions) | 3 | 7982 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.71, 1.17] |
| 7 Gonorrhoea prevalence | 5 | 10746 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.67, 2.02] |
| 8 Chlamydia prevalence | 5 | 9623 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.75, 1.18] |
| 9 Trichomona prevalence (only in women) | 2 | 916 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.77, 1.30] |
| 10 Trichomona prevalence (both sexes) | 1 | 2894 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.07 [0.02, 0.18] |
| 11 Composite score 1: HIV, HSV‐2, syphilis, gonorrhea, chlamydia, trichomonas (in women) | 1 | 8377 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.84, 1.06] |
| 12 Composite score 2: HIV, syphilis, gonorrhea, chlamydia, trichomonas | 1 | 2441 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.59, 1.04] |
| 13 Composite score 3: gonorrhea, chlamydia and trichomonas (all results assessed in women) | 1 | 669 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.72, 1.36] |
| 14 Two or more sexual partners in last year | 5 | 4008 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.78, 1.04] |
| 15 Use of condom during last sexual intercourse | 3 | 1898 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [1.03, 1.40] |
| 16 Use of condom during last sexual intercourse with non‐regular partner | 1 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.78, 1.72] |
| 17 Unprotected sex with regular partner in past three years | 1 | 103 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.91, 1.16] |
| 18 Correct condom use at last sexual intercourse at 12 months | 1 | 489 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.85, 1.12] |
| 19 Correct condom use at last sexual intercourse 24 months | 1 | 386 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.84, 1.12] |
| 20 Consistent condom use at 6 months | 1 | 726 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.38, 1.05] |
| 21 Consistent condom use at 12 months | 1 | 566 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.53, 1.60] |
| 22 Knowledge: Condom can prevent HIV | 4 | 2004 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [1.03, 1.29] |
| 23 Knowledge: Condom can prevent STIs | 3 | 1231 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [1.07, 1.41] |
| 24 Condom self efficacy | 1 | 699 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.94, 1.41] |
| 25 HIV self efficacy | 1 | 329 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.68, 1.46] |
| 26 Self efficacy | 1 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.90, 1.17] |
Comparison 2. Stratified analysis: Condom promotion along with free massive condom distribution.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 HIV incidence (person/years) | 2 | 7735 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.57, 2.17] |
| 2 HIV prevalence | 1 | 3354 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.67, 1.47] |
| 3 HSV‐2 prevalence | 1 | 733 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.76, 1.15] |
| 4 Syphilis prevalence (1:8 dilutions) | 2 | 4645 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.72, 1.20] |
| 5 Gonorrhoea prevalence | 3 | 6652 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.66, 2.38] |
| 6 Chlamydia prevalence | 3 | 6269 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.83, 1.66] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Cowan 2010.
| Methods | Cluster randomized controlled trial | |
| Participants | 6791 young Zimbabweans who were attending Form 2 at secondary school (intervention 3381, control 3410) | |
| Interventions | The intervention aims to change societal behaviors through social learning theory. It has three components: 1. A program addressed to young people, with the objective of increasing their knowledge about HIV prevention 2. A program addressed to parents and the community, with the objective of enhancing their knowledge on reproductive health, improving communication between parents and children, and developing community support to reproductive health in adolescents 3. A program which aimed to train nurses and other medical staff, in order to provide better health care access to young people Control: Same intervention delayed for a period of 4 years |
|
| Outcomes | Primary outcomes: Prevalence of HIV and of HSV‐2 Secondary outcomes:
|
|
| Notes | Assessment: Baseline survey, interim and representative population‐based survey (3 years after baseline survey), and final survey (4 years after baseline survey) Funding: The trial was funded by the National Institute of Mental Health, additional funding was received from DFID Zimbabwe |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Although it states that the venues were selected randomly, the study does not provide the specific technique of randomization that was used |
| Allocation concealment (selection bias) | Low risk | Lack of concealment would not affect the outcome |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding was not feasible. However, since the outcome was biological, it was unlikely that the outcome would have been influenced by the lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not stated, but it was unlikely that the outcome was influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | It provides detailed information of attrition causes (death, migration, not reached, away long‐term) and the causes of attrition between arms were relatively symmetric. Attrition after 4 years of follow‐up was 31% in the control group and 31% in the intervention group. Due to attrition, the author decided to treat the study as cross‐sectional |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Other bias | Low risk | a. Different individual recruitment: No b. Cluster imbalance: No Baseline characteristics were comparable across groups c. Loss of cluster: No d. Statistical analysis: Performed correctly e. Secular trend: Unclear Statistical methods that allow for clustering of events within the community was used for the analysis |
Doyle 2010.
| Methods | Community randomized trial | |
| Participants | 9645 Tanzanian adolescents, aged 14 years or more who were in Years 4–6 of all 121 government primary schools within the 20 trial communities (intervention 4870, comparison 4775) | |
| Interventions | Intervention: MEMA kwa Vijana interventions: 1. Activities addressed to the community 2. Sexual health education to students in Years 5‐7 at primary school, provided by teachers, supported by peers 3. Training and supervision in 'youth‐friendly' services to health workers 4. Social marketing of condoms, supported by peers Comparison: Standard activities |
|
| Outcomes | Primary outcomes:
Secondary outcomes:
|
|
| Notes | Assessment: Baseline assessment, interim follow‐up survey (2 years after recruitment), final follow‐up (approximately 3 years after recruitment), long‐term assessment (> 8 years post‐intervention) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The specific technique of randomization was not specified |
| Allocation concealment (selection bias) | Low risk | Lack of concealment would not affect the outcome |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Since the outcome was biological, it was unlikely that the outcome would have been influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not stated. However, it was unlikely that the outcome measurement was influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Attrition was balanced among the two groups. The reasons were the same in each group: death, absence or migration However, after 9 years of follow‐up attrition was 60% in the control group and 61% in the intervention group |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | a. Different individual recruitment: No b. Cluster imbalance: No Baseline characteristics were balanced across groups c. Loss of cluster: No d. Statistical analysis: Performed correctly e. Secular trend: Unclear The data were analysed as described for stratified cluster randomized trials |
Feldblum 2001.
| Methods | Cluster randomized controlled trial | |
| Participants | 1929 women employees, spanning from 18 to 50 years old (intervention 969, control 960) | |
| Interventions | Intervention: Education program (using Information Education Communication concept, social learning theory, and cultural adaptation) on prevention and management of STI. Provision of male and female condoms Control: Education program (without mentioning female condoms), and male condoms only |
|
| Outcomes | Trichomonas, chlamydia and gonorrhoea | |
| Notes | Baseline assessment, first follow‐up (6 months after baseline assessment), and second follow‐up (12 months after baseline assessment) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computerized random number algorithm was used |
| Allocation concealment (selection bias) | Low risk | Although it was not performed, it is unlikely that lack of concealment affects the outcome |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding was not feasible. However, it is unlikely that this feature had an important impact on the outcomes since these are biological‐based |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not stated, but since the outcomes were biological, it was unlikely they were influenced by the lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | First follow‐up (6 month after baseline assessment): Numbers of loss to follow‐up were balance and less than 20% (intervention 9.08%, control 10.83%), but the reasons were not stated Second follow‐up (14 months after baseline assessment): Numbers of loss to follow‐up were balance and less than 30% (intervention 17.75%, control 16.87%), but the reasons were not stated |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | a. Different individual recruitment: No Permanent female employees were recruited using randomly sorted employee rosters b. Cluster imbalance: No Baseline characteristics were comparable across groups c. Loss of cluster: No There was no loss of cluster d. Statistical analysis: Adequate |
Garcia 2012.
| Methods | Community randomized controlled trial | |
| Participants | 15,261 young adults aged 18‐29 years old in 24 Peruvian cities who have lived in the city for 6 months (intervention 7626, control 8021), and female sexual workers (FSWs) | |
| Interventions | Intervention: Internet courses for health workers, social marketing of cheap condoms and STIs treatment strategies, healthcare staff training on management of STIs, regular visits of 'prevention salespersons', educational campaigns to the entire community, and recognition of STIs by pharmacy employees. All these activities were developed in order to improve the syndromic management of STIs Control: Standard care |
|
| Outcomes | Prevalence at a community level (mainly in young adults, but also in FSW) of the following STIs: gonorrhoea, syphilis, chlamydia and vaginal trichomoniasis | |
| Notes | Assessment: Baseline surveys in 2002, and subsequent outcome surveys in 2005 and 2006 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Statistical software was used to randomly allocate cities either to the intervention or control group |
| Allocation concealment (selection bias) | Low risk | Allocation concealment was not applied, but lack of concealment would not affect the outcome |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Investigators were blinded regarding what cities were receiving the intervention until the surveys and laboratory tests were completed |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Members analyzing laboratory data were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated (cross‐sectional) |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Other bias | Low risk | a. Different individual recruitment: No b. Cluster imbalance: No Baseline characteristics were comparable across groups, even though some imbalances existed c. Loss of cluster: No d. Statistical analysis: Performed correctly e. Secular trend: Unclear |
Gregson 2007.
| Methods | Cluster randomized controlled trial | |
| Participants | 9454 Zimbabweans, males aged 17‐54 years old and females aged 15–44 years old from the included communities (intervention 4792, control 4662) | |
| Interventions | Intervention: The program included three interventions: strengthening of the syndromic management of STIs, distribution and use of condoms among sex workers and their clients and also among the general population –all supported by income‐generating projects – and finally, activities in healthcare facilities in order to boost STI treatment and safer sexual behaviors Control: Both intervention and control communities received standard services provided by the government such as condoms from private and public institutions, frequent activities to promote awareness regarding HIV/AIDS in the communities (e.g leaflets), and syndromic STI management. Social marketing of condoms (male and female) was also developed |
|
| Outcomes | Primary outcome: Outcomes: community‐level HIV incidence Secondary outcomes (measured at the community and individual level):
|
|
| Notes | Interventions were conducted in two rounds Assessment: Baseline assessment, assessment of first round (three years after baseline assessment), final assessment (assessment of second round) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Communities were randomly assigned to either intervention or control groups by tossing a coin |
| Allocation concealment (selection bias) | Low risk | Not performed, but lack of concealment would not affect the outcome |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Since the outcome was biological, It was unlikely that the outcome was influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Most research staff were blind to the individuals' HIV status |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Follow‐up (3 years after baseline assessment) Numbers of loss to follow‐up were balanced but were higher than 30% (intervention and control 45%). Similar reasons across groups |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Other bias | Low risk | a. Different individual recruitment: No b. Cluster imbalance: No There were some differences across groups, but their magnitude was small enough to be considered not significant c. Loss of cluster: No d. Statistical analysis: Performed correctly e. Secular trend: Unclear |
Jewkes 2008.
| Methods | Cluster randomised trial | |
| Participants | 2776 men and women volunteers from South Africa, aged 16‐23 years old, normally resident in the villages (Intervention 1409, control 1367) | |
| Interventions | Intervention: The Stepping Stones Program based its interventions on participatory learning approaches such as role plays, dramas, critical reflection and participants' personal experiences. It also included concepts related to relationships, contraception, pregnancy, STIs and HIV, taking risks and its consequences, condom use, safer sex, gender violence, communication skills, and motivations for sexual behavior Comparison: A three‐hour session on HIV, safer sex, and condoms |
|
| Outcomes | Primary outcome: Incidence of HIV Other outcomes:
|
|
| Notes | Assessment: Baseline assessment, first follow‐up (12 months after baseline assessment), second follow‐up (24 months after baseline assessment) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Each village was numbered and listed, and the study arm allocation was determined using computer‐generated randomization with equal numbers of villages allocated to each arm in each stratum |
| Allocation concealment (selection bias) | Low risk | Not performed, but lack of concealment would not affect the outcome (the project manager and field work coordinators in Mthatha identified and randomized the clusters and then enrolled participants) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding was not feasible. However, since the outcome was biological, it was unlikely that the outcome would have been influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All blood tests were conducted blind to the treatment arm |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | First follow‐up (12 months after baseline assessment): Numbers of loss to follow‐up were balanced and less than 30% (intervention: women 24.2%, men 24.9%; control: women 24.7%, men 28.2%), similar reasons across groups Second follow‐up (24 months after baseline assessment): Numbers of loss to follow‐up were balanced and less than 30% (intervention: women 26.9%, men 30.5%; total: 28.7%; control: women 24%, men 30.8%; total: 27.4%), similar reasons across groups |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | a. Different individual recruitment: No b. Cluster imbalance: No Baseline characteristics were balanced across groups c. Loss of cluster: No d. Statistical analysis: Performed correctly e. Secular trend: Unclear |
Kamali 2003.
| Methods | Community randomized trial | |
| Participants | Total: 14554 Ugandans Intervention (behavioural; group A): 4931 Intervention (behavioural + STD treatment; group B): 4787 Control (group C): 4836 |
|
| Interventions | Intervention The program was based on the behavioural change for an intervention model that includes motivation, knowledge, skills and attitudes development. The community was targeted though a variety of approaches, including communal and one‐to‐one activities, role plays, leaflets, and others Healthcare staff received training along with the provision of medication. These activities were supported by community health education. Group B: Same as the intervention for Group A, plus improved management of STIs Group C (control): The activities developed in this group were chosen by the community, and were related to general health topics. The activities in this group included taking care of elderly people, profit‐making and self support groups, seminars about general health issues, in addition to general health programs provided by the government Social marketing of female and male condoms along with voluntary HIV counselling were provided to three groups (for ethical considerations) |
|
| Outcomes | Primary outcome: Incidence of HIV‐1 Secondary outcomes:
|
|
| Notes | Interventions were undertaken over three rounds: Round 1 (April 1994 to November 1996): Initial cohort members Round 2 (December 1996 to December 1998): New individuals were enrolled (either missed at baseline, new immigrants, children at 13 years of age, or those who initially refused Round 3 (January 1999 to June 2000): Only those who previously provided a blood sample at either of the first two rounds were eligible for participation Assessment: Effects of the interventions were assessed through three laboratory tests and baseline surveys during Rounds 1, 2 and 3 covering knowledge, attitudes, behavior, and practices |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The names of communities within each group of three were written on individual cards, mixed and selected randomly: the first from each group was assigned to arm A (IEC alone), the second to arm B (IEC and STI management) and the third to arm C |
| Allocation concealment (selection bias) | Low risk | Lack of concealment would not affect the outcome |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding was not feasible, but since the outcome is biological, it is unlikely that it is influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The trial research team remained blind to the data until the end of the study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Round 1 to Round 2 (after 2 years): Numbers of loss to follow‐up were balanced and less than 30% (Group A: 27%, Group B: 25%, Group C: 25%), similar reason across groups Round 2 to Round 3 (after 2 years): Numbers of loss to follow‐up were balanced and less than 30% (Group A: 18%, Group B: 19%, Group C: 20%), similar reason across groups Round 1 to Round 3 (after 5 years) Numbers of loss to follow‐up were balanced but more than 30% (Group A: 68%, Group B: 67%, Group C: 68%), similar reason across groups |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Other bias | Low risk | a. Different individual recruitment: Unclear, possibly no b. Cluster imbalance: No Baseline characteristics were balanced across groups c. Loss of cluster: No d. Statistical analysis: Performed correctly e. Secular trend: Unclear |
NIMH 2010.
| Methods | Group randomized trial | |
| Participants | 18,147 people (intervention 9044, control 9103) from China, India, Peru, Russia, and Zimbabwe | |
| Interventions | Intervention: The intervention was based on community popular opinion leaders, people defined as natural leaders of the communities ‐ who conveyed an educational message about HIV/AIDS, including condom use and in general safer sex practices. These community popular opinion leaders reached the community in public venues where people tend to gather. The objective was to change people’s behaviours by using the innovation diffusion theory which posits that innovations and changes often originate within a subset of the population, made up of its opinion leaders whose views are then adopted by others in the community. Because the intervention was carried out in different cultural settings, the content of the program was tailored to fit each local community Control: General information and education about HIV/AIDS was communicated through brochures and educational materials, along with HIV/STI counselling, testing, and ‐ if positive ‐ treatment. This group was also given free or inexpensive condoms |
|
| Outcomes | Primary outcomes: Behavioral: change between the 24‐month follow‐up assessment and baseline in the proportion of participants reporting unprotected acts with non‐spousal/non live‐in partners in the past 3 months Biological: the incidence of any new STIs, including chlamydia, gonorrhoea, HIV, HSV‐2, syphilis, and ‐ for women ‐trichomoniasis observed across the 12‐month and 24‐month follow‐up points |
|
| Notes | Assessment: Baseline, 2‐month follow up, and 24‐month follow‐up |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The randomization method was not described |
| Allocation concealment (selection bias) | Low risk | Unclear, but lack of concealment would not affect the outcome |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Since the outcome is biological, it is unlikely that the outcome is influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessment was blinded to site investigators |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | First follow‐up (12 months after baseline assessment): Numbers of loss to follow‐up were balanced and less than 30% (intervention 15.7%, control 15.6%), reasons were not stated. Second follow‐up (24 months after baseline assessment): Numbers of loss to follow‐up were balanced and less than 30% (intervention: 18.4%, control 17.6%), but the specific reasons were not stated |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | a. Different individual recruitment: Unclear, possibly no b. Cluster imbalance: No Baseline characteristics were balanced across groups c. Loss of cluster: No. However, after initial randomization, 10 venues in China and 4 venues in India were replaced due to the closure of food markets in China and the 2004 tsunami that destroyed 4 venues in India d. Statistical analysis: Performed correctly e. Secular trend: Unclear |
Ross 2007.
| Methods | Community randomized trial | |
| Participants | 9645 adolescents from Tanzania, aged 14 years or more who were in Years 4–6 of all 121 government primary schools within the 20 trial communities (intervention 4870, comparison 4775) | |
| Interventions | Intervention: MEMA kwa Vijana interventions: ‐ Community activities ‐ Teacher‐led, peer‐assisted sexual health education in Years 5–7 of primary school ‐ Training and supervision of health workers to provide a ‘youth‐friendly’ sexual health service ‐ Condom social marketing Comparison: Standard activities |
|
| Outcomes | Primary outcomes:
Secondary outcomes:
|
|
| Notes | Assessment: Baseline assessment, interim follow‐up survey (2 years after recruitment), and final follow‐up (approximately 3 years after recruitment) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A system of constrained randomization was used to allocate communities to the two study arms, ensuring adequate balance of important factors. There were 28,000 ways of allocating half the communities in each stratum to the intervention arm. A computer program tested whether each of these allocations satisfied balance criteria, and one was randomly chosen at a meeting attended by senior government officials |
| Allocation concealment (selection bias) | Low risk | Lack of concealment would not affect the outcome |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding was not feasible. However, since the outcome was biological it was unlikely that the outcome was influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not stated, but it is unlikely that it is influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Final follow‐up (after 3 years) Numbers of loss to follow‐up were balanced and less than 30% (intervention 28%, comparison 26%); reasons were similar across groups |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | a. Different individual recruitment: No All those aged 14 years or more (mean 15.7 years) in late 1998, who were in years 4–6 of all 121 government primary schools within the 20 trial communities (and about to enter years 5–7) were eligible for enrolment b. Cluster imbalance: No Baseline characteristics were balance across groups c. Loss of cluster: No d. Statistical analysis: Performed correctly e. Secular trend: Unclear |
AIDS: Acquired immunodeficiency syndrome
DFID: Department for International Development
FSW: Female sex workers
HIV: Human immunodeficiency virus
HSV‐2: Herpes simplex virus 2
IEC: Information, education and communication
MEMA kwa vijana: It translates from the local language “good things for young people”
STI: Sexually transmitted infection
STD: Sexually transmitted disease
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Arnold 2012 | No biological outcomes. The units of randomisation were schools instead of entire communities |
| Bassett 1998 | Lacked key information to properly assess inclusion criteria, risk of bias and risk ratio. The author could not be reached |
| Basu 2004 | No biological outcomes |
| Beattie 2010 | Not randomized controlled trial |
| Bolu 2004 | Clinical setting, not community based |
| Boyer 2005 | Military setting with platoons as the unit for randomisation; not a community study; military population ‐(military women) not representative of general population |
| Campos 2013 | No HIV outcomes. Study is a subanalysis of Garcia 2012 |
| Celentano 1998 | Not randomized controlled trial; Observational, cohort study. The units of randomisation were military bases |
| DiClemente 2009 | Clinical setting; not community based |
| Doyle 2011 | Subanalysis from Doyle 2010 |
| Duan 2013 | No biological outcomes |
| El‐Bassel 2010 | Conducted in health facilities, focusing on couples and groups; not a community study |
| Fontanet 1998 | Randomization process took place in brothels, instead of an entire geographical community |
| Grosskurth 1995 | The objective of the study was not to distribute or promote condoms (the study aimed to improve the management of STIs and encourage people to seek medical attention) |
| Kalichman 2013 | No biological outcomes |
| Kamb 1998 | Face‐to‐face counselling in a clinical setting; not community based |
| Kennedy 2012 | No biological outcomes |
| Kerrigan 2006 | Not randomized controlled trial |
| Kim 2007 | No assessment of biological outcome; substudy from “Effect of a structural intervention for the prevention of intimate‐partner violence and HIV in rural South Africa: a cluster randomised trial”. |
| Kim 2009 | Biological data not provided |
| Koblin 2004 | Clinical based with one‐to‐one counselling; not community based |
| Labbe 2012 | The main intervention was provision of antibiotics. The units of randomisation were brothels instead of entire communities |
| Larke 2010 | Self reported outcome and sub‐study from “Biological and behavioural impact of an adolescent sexual health intervention in Tanzania: a community‐randomized trial” |
| Mathews 2012 | No biological outcomes; The units of randomisation were schools instead of entire communities |
| Maticka‐Tyndale, E. 2011 | No biological outcomes |
| Metcalf 2005 | One‐to‐one counselling; not community based |
| Okonofua 2003 | Self reported STI symptoms: no biological markers used |
| Padian 2007 | Clinical study (clinical setting); not community based |
| Pronyk 2006 | Lack of information in the study and protocol about the extent of condom promotion; no response from author |
| Pronyk 2008 | Biological outcomes not assessed; promotion of condom use unclear; secondary analysis from “Effect of a structural intervention for the prevention of intimate‐partner violence and HIV in rural South Africa: a cluster randomised trial” |
| Quigley 2004 | It is a secondary study from “Syndromic management of sexually transmitted infections and behaviour change interventions on transmission of HIV‐1 in rural Uganda: a community randomised trial” |
| Rotheram‐Borus 2011 | Substudy from China included in "The NIMH Collaborative HIV/STD Prevention Trial Group, 2010" |
| Sawaya 2008 | Clinical setting; not community based |
| Shain 2004 | Personal counselling; not community intervention |
| Spielberg 2013 | No biological outcomes |
| Strathdee 2013 | Not a community‐level study. Randomization conducted on female sex workers |
| Van der Straten 2009 | Clinical trial, not community based; no biological outcome |
| Wawer 1999 | Condom promotion was not one of the goals of the study. Instead, its objective was to determine if HIV transmission could be reduced through the control of sexually transmitted diseases, using home‐based mass antibiotic treatment |
FSW: Female sex worker
HIV: Human immunodeficiency virus
STD: Sexually transmitted disease
Characteristics of studies awaiting assessment [ordered by study ID]
Wagman ongoing.
| Methods | Cluster randomized controlled trial, "The SHARE Project" |
| Participants | Not clear |
| Interventions | Intimate partner violence prevention program along with routine HIV services |
| Outcomes | Physical violence, HIV incidence |
| Notes | The available text in the ClinicalTrials.gov's webpage with the name "Assessing the Impact of an Intervention to Prevent Intimate Partner Violence and HIV in Uganda" (ID: NCT02050763) does not provide proper information about the study's design or its results. The only full‐text study related to this clinical trial and that is available in other databases is "A Public Health Approach to Intimate Partner Violence Prevention in Uganda" by Wagman JA, published in 2012. However, this document only describes a theoretical framework, the implementation and design of the project and the challenges faced by the research team. It is a descriptive document without quantitative results. Thus, no proper classification of the study was feasible (it is unclear if the study was implemented at a community level, the specific type of interventions implemented, if condoms were delivered and if the study collected HIV samples after the intervention(s) took place) |
Characteristics of ongoing studies [ordered by study ID]
Vermund ongoing.
| Trial name or title | Can combination prevention strategies reduce HIV transmission in generalized epidemic settings in Africa? The HPTN 071 (PopART) study plan in South Africa and Zambia |
| Methods | Comunity‐RCT |
| Participants | 1.2 million individuals |
| Interventions | ART therapy, male circumcision, treatment of STIs, behavioral interventions and condom distribution |
| Outcomes | HIV incidence |
| Starting date | 2013 |
| Contact information | Nulda Beyers, nb@sun.ac.za |
| Notes | Ongoing study in which several proven interventions to reduce HIV transmission will be assessed at a community‐structural level. The study is also awaiting for classification given the broad spectrum of interventions implemented. |
HIV: Human immunodeficiency virus
IDU: Intravenous drug user
MSM: Men who have sex with men
RCT: Randomized controlled trial
STI: Sexually transmitted infections
Differences between protocol and review
Title in the Review
The title slightly changed since the Protocol was published, from "Structural and community‐level interventions for increasing condom use to prevent HIV and other sexually transmitted infections" to " Structural and community‐level interventions for increasing condom use to prevent the transmission of HIV and other sexually transmitted infections."
Authors
The authors changed from “Nababan H, Ota E, Wariki WMV, Koyanagi A, Ezoe S, Shibuya K, Tobe‐Gai R” in the last published protocol, to “Moreno R, Nababan HY, Ota E, Wariki WMV, Ezoe S, Gilmour S, Shibuya K” in this document.
Type of interventions
When a trial did not explicitly mention condom promotion but the intervention suggested it, authors were contacted (via email) in order to clarify whether condom promotion occurred or not.
Population
The protocol mentioned examples of 'communities', but without specifying the definition used during study selection. Nonetheless, this concept was crucial during the selection of trials as each study defined the term 'community' in a different manner. Therefore, the selection criterion was based on the most accepted and common concept of community: “a geographic unit, such as counties, cities or villages” (Atienza 2002). Thus, trials conducted exclusively in 'smaller communities' such as schools, workplaces, brothels or others alike were ruled out.
Outcomes
The original primary outcomes were: reported condom use, HIV incidence or prevalence and STI incidence or prevalence. However, during the review process it was decided that in order to give strength to the review, intermediate outcomes (i.e. reported condom use) should be avoided as primary outcomes and as selection criteria. Instead, only those studies reporting biological data were included. Studies that solely contained self reported STIs without laboratory confirmation were excluded as well.
The initial secondary outcomes for this review were: knowledge about condom use (both self reported and tested), attitudes towards condom use (both self reported and tested), condom acquisition/procurement, stated intention to use condoms, negotiation skills around condom use (both self reported and tested), changes of other STI risk‐related practices, including number of partners, process indicators reporting on satisfaction with and/or acceptability of aspects of the intervention (including the mode of condom distribution as well as the structure and/or content of the condom promotion strategy), reported harms related to condom use, such as domestic violence, abuse or allergic reactions to latex, and comparative costs of interventions. However, for several reasons not all these outcomes were included in this review: first, most of these results were not reported in the original papers; second, if they were reported, it was difficult to include these results in a meta‐analysis given the different presentation of the data; third, in this review 26 different results were already presented, so the addition of extra outcomes would have made the document difficult to follow given its extension.
Subgroup analysis and investigation of heterogeneity
The protocol stated that when possible, stratified analysis would be undertaken among participant subgroups. Subgroups included age, gender, and primary motivation for condom use (contraception and prophylaxis versus prophylaxis only). Additional stratified analyses divided studies according to methodological quality, when sufficient numbers were available. However, since outcomes were homogeneous for all results, subanalysis was deemed unnecessary.
Correction for cluster effect
In the original protocol it was stated that different statistical methods would be used when needed, without mentioning a specific strategy to calculate cluster effect and ICCs. Since it was not possible to find ICCs from authors, and a unique ICC value applied to all results taken from a trial with the same total population is inaccurate, we decided to use a linear regression model to find the missing ICCs.
Contributions of authors
Herfina Nababan (HN), Erika Ota (EO) and Windy Wariki (WW) designed, set up, and drafted the protocol. HN and Ralfh Moreno (RM) conducted both the selection of the articles and the risk of bias assessment. Any disagreement between HN and RM was resolved by EO. RM developed the protocol, and wrote the document. StuartGilmour (SG), Satoshi Ezoe (SE), and Jenji Shibuya (KS) commented upon and revised the article. All authors have approved the final review.
Sources of support
Internal sources
Department of Global Health Policy, Graduate School of Medicine, The University of Tokyo, Tokyo, Japan.
External sources
Ministry of Health, Labour and Welfare, Health Labour Sciences Research Grant (No.13800128), Japan.
Declarations of interest
Ralfh Moreno declared not having any conflict of interest.
Herfina Nababan declared not having any conflict of interest.
Erika Ota declared not having any conflict of interest.
Windy Wariki declared not having any conflict of interest.
Satoshi Ezoe declared not having any conflict of interest.
Stuart Gilmour declared not having any conflict of interest.
Kenji Shibuya declared not having any conflict of interest.
New
References
References to studies included in this review
Cowan 2010 {published data only}
- Cowan FM, Pascoe SJ, Langhaug LF, Mavhu W, Chidiya S, Jaffar S, et al. The Regai Dzive Shiri project: results of a randomized trial of an HIV prevention intervention for youth. AIDS 2010;24(16):2541‐52. [1473‐5571: (Electronic)] [DOI] [PMC free article] [PubMed] [Google Scholar]
Doyle 2010 {published data only}
- Doyle AM, Ross DA, Maganja K, Baisley K, Masesa C, Andreasen A, et al. Vijana Trial Study Group. Long‐term biological and behavioural impact of an adolescent sexual health intervention in Tanzania: follow‐up survey of the community‐based MEMA kwa Vijana Trial. PLoS Medicine 2010;7(6):e1000287. [DOI] [PMC free article] [PubMed] [Google Scholar]
Feldblum 2001 {published data only}
- Feldblum PJ, Kuyoh MA, Bwayo JJ, Omari M, Wong EL, Tweedy KG, et al. Female condom introduction and sexually transmitted infection prevalence: results of a community intervention trial in Kenya. AIDS 2001;15(8):1037‐44. [0269‐9370: (Print)] [DOI] [PubMed] [Google Scholar]
- Welsh MJ, Feldblum PJ, Kuyoh MA, Mwarogo P, Kungu D. Condom use during a community intervention trial in Kenya. International Journal of STD & AIDS 2001;12(7):469‐74. [DOI] [PubMed] [Google Scholar]
Garcia 2012 {published data only}
- Garcia PJ, Holmes KK, Carcamo CP, Garnett GP, Hughes JP, Campos PE, et al. Prevention of sexually transmitted infections in urban communities (Peru PREVEN): a multicomponent community‐randomised controlled trial. Lancet 2012;379(9821):1120‐8. [1474‐547X: (Electronic)] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gregson 2007 {published data only}
- Gregson S, Adamson S, Papaya S, Mundondo J, Nyamukapa CA, Mason PR, et al. Impact and process evaluation of integrated community and clinic‐based HIV‐1 control: a cluster‐randomised trial in eastern Zimbabwe. PLoS Medicine 2007;4(3):e102. [1549‐1676: (Electronic)] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jewkes 2008 {published data only}
- Jewkes R, Nduna M, Levin J, Jama N, Dunkle K, Puren A, et al. Impact of Stepping Stones on incidence of HIV and HSV‐2 and sexual behaviour in rural South Africa: cluster randomised controlled trial. BMJ 2008;337:a506. [1756‐1833: (Electronic)] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kamali 2003 {published data only}
- Kamali A, Quigley M, Nakiyingi J, Kinsman J, Kengeya‐Kayondo J, Gopal R, et al. Syndromic management of sexually‐transmitted infections and behaviour change interventions on transmission of HIV‐1 in rural Uganda: a community randomised trial. Lancet 2003;361(9358):645‐52. [0140‐6736: (Print)] [DOI] [PubMed] [Google Scholar]
NIMH 2010 {published data only}
- NIMH Collaborative HIV/STD Prevention Trial Group. Results of the NIMH collaborative HIV/sexually transmitted disease prevention trial of a community popular opinion leader intervention. Journal of Acquired Immune Deficiency Syndromes 2010;54(2):204‐14. [1944‐7884: (Electronic)] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ross 2007 {published data only}
- Ross DA, Changalucha J, Obasi AI, Todd J, Plummer ML, Cleophas‐Mazige B, et al. Biological and behavioural impact of an adolescent sexual health intervention in Tanzania: a community‐randomized trial. AIDS 2007;21(14):1943‐55. [0269‐9370: (Print)] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Arnold 2012 {published data only}
- Arnold R, Maticka‐Tyndale E, Tenkorang E, Holland D, Gaspard A, Luginaah I. Evaluation of school‐ and community‐based HIV prevention interventions with junior secondary school students in Edo State, Nigeria. African Journal of Reproductive Health 2012;16(2):103‐25. [PubMed] [Google Scholar]
Bassett 1998 {published data only}
- Bassett M. Impact of peer education on HIV infection in Zimbabwe. Sexual Health Exchange 1998;4:14‐15. [PubMed] [Google Scholar]
Basu 2004 {published data only}
- Basu I, Jana S, Rotherman‐Borus MJ, Swendeman D, Lee SJ, Newman P, et al. HIV prevention among sex workers in India. Journal of Acquired Immune Deficiency Syndromes 2004;36(3):845‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Beattie 2010 {published data only}
- Beattie TS, Bhattacharjee P, et al. Violence against female sex workers in Karnataka state, south India: impact on health, and reductions in violence following an intervention program. BMC Public Health 2010;10:476. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bolu 2004 {published data only}
- Bolu OO, Lindsey C, Kamb ML, Kent C, Zenilman J, Douglas JM, et al: Project RESPECT Study Group. Is HIV/sexually transmitted disease prevention counseling effective among vulnerable populations?: a subset analysis of data collected for a randomized, controlled trial evaluating counseling efficacy (Project RESPECT). Sexually Transmitted Diseases 2004;31(8):469‐74. [DOI] [PubMed] [Google Scholar]
Boyer 2005 {published data only}
- Boyer CB, Shafer MA, Shaffer RA, Brodine SK, Pollack LM, Betsinger K, et al. Evaluation of a cognitive‐behavioral, group, randomized controlled intervention trial to prevent sexually transmitted infections and unintended pregnancies in young women. Preventive Medicine 2005;40(4):420‐31. [0091‐7435: (Print)] [DOI] [PubMed] [Google Scholar]
Campos 2013 {published data only}
- Campos PE, Buffardi A L, Carcamo CP, Garcia PJ, Buendia C, Chiappe M, et al. Reaching the unreachable: providing STI control services to female sex workers via mobile team outreach. PLoS One 2013;8(11):e81041. [DOI] [PMC free article] [PubMed] [Google Scholar]
Celentano 1998 {published data only}
- Celentano D D, Nelson KE, Lyles CM, Beyrer C, Eiumtrakul S, Go VF, et al. Decreasing incidence of HIV and sexually transmitted diseases in young Thai men: evidence for success of the HIV/AIDS control and prevention program.. AIDS 1998;12(5):F29‐36. [DOI] [PubMed] [Google Scholar]
DiClemente 2009 {published data only}
- DiClemente RJ, Wingood GM, Rose ES, Sales JM, Lang DL, Caliendo AM, et al. Efficacy of sexually transmitted disease/human immunodeficiency virus sexual risk‐reduction intervention for African American adolescent females seeking sexual health services: a randomized controlled trial. Archives of Pediatrics & Adolescent Medicine 2009;163(12):1112‐21. [DOI] [PubMed] [Google Scholar]
Doyle 2011 {published data only}
- Doyle AM, Weiss HA, Maganja K, Kapiga S, McCormack S, Watson‐Jones D, et al. The long‐term impact of the MEMA kwa Vijana adolescent sexual and reproductive health intervention: Effects of dose and time since exposure to intervention. Sexually Transmitted Infections 2011;87:A215. [DOI] [PMC free article] [PubMed] [Google Scholar]
Duan 2013 {published data only}
- Duan Y, Zhang H, Wang J, Wei S, Yu F, She M. Community‐based peer intervention to reduce HIV risk among men who have sex with men in Sichuan province, China. AIDS Education and Prevention 2013;25(1):38‐48. [DOI] [PubMed] [Google Scholar]
El‐Bassel 2010 {published data only}
- El‐Bassel N, Jemmott JB, Landis JR, Pequegnat W, Wingood GM, Wyatt GE, et al: NIMH Multisite HIV/STD Prevention Trial for African American Couples Group. National Institute of Mental Health Multisite Eban HIV/STD prevention intervention for African American HIV serodiscordant couples: a cluster randomized trial. Archives of Internal Medicine 2010;170(17):1594‐1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fontanet 1998 {published data only}
- Fontanet AL, Saba J, Chandelying V, Sakondhavat C, Bhiraleus P, Rugpao S, et al. Protection against sexually transmitted diseases by granting sex workers in Thailand the choice of using the male or female condom: results from a randomized controlled trial. AIDS 1998;12(14):1851‐9. [DOI] [PubMed] [Google Scholar]
Grosskurth 1995 {published data only}
- Grosskurth H, Mosha F, Todd J, Mwijarubi E, Klokke A, Senkoro K, et al. Impact of improved treatment of sexually transmitted diseases on HIV infection in rural Tanzania: randomised controlled trial. Lancet 1995;346(8974):530‐6. [DOI] [PubMed] [Google Scholar]
Kalichman 2013 {published data only}
- Kalichman SC, Simbayi LC, Cain D, Carey KB, Carey MP, Eaton L, et al. Randomized community‐level HIV prevention intervention trial for men who drink in South African alcohol‐serving venues. European Journal of Public Health 2013;18:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kamb 1998 {published data only}
- Kamb ML, Fishbein M, Douglas JM Jr, Rhodes F, Rogers J, Bolan G, et al. Efficacy of risk‐reduction counseling to prevent human immunodeficiency virus and sexually transmitted diseases: a randomized controlled trial. Project RESPECT Study Group. JAMA 1998;280(13):1161‐7. [DOI] [PubMed] [Google Scholar]
Kennedy 2012 {published data only}
- Kennedy SB. Community trial to prevent HIV/STDs among rural youths in post‐conflict Liberia. Tropical Medicine and International Health 2012;17:10. [Google Scholar]
Kerrigan 2006 {published data only}
- Kerrigan D, Moreno L, Rosario S, Gomez B, Jerez H, Barrington C, et al. Environmental‐structural interventions to reduce HIV/STI risk among female sex workers in the Dominican Republic. American Journal of Public Health 2006;96(1):120‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2007 {published data only}
- Kim JC, Watts CH, Hargreaves JR, Ndhlovu LX, Phetla G, Morison LA, et al. Understanding the impact of a microfinance‐based intervention on women's empowerment and the reduction of intimate partner violence in South Africa. American Journal of Public Health 2007;97(10):1794‐802. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2009 {published data only}
- Kim J1, Ferrari G, Abramsky T, Watts C, Hargreaves J, Morison L, et al. Assessing the incremental effects of combining economic and health interventions: the IMAGE study in South Africa. Bulletin of the World Health Organization 2009;87(11):824‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Koblin 2004 {published data only}
- Koblin B, Chesney M, Coates T, EXPLORE Study Team. Effects of a behavioural intervention to reduce acquisition of HIV infection among men who have sex with men: the EXPLORE randomised controlled study. Lancet 2004;364(9428):41‐50. [DOI] [PubMed] [Google Scholar]
Labbe 2012 {published data only}
- Labbé AC1, Pépin J, Khonde N, Dzokoto A, Méda H, Asamoah‐Adu C, et al. Periodical antibiotic treatment for the control of gonococcal and chlamydial infections among sex workers in Benin and Ghana: a cluster‐randomized placebo‐controlled trial. Sexually Transmitted Diseases 2012;39(4):253‐9. [DOI] [PubMed] [Google Scholar]
Larke 2010 {published data only}
- Larke N, Cleophas‐Mazige B, Plummer ML, Obasi AI, Rwakatare M, Todd J, et al. Impact of the MEMA kwa Vijana adolescent sexual and reproductive health interventions on use of health services by young people in rural Mwanza, Tanzania: results of a cluster randomized trial. Journal of Adolescent Health 2010;47(5):512‐22. [DOI] [PubMed] [Google Scholar]
Mathews 2012 {published data only}
- Mathews C, Aarø LE, Grimsrud A, Flisher AJ, Kaaya S, Onya H, et al. Effects of the SATZ teacher‐led school HIV prevention programmes on adolescent sexual behaviour: cluster randomised controlled trials in three sub‐Saharan African sites. International Health 2012;4(12):111‐22. [DOI] [PubMed] [Google Scholar]
Maticka‐Tyndale, E. 2011 {published data only}
- Maticka‐Tyndale E, Onokheroya A. HIV prevention for rural youth in Nigeria: Evaluation results of school‐ and community‐based programming. Journal of Sexual Medicine 2011;8:128. [Google Scholar]
Metcalf 2005 {published data only}
- Metcalf CA1, Douglas JM Jr, Malotte CK, Cross H, Dillon BA, Paul SM, et al. RESPECT‐2 Study Group. Relative efficacy of prevention counseling with rapid and standard HIV testing: a randomized, controlled trial (RESPECT‐2). Sexually Transmitted Diseases 2005;32(2):130‐8. [DOI] [PubMed] [Google Scholar]
Okonofua 2003 {published data only}
- Okonofua FE1, Coplan P, Collins S, Oronsaye F, Ogunsakin D, Ogonor JT, et al. Impact of an intervention to improve treatment‐seeking behavior and prevent sexually transmitted diseases among Nigerian youths. International Journal of Infectious Diseases 2003;7(1):61‐73. [DOI] [PubMed] [Google Scholar]
Padian 2007 {published data only}
- Padian NS, Straten A, Ramjee G, Chipato T, Bruyn G, Blanchard K, et al. MIRA Team. Diaphragm and lubricant gel for prevention of HIV acquisition in southern African women: a randomised controlled trial. Lancet 2007;370(9583):251‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pronyk 2006 {published data only}
- Pronyk PM, Hargreaves JR, Kim JC, Morison LA, Phetla G, Watts C, et al. Effect of a structural intervention for the prevention of intimate‐partner violence and HIV in rural South Africa: a cluster randomised trial. Lancet 2006;368(9551):1973‐83. [1474‐547X: (Electronic)] [DOI] [PubMed] [Google Scholar]
Pronyk 2008 {published data only}
- Pronyk PM1, Kim JC, Abramsky T, Phetla G, Hargreaves JR, Morison LA, et al. A combined microfinance and training intervention can reduce HIV risk behaviour in young female participants. AIDS 2008;22(13):1659‐65. [DOI] [PubMed] [Google Scholar]
Quigley 2004 {published data only}
- Quigley MA1, Kamali A, Kinsman J, Kamulegeya I, Nakiyingi‐Miiro J, Kiwuwa S, et al. The impact of attending a behavioural intervention on HIV incidence in Masaka, Uganda. AIDS 2004;18(15):2055‐63. [DOI] [PubMed] [Google Scholar]
Rotheram‐Borus 2011 {published data only}
- Rotheram‐Borus MJ, Wu Z, Liang LJ, Li L, Detels R, Guan J, et al: NIMH Collaborative HIV/STD Prevention Trial Group. Reductions in sexually transmitted infections associated with popular opinion leaders in China in a randomised controlled trial. Sexually Transmitted Infections 2011;87(4):337‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sawaya 2008 {published data only}
- Sawaya GF, Chirenje MZ, Magure MT, Tuveson JL, Ma Y, Shiboski SC, et al. Effect of diaphragm and lubricant gel provision on human papillomavirus infection among women provided with condoms: a randomized controlled trial. Obstetrics & Gynecology 2008;112(5):990‐7. [DOI] [PubMed] [Google Scholar]
Shain 2004 {published data only}
- Shain RN, Piper JM, Holden AE, Champion JD, Perdue ST, Korte JE, et al. Prevention of gonorrhea and chlamydia through behavioral intervention: results of a two‐year controlled randomized trial in minority women. Sexually Transmitted Diseases 2004;31(7):401‐8. [DOI] [PubMed] [Google Scholar]
Spielberg 2013 {published data only}
- Spielberg F, Crookston BT, Chanani S, Kim J, Kline S, Gray BL. Leveraging microfinance to impact HIV and financial behaviors among adolescents and their mothers in West Bengal: a cluster randomized trial. International Journal of Adolescent Medicine and Health 2013;25(2):157‐66. [DOI] [PubMed] [Google Scholar]
Strathdee 2013 {published data only}
- Strathdee SA, Abramovitz D, Lozada R, Martinez G, Rangel MG, Vera A, et al. Reductions in HIV/STI incidence and sharing of injection equipment among female sex workers who inject drugs: results from a randomized controlled trial. PLoS One 2013;8(6):e65812. [DOI] [PMC free article] [PubMed] [Google Scholar]
Van der Straten 2009 {published data only}
- Straten A, Cheng H, Moore J, Kacanek D, Blanchard K, Bruyn G, et al. The use of the diaphragm instead of condoms in a phase III diaphragm trial. AIDS and Behavior 2009;13(3):564‐72. [DOI] [PubMed] [Google Scholar]
Wawer 1999 {published data only}
- Wawer MJ, Sewankambo NK, Serwadda D, Quinn TC, Paxton LA, Kiwanuka N, et al. Control of sexually transmitted diseases for AIDS prevention in Uganda: a randomised community trial. Rakai Project Study Group. Lancet 1999;353(9152):525‐35. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Wagman ongoing {published and unpublished data}
References to ongoing studies
Vermund ongoing {published data only}
- Can combination prevention strategies reduce HIV transmission in generalized epidemic settings in Africa? The HPTN 071 (PopART) study plan in South Africa and Zambia. Ongoing study 2013. [DOI] [PMC free article] [PubMed]
Additional references
Abdullah 2002
- Abdullah AS, Fielding R, Hedley AJ, Ebrahim SH, Luk YK. Reasons for not using condoms among the Hong Kong Chinese population: implications for HIV and STD prevention. Sexually Transmitted Infections 2002;78(3):180‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Adam 2009
- Adam PC, Wit JB, Toskin I, Mathers BM, Nashkhoev M, Zablotska I, et al. Estimating levels of HIV testing, HIV prevention coverage, HIV knowledge, and condom use among men who have sex with men (MSM) in low‐income and middle‐income countries. Journal of Acquired Immune Deficiency Syndromes 2009;52 Suppl 2:S143‐51. [DOI] [PubMed] [Google Scholar]
Adimora 2010
- Adimora AA, Auerbach JD. Structural interventions for HIV prevention in the United States. Journal of Acquired Immune Deficiency Syndromes 2010; Vol. 55:S132‐5. [1525‐4135] [DOI] [PMC free article] [PubMed]
Akinyemi 2010
- Akinyemi JO, Awolude OA, Adewole IF, Kanki PJ. Condom use among antiretroviral therapy patients in Ibadan, Nigeria. Journal of Infection in Developing Countries 2010;4(8):495‐502. [1972‐2680: (Electronic)] [DOI] [PubMed] [Google Scholar]
Anglemyer 2011
- Anglemyer A, Rutherford GW, Horvath T, Baggaley RC, Egger M, Siegfried N. Antiretroviral therapy for prevention of HIV transmission in HIV‐discordant couples. Cochrane Database of Systematic Reviews 2013, Issue 4. [DOI: 10.1002/14651858.CD009153] [DOI] [PMC free article] [PubMed] [Google Scholar]
Arora 2012
- Arora P, Nagelkerke NJ, Jha P. A systematic review and meta‐analysis of risk factors for sexual transmission of HIV in India. PLoS One 2012;7(8):e44094. [DOI] [PMC free article] [PubMed] [Google Scholar]
Atienza 2002
- Atienza AA, King AC. Community‐based health intervention trials: an overview of methodological issues. Epidemiologic Reviews 2002;24(1):72‐9. [DOI] [PubMed] [Google Scholar]
Bandura 1986
- Bandura A. Social Foundations of Thought and Action: A Social Cognitive Theory. 1st Edition. Englewood Cliffs, NJ: Prentice Hall, 1986. [Google Scholar]
Barnett 2006
- Barnett T, Whiteside A. AIDS in the Twenty‐First Century: Disease and Globalization. 2nd Edition. New York: Palgrave Macmillan, 2006. [Google Scholar]
Berhan 2012
- Berhan A, Berhan Y. Is the sexual Behaviour of HIV patients on antiretroviral therapy safe or risky in Sub‐Saharan Africa? Meta‐analysis and meta‐regression. AIDS Research and Therapy 2012;9(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bertrand 2006
- Bertrand JT, O'Reilly K, Denison J, Anhang R, Sweat M. Systematic review of the effectiveness of mass communication programs to change HIV/AIDS‐related behaviors in developing countries. Health Education Research 2006;21(4):567‐97. [0268‐1153: (Print)] [DOI] [PubMed] [Google Scholar]
Blankenship 2000
- Blankenship KM, Bray SJ, Merson MH. Structural interventions in public health. AIDS 2000;14 Suppl 1:S11‐21. [DOI] [PubMed] [Google Scholar]
Blankenship 2010
- Blankenship KM, Biradavolu MR, Jena A, George A. Challenging the stigmatization of female sex workers through a community‐led structural intervention: learning from a case study of a female sex worker intervention in Andhra Pradesh, India. AIDS Care: Psychological and Socio‐medical Aspects of AIDS/HIV 2010;22(1 supp 2):1629‐36. [0954‐0121] [DOI] [PubMed] [Google Scholar]
Britannica 2003
- Islam | Christianity. Encyclopædia Britannica 2003:306.
Charania 2011
- Charania MR, Crepaz N, Guenther‐Gray C, Henny K, Liau A, Willis LA, et al. Efficacy of structural‐level condom distribution interventions: a meta‐analysis of U.S. and international studies, 1998‐2007. AIDS and Behavior 2011;15(7):1283‐97. [1573‐3254: (Electronic)] [DOI] [PMC free article] [PubMed] [Google Scholar]
Coates 2008
- Coates TJ, Richter L, Caceres C. Behavioural strategies to reduce HIV transmission: how to make them work better. The Lancet 2008;372(9639):669‐84. [0140‐6736] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cohen 2006
- Cohen DA, Wu SY, Farley TA. Structural interventions to prevent HIV/sexually transmitted disease: are they cost‐effective for women in the southern United States?. Sexually Transmitted Diseases 2006;33(7 Suppl):S46‐9. [0148‐5717: (Print)] [DOI] [PubMed] [Google Scholar]
Cohen 2011
- Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. HPTN 052 Study Team. Prevention of HIV‐1 infection with early antiretroviral therapy. The New England Journal of Medicine 2011;365(6):493‐505. [DOI] [PMC free article] [PubMed] [Google Scholar]
Crepaz 2004
- Crepaz N, Hart TA, Marks G. Highly active antiretroviral therapy and sexual risk behavior. JAMA 2004;292(2):224‐36. [DOI] [PubMed] [Google Scholar]
Crosby 2005
- Crosby R, Yarber WL, Sanders SA, Graham CA. Condom discomfort and associated problems with their use among university students. The Journal of American College Health 2005;54(3):143‐7. [0744‐8481: (Print)] [DOI] [PubMed] [Google Scholar]
D'Amico 2012
- D'Amico EJ, Tucker JS, Miles JN, Zhou AJ, Shih RA, Green HD Jr. Preventing alcohol use with a voluntary after‐school program for middle school students: results from a cluster randomized controlled trial of CHOICE. Prevention Science 2012;13(4):415‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
da Silveira 2005
- Silveira MF, dos Santos IS, Beria JU, Horta BL, Tomasi E, Victora CG. Factors associated with condom use in women from an urban area in southern Brazil. Cadernos de Saúde Pública 2005;21(5):1557‐64. [0102‐311X: (Print)] [DOI] [PubMed] [Google Scholar]
Dandona 2005
- Dandona R, Dandona L, Gutierrez J, Kumar A, McPherson S, Samuels F, et al. High risk of HIV in non‐brothel based female sex workers in India. BMC Public Health 2005;5(1):87. [1471‐2458] [DOI] [PMC free article] [PubMed] [Google Scholar]
Donner 2000
- Donner A, Klar N. Design and Analysis of Cluster Randomization Trials in Health Research. Chichester: John Wiley & Sons, Ltd, 2000. [Google Scholar]
Essien 2005
- Essien E, Meshack A, Peters R, Ogungbade G, Osemene N. Strategies to prevent HIV transmission among heterosexual African‐American men. BMC Public Health 2005;5(1):3. [1471‐2458] [DOI] [PMC free article] [PubMed] [Google Scholar]
Foss 2007
- Foss AM, Hossain M, Vickerman PT, Watts CH. A systematic review of published evidence on intervention impact on condom use in sub‐Saharan Africa and Asia. Sexually Transmitted Infections 2007;83(7):510‐6. [1472‐3263: (Electronic)] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gorbach 2005
- Gorbach PM, Drumright LN, Holmes KK. Discord, discordance, and concurrency: comparing individual and partnership‐level analyses of new partnerships of young adults at risk of sexually transmitted infections. Sexually Transmitted Diseases 2005;32(1):7‐12. [DOI] [PubMed] [Google Scholar]
Gupta 2008
Hearst 2004
- Hearst N, Chen S. Condom promotion for AIDS prevention in the developing world: is it working?. Studies in Family Planning 2004;35(1):39‐47. [0039‐3665: (Print)] [DOI] [PubMed] [Google Scholar]
Hecht 2009
- Hecht R, Bollinger L, Stover J, McGreevey W, Muhib F, Madavo CE, et al. Critical choices in financing the response to the global HIV/AIDS pandemic. Health Affairs 2009;28(6):1591‐605. [DOI] [PubMed] [Google Scholar]
Herbst 2007a
- Herbst JH, Beeker C, Mathew A, McNally T, Passin WF, Kay LS, et al. The effectiveness of individual‐, group‐, and community‐level HIV behavioral risk‐reduction interventions for adult men who have sex with men: a systematic review. American Journal of Preventive Medicine 2007;32(4 Suppl):S38‐67. [0749‐3797: (Print)] [DOI] [PubMed] [Google Scholar]
Herbst 2007b
- Herbst JH, Kay LS, Passin WF, Lyles CM, Crepaz N, Marin BV. A systematic review and meta‐analysis of behavioral interventions to reduce HIV risk behaviors of Hispanics in the United States and Puerto Rico. AIDS and Behavior 2007;11(1):25‐47. [1090‐7165: (Print)] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Holmes 2004
- Holmes KK, Levine R, Weaver M. Effectiveness of condoms in preventing sexually transmitted infections. Bulletin of the World Health Organization 2004;82(6):454‐61. [PMC free article] [PubMed] [Google Scholar]
HUMANAE VITAE 1968
- Pope PAUL VI. Of Human Life [HUMANAE VITAE]. Encyclical Letter of his Holiness Pope Paul Vl 1968.
Jadack 1997
- Jadack RA, Fresia A, Rompalo AM, Zenilman J. Reasons for not using condoms of clients at urban sexually transmitted diseases clinics. Sexually Transmitted Diseases 1997;24(7):402‐8. [DOI] [PubMed] [Google Scholar]
Johnson 2008
- Johnson WD, Diaz RM, Flanders WD, Goodman M, Hill AN, Holtgrave D, et al. Behavioral interventions to reduce risk for sexual transmission of HIV among men who have sex with men. Cochrane Database of Systematic Reviews 2008, Issue 3. [DOI: 10.1002/14651858.CD001230] [DOI] [PubMed] [Google Scholar]
Jones 2012
- Jones J, Mosher W, Daniels K. Current contraceptive use in the United States, 2006–2010, and changes in patterns of use since 1995. National Health Statistics Reports 2012;60:25. [PubMed] [Google Scholar]
Killip 2004
- Killip S, Mahfoud Z, Pearce K. What is an intracluster correlation coefficient? Crucial concepts for primary care researchers. The Annals of Family Medicine 2004;2(3):204‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Koenig 2012
- Koenig HG, King DE, Benner V. Handbook of Religion and Health. 2nd Edition. New York: Oxford University Press, 2012. [Google Scholar]
Kumar 2006
- Kumar GA, Dandona R, Gutierrez JP, McPherson S, Bertozzi SM, Dandona L. Access to condoms for female sex workers in Andhra Pradesh. The National Medical Journal of India 2006;19(6):306‐12. [0970‐258X: (Print)] [PubMed] [Google Scholar]
Lau 2008
- Lau JT1, Wang M, Wong HN, Tsui HY, Jia M, Cheng F, et al. Prevalence of bisexual behaviors among men who have sex with men (MSM) in China and associations between condom use in MSM and heterosexual behaviors. Sexually Transmitted Diseases 2008;35(4):406‐13. [DOI] [PubMed] [Google Scholar]
Lefkowitz 2004
- Lefkowitz ES, Gillen MM, Shearer CL, Boone TL. Religiosity, sexual behaviors, and sexual attitudes during emerging adulthood. Journal of Sex Research 2004;41(2):150‐9. [0022‐4499: (Print)] [DOI] [PubMed] [Google Scholar]
Lyles 2007
- Lyles CM, Kay LS, Crepaz N, Herbst JH, Passin WF, Kim AS, et al. Best‐evidence interventions: findings from a systematic review of HIV behavioral interventions for US populations at high risk, 2000‐2004. American Journal of Public Health 2007;97(1):133‐43. [1541‐0048: (Electronic)] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mah 2010
- Mah TL, Halperin DT. Concurrent sexual partnerships and the HIV epidemics in Africa: evidence to move forward. AIDS and Behavior 2010;14(1):11‐6; discussion 34‐7. [DOI] [PubMed] [Google Scholar]
Majoko 2007
- Majoko F, Munjanja SP, Nystrom L, Mason E, Lindmark G. Randomised controlled trial of two antenatal care models in rural Zimbabwe. BJOG: An International Journal of Obstetrics and Gynaecology 2007;114(7):802‐11. [DOI] [PubMed] [Google Scholar]
Maticka‐Tyndale 2012
- Maticka‐Tyndale E. Condoms in sub‐Saharan Africa. Sexual Health 2012;9(1):59‐72. [DOI] [PubMed] [Google Scholar]
Matson 2010
- Matson PA, Adler NE, Millstein SG, Tschann JM, Ellen JM. Developmental changes in condom use among urban adolescent females: influence of partner context. Journal of Adolescent Health 2011;48(4):386‐90. [1054‐139X] [DOI] [PMC free article] [PubMed] [Google Scholar]
Medley 2004
- Medley A, Garcia‐Moreno C, McGill S, Maman S. Rates, barriers and outcomes of HIV serostatus disclosure among women in developing countries: implications for prevention of mother‐to‐child transmission programmes. Bulletin of the World Health Organization 2004;82(4):299‐307. [0042‐9686: (Print)] [PMC free article] [PubMed] [Google Scholar]
Medley 2009
- Medley A, Kennedy C, O'Reilly K, Sweat M. Effectiveness of peer education interventions for HIV prevention in developing countries: a systematic review and meta‐analysis. AIDS Education and Prevention 2009;21(3):181‐206. [1943‐2755: (Electronic)] [DOI] [PMC free article] [PubMed] [Google Scholar]
Michielsen 2010
- Michielsen K, Chersich MF, Luchters S, Koker P, Rossem R, Temmerman M. Effectiveness of HIV prevention for youth in sub‐Saharan Africa: systematic review and meta‐analysis of randomized and nonrandomized trials. AIDS 2010;24(8):1193‐202. [1473‐5571: (Electronic)] [DOI] [PubMed] [Google Scholar]
Ng 2011
- Ng BE, Butler LM, Horvath T, Rutherford GW. Population‐based biomedical sexually transmitted infection control interventions for reducing HIV infection. Cochrane Database of Systematic Reviews 2011, Issue 3. [DOI: 10.1002/14651858.CD001220] [DOI] [PubMed] [Google Scholar]
Noar 2008
- Noar SM. Behavioral interventions to reduce HIV‐related sexual risk behavior: review and synthesis of meta‐analytic evidence. AIDS and Behavior 2008;12(3):335‐53. [DOI] [PubMed] [Google Scholar]
Ojo 2011
- Ojo O, Verbeek JH, Rasanen K, Heikkinen J, Isotalo LK, Mngoma N, et al. Interventions to reduce risky sexual behaviour for preventing HIV infection in workers in occupational settings. Cochrane Database of Systematic Reviews 2011, Issue 12. [DOI: 10.1002/14651858.CD005274] [DOI] [PubMed] [Google Scholar]
Ortblad 2013
- Ortblad KF, Lozano R, Murray CJ. The burden of HIV: insights from the Global Burden of Disease Study 2010. AIDS 2013;27(13):2003‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ota 2011
- Ota E, Wariki WM, Mori R, Hori N, Shibuya K. Behavioral interventions to reduce the transmission of HIV infection among sex workers and their clients in high‐income countries. Cochrane Database of Systematic Reviews 2011, Issue 12. [DOI: 10.1002/14651858.CD006045] [DOI] [PubMed] [Google Scholar]
Painter 2001
- Painter TM. Voluntary counseling and testing for couples: a high‐leverage intervention for HIV/AIDS prevention in sub‐Saharan Africa. Social Science & Medicine 2001;53(11):1397‐411. [DOI] [PubMed] [Google Scholar]
Paul‐Ebhohimhen 2008
- Paul‐Ebhohimhen VA, Poobalan A, Teijlingen ER. A systematic review of school‐based sexual health interventions to prevent STI/HIV in sub‐Saharan Africa. BMC Public Health 2008;8:4. [1471‐2458: (Electronic)] [DOI] [PMC free article] [PubMed] [Google Scholar]
Potts 2008
- Potts M, Halperin DT, Kirby D, Swidler A, Marseille E, Klausner JD, et al. Reassessing HIV prevention. Science 2008;320(5877):749‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pulerwitz 2006
- Pulerwitz J, Barker G, Segundo M, Nascimento M. Promoting gender equity among young Brazilian men as an HIV prevention strategy (Horizons Research Summary). www.popcouncil.org/uploads/pdfs/horizons/brgndrnrmssum.pdf (accessed June 2013).
Randall 1991
- Randall D, Fernandes M. The social desirability response bias in ethics research. Journal of Business Ethics 1991;10(11):805‐17. [Google Scholar]
Reece 2010
- Reece M, Herbenick D, Schick V, Sanders SA, Dodge B, Fortenberry JD. Condom use rates in a national probability sample of males and females ages 14 to 94 in the United States. The Journal of Sexual Medicine 2010;7 Suppl 5:266‐76. [DOI] [PubMed] [Google Scholar]
Reniers 2010
- Reniers G, Watkins S. Polygyny and the spread of HIV in sub‐Saharan Africa: a case of benign concurrency. AIDS 2010;24(2):299‐307. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rojanapithayakorn 1996
- Rojanapithayakorn W, Hanenberg R. The 100% condom program in Thailand. AIDS 1996;10(1):1‐7. [DOI] [PubMed] [Google Scholar]
Roth 2001
- Roth J, Krishnan SP, Bunch E. Barriers to condom use: results from a study in Mumbai (Bombay), India. AIDS Education and Prevention 2001;13(1):65‐77. [DOI] [PubMed] [Google Scholar]
Sangani 2004
- Sangani P, Rutherford G, Wilkinson D. Population‐based interventions for reducing sexually transmitted infections, including HIV infection. Cochrane Database of Systematic Reviews 2004, Issue 2. [DOI: 10.1002/14651858.CD001220] [DOI] [PubMed] [Google Scholar]
Sarkar 2008
- Sarkar NN. Barriers to condom use. The European Journal of Contraception and Reproductive Health Care 2008;13(2):114‐22. [DOI] [PubMed] [Google Scholar]
Senn 2009
- Senn TE, Carey MP, Vanable PA, Coury‐Doniger P, Urban M. Sexual partner concurrency among STI clinic patients with a steady partner: correlates and associations with condom use. Sexually Transmitted Infections 2009;85(5):343‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shahmanesh 2008
- Shahmanesh M, Patel V, Mabey D, Cowan F. Effectiveness of interventions for the prevention of HIV and other sexually transmitted infections in female sex workers in resource poor setting: a systematic review. Tropical Medicine & International Health 2008;13(5):659‐79. [1365‐3156: (Electronic)] [DOI] [PubMed] [Google Scholar]
Shisana 2001
- Shisana O, Simbayi L. Nelson Mandela HSRC Study of HIV/AIDS: Full Report. South African national HIV prevalence, behavioural risks and mass media. Household Survey 2002. 1st Edition. Cape Town: The Human Science Research Council 2002, 2002. [Google Scholar]
Siegfried 2009
- Siegfried N, Muller M, Deeks JJ, Volmink J. Male circumcision for prevention of heterosexual acquisition of HIV in men. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD003362.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Subramanian 2008
- Subramanian T, Gupte MD, Paranjape RS, Brahmam GN, Ramakrishnan L, Adhikary R, et al. HIV, sexually transmitted infections and sexual behaviour of male clients of female sex workers in Andhra Pradesh, Tamil Nadu and Maharashtra, India: results of a cross‐sectional survey. AIDS 2008;22 Suppl 5:S69‐79. [DOI] [PubMed] [Google Scholar]
Suleman 2012
- Otho SM, Perveen S, Abbas Q. FSWs typology and condoms use among HIV high risk groups in Sindh, Pakistan: a developing country perspective. ISDS Annual Conference Proceedings. 2012; Vol. ISSN 1947‐2579.
Sumartojo 2000
- Sumartojo E, Doll L, Holtgrave D, Gayle H, Merson M. Enriching the mix: incorporating structural factors into HIV prevention. AIDS 2000;14 Suppl 1:S1‐2. [DOI] [PubMed] [Google Scholar]
Sunmola 2005
- Sunmola AM. Sexual practices, barriers to condom use and its consistent use among long distance truck drivers in Nigeria. AIDS Care 2005;17(2):208‐21. [DOI] [PubMed] [Google Scholar]
Swerissen 2004
- Swerissen H, Crisp BR. The sustainability of health promotion interventions for different levels of social organization. Health Promotion International 2004;19(1):123‐30. [DOI] [PubMed] [Google Scholar]
Taljaard 2008
- Taljaard M, Donner A, Villar J, Wojdyla D, Velazco A, Bataglia V, et al. Intracluster correlation coefficients from the 2005 WHO Global Survey on Maternal and Perinatal Health: implications for implementation research. Paediatric and Perinatal Epidemiology 2008;22(2):117‐25. [DOI] [PubMed] [Google Scholar]
Thomsen 2004
- Thomsen S, Stalker M, Toroitich‐Ruto C. Fifty ways to leave your rubber: how men in Mombasa rationalise unsafe sex. Sexually Transmitted Infections 2004;80(6):430‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Townsend 2013
- Townsend L, Mathews C, Zembe Y. A systematic review of behavioral interventions to prevent HIV infection and transmission among heterosexual, adult men in low‐and middle‐Income countries. Prevention Science 2013;14(1):88‐105. [DOI] [PubMed] [Google Scholar]
UNAIDS 1999
- UNAIDS. Acting early to prevent AIDS: The case of Senegal. www.data.unaids.org/publications/irc‐pub04/una99‐34_en.pdf (accessed 1 December 2013).
UNAIDS 2007
- UNAIDS/WHO/London School of Hygiene, Tropical Medicine. Male circumcision: global trends and determinants of prevalence, safety and acceptability. www.who.int/hiv/pub/malecircumcision/globaltrends/en/ (accessed 15 June 2013).
UNAIDS 2013
- UNAIDS 2013. Global Report: UNAIDS report on the global AIDS epidemic 2013. www.unaids.org/en/media/unaids/contentassets/documents/epidemiology/2013/gr2013/unaids_global_report_2013_en.pdf (accessed 20 February 2014).
Underhill 2008
- Underhill K, Montgomery P, Operario D. Abstinence‐plus programs for HIV infection prevention in high‐income countries. Cochrane Database of Systematic Reviews 2008, Issue 1. [DOI: 10.1002/14651858.CD007006] [DOI] [PubMed] [Google Scholar]
USAID 2002
- USAID. What Happened in Uganda? Declining HIV Prevalance, Behavior Change, and the National Response. www.unicef.org/lifeskills/files/WhatHappenedInUganda.pdf (accessed July 26 2013).
Wang 2011
- Wang K, Brown K, Shen SY, Tucker J. Social network‐based interventions to promote condom use: a systematic review. AIDS and Behavior 2011;15(7):1298‐308. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wariki 2012
- Wariki WM, Ota E, Mori R, Koyanagi A, Hori N, Shibuya K. Behavioral interventions to reduce the transmission of HIV infection among sex workers and their clients in low‐ and middle‐income countries. Cochrane Database of Systematic Reviews 2012; Vol. 2. [DOI: 10.1002/14651858] [DOI] [PMC free article] [PubMed]
Weiss 1993
- Weiss RA. How does HIV cause AIDS?. Science 1993;260(5112):1273‐9. [DOI] [PubMed] [Google Scholar]
Weiss 2000
- Weiss HA, Quigley MA, Hayes RJ. Male circumcision and risk of HIV infection in sub‐Saharan Africa: a systematic review and meta‐analysis. AIDS 2000;14(15):2361‐70. [DOI] [PubMed] [Google Scholar]
Weller 2002
- Weller S, Davis K. Condom effectiveness in reducing heterosexual HIV transmission. Cochrane Database of Systematic Reviews 2002, Issue 3. [DOI: 10.1002/14651858.CD003255] [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2012
- WHO. Social determinants of health. www.who.int/social_determinants/B_132_14‐en.pdf (accessed 3 March 2014).
Xia 2005
- Xia G, Yang X. Risky sexual behavior among female entertainment workers in China: implications for HIV/STD prevention intervention. AIDS Education and Prevention 2005;17(2):143‐56. [DOI] [PubMed] [Google Scholar]


