Summary
To expand on the work of previous meetings, a virtual Baveno VII workshop was organised for October 2021. Among patients with compensated cirrhosis or compensated advanced chronic liver disease (cACLD – defined at the Baveno VI conference), the presence or absence of clinically significant portal hypertension (CSPH) is associated with differing outcomes, including risk of death, and different diagnostic and therapeutic needs. Accordingly, the Baveno VII workshop was entitled “Personalized Care for Portal Hypertension”. The main fields of discussion were the relevance and indications for measuring the hepatic venous pressure gradient as a gold standard, the use of non-invasive tools for the diagnosis of cACLD and CSPH, the impact of aetiological and non-aetiological therapies on the course of cirrhosis, the prevention of the first episode of decompensation, the management of an acute bleeding episode, the prevention of further decompensation, as well as the diagnosis and management of splanchnic vein thrombosis and other vascular disorders of the liver. For each of these 9 topics, a thorough review of the medical literature was performed, and a series of consensus statements/recommendations were discussed and agreed upon. A summary of the most important conclusions/recommendations derived from the workshop is reported here. The statements are classified as unchanged, changed, and new in relation to Baveno VI.
Keywords: Cirrhosis, diagnosis, decompensation, treatment, recommendations
Introduction
Portal hypertension is a major consequence of cirrhosis and is responsible for its most severe complications, including ascites, bleeding from gastro-oesophageal varices and encephalopathy. The evaluation of diagnostic tools and the design and conduct of high-quality clinical trials for the treatment of portal hypertension and its complications have always been difficult. Awareness of these difficulties has led to the organisation of a series of consensus meetings. The first one was organised by Andrew Burroughs in Groningen, the Netherlands in 1986.1 After Groningen, other meetings followed, in Baveno, Italy in 1990 (Baveno I)2 and in 1995 (Baveno II)3,4; in Milan, Italy in 19925; in Reston, the United States,6 in 1996; in Stresa, Italy, in 2000 (Baveno III)7,8; in Baveno in 2005 (Baveno IV)9,10; in Atlanta, the United States in 200711; in Stresa in 2010 (Baveno V)12,13; and in Baveno in 2015 (Baveno VI).14,15
The aims of these meetings were to develop definitions of key events in portal hypertension, to review the existing evidence on the natural history, the diagnosis, and the therapeutic modalities of portal hypertension, and to issue evidence-based recommendations for the conduct of clinical trials and the management of patients. All these meetings were successful and produced consensus recommendations that referred mostly to the management of varices and variceal haemorrhage.
To continue and expand on the work of previous meetings, a Baveno VII workshop was planned for March 20–21, 2020. This would also include recommendations on other complications of cirrhosis and portal hypertension besides variceal haemorrhage. However, the COVID-19 pandemic and the consequent lockdown forced the organisers to postpone the workshop until the end of October 2021 and to change the format from a face-to-face to a virtual meeting. Despite these limitations, many of the experts responsible for the major recent achievements in the field of portal hypertension and its complications participated in the workshop. Many of them had attended the previous meetings.
Importantly, following the spirit of the Baveno meetings, the Baveno Cooperation was formed in 2016 with the aim of expanding the scope of such meetings towards the continuous collaboration of experts in portal hypertension and to the establishment of a continuous, high-quality research agenda. In 2019, the European Association for the Study of the Liver (EASL) endorsed the Baveno Cooperation as an official EASL consortium.
Patients with cirrhosis transition through different prognostic stages, the main ones being the compensated and decompensated stages. Transition from the compensated to the decompensated stage is clinically marked by the development of complications such as ascites, variceal haemorrhage and overt hepatic encephalopathy. Because “cirrhosis” implies a pathological (invasive) diagnosis, at the Baveno VI conference, the concept of compensated advanced chronic liver disease (cACLD) was put forward based on non-invasive tests (NITs) that would predict the development of complications of cirrhosis. Among patients with compensated cirrhosis or cACLD, at least two different stages have been identified based on the presence or absence of clinically significant portal hypertension (CSPH). The various disease stages are associated with differing outcomes, including risk of death, and therefore patients in different stages have different diagnostic and therapeutic needs. Accordingly, the Baveno VII workshop was entitled “Personalized Care for Portal Hypertension”. The main fields of discussion were the relevance and indications for measuring the hepatic venous pressure gradient (HVPG) as a gold standard, the use of non-invasive tools for the diagnosis of cACLD and CSPH, the impact of aetiological and non-aetiological therapies on the course of cirrhosis, the prevention of the first episode of decompensation, the management of an acute bleeding episode, the prevention of further decompensation, as well as the diagnosis and management of splanchnic vein thrombosis and other vascular disorders of the liver. For each of these 9 topics, a thorough review of the medical literature was performed, and a series of consensus statements/recommendations were discussed and agreed upon. Whenever applicable, the level of existing evidence was evaluated, and the recommendations were ranked according to the GRADE system,16 according to which the scientific evidence was graded from A (high) to D (very low). The strength of the recommendations was graded 1 (strong) and 2 (weak). The presentations made during the workshop are reported ‘in extenso’ in the Baveno VII proceedings book.17 A summary of the most important conclusions/recommendations derived from the workshop is reported here. The statements are classified as unchanged, changed, and new in relation to Baveno VI.
1). HVPG as a gold standard
Description of HVPG measurement
-
1.1.
The use of an end-hole, compliant balloon occlusion catheter reduces the random error of wedged hepatic vein pressure (WHVP) measurements and is preferred over the use of a conventional straight catheter. (A.1) (New)
-
1.2.
A small volume of contrast medium should be injected when the occlusion balloon is inflated to confirm a satisfactory occluded position and to exclude the presence of hepatic venous-to-venous communications. (A.1) (New)
-
1.3.
Hepatic venous-to-venous communications may result in underestimation of the WHVP and must be reported. (A.1) (New)
-
1.4.
Deep sedation during liver haemodynamic measurement may cause inaccurate HVPG values.(B.1) If light sedation is required, low dose midazolam (0.02 mg/kg) does not modify the HVPG and is acceptable. (B.1) (New)
-
1.5.
Slow speed (up to 7.5 mm/s) permanent tracings of pressures, recorded either on paper or electronically, are recommended. Digital, on-screen, readings are much less accurate and should not be used. (A.1) (New)
-
1.6.
To properly reflect portal venous pressure, WHVP requires a stabilisation time. Recording of WHVP requires a minimum of 1 minute, with particular attention to stability during the last 20–30 seconds. WHVP should be recorded in triplicate. (D.1) (New)
-
1.7.
The wedged to free hepatic vein pressure gradient has superior clinical prognostic value than wedged to right atrial pressure gradient and should be used as the standard reference.(B.1) Right atrial pressure can be measured to rule out a post-hepatic component of portal hypertension. (B.1) (New)
-
1.8.
Free hepatic vein pressure must be measured in the hepatic vein within 2–3 cm of its confluence with the inferior vena cava (IVC). IVC pressure should be measured as an internal control, at the level of the hepatic vein ostium. If the free hepatic vein pressure is more than 2 mmHg above IVC pressures, the presence of a hepatic vein outflow obstruction should be ruled out by injecting a small amount of contrast medium. (A.1) (New)
Diagnosis of CSPH in patients with cirrhosis
-
1.9.
HVPG values >5 mmHg indicate sinusoidal portal hypertension. (A.1) (Unchanged)
-
1.10.
In patients with viral- and alcohol-related cirrhosis, HVPG measurement is the gold-standard method to determine the presence of “clinically significant portal hypertension” (CSPH), which is defined as an HVPG ≥10 mmHg. (A.1) (Changed)
-
1.11.
In patients with primary biliary cholangitis, there may be an additional pre-sinusoidal component of portal hypertension that cannot be assessed by HVPG.(B.1) As such, in these patients, HVPG may underestimate the prevalence and severity of PH. (B.1) (New)
-
1.12.
In patients with non-alcoholic steatohepatitis (NASH)-related cirrhosis, although an HVPG ≥10 mmHg remains strongly associated with the presence of clinical signs of portal hypertension, these signs can also be present in a small proportion of patients with HVPG values <10 mmHg. (C.2) (New)
-
1.13.
In patients with chronic liver disease and clinical signs of portal hypertension (gastro-oesophageal varices, ascites, portosystemic collateral vessels) but with HVPG <10 mmHg, porto-sinusoidal vascular disorder (PSVD) must be ruled out. (B.1) (New)
-
1.14.
In alcohol-related or viral cirrhosis, a decrease in HVPG in response to non-selective beta-blockers (NSBBs) is associated with a significant reduction in the risk of variceal bleeding or of other decompensating events. (A.1) (Changed)
Inclusion of HVPG assessment in trial design
-
1.15.
HVPG measurements should be encouraged in clinical trials investigating novel therapies but are not essential if portal hypertension-associated endpoints are well defined. (B.1) (Unchanged)
-
1.16.
In viral, alcohol-related, and reasonably in NASH-related cirrhosis, HVPG response assessment is recommended as a surrogate endpoint in phase II clinical trials where a low rate of events is expected. (D.2) (Changed)
-
1.17.
Test-retest reliability of HVPG measurement is excellent but influenced by the stage of liver disease (lower in decompensated patients) and its aetiology (higher in patients with alcohol-related disease). This should be taken into consideration when designing clinical trials based on HVPG assessment. (C.1) (New)
Assessment of surgical risks
-
1.18.
The presence of CSPH, determined either by HVPG ≥10 mmHg or by clinical manifestations of portal hypertension, is associated with a higher risk of decompensation and mortality in patients with cirrhosis undergoing liver resection for hepatocellular carcinoma (HCC). (A.1) (New)
-
1.19.
In candidates for non-hepatic abdominal surgery, a HVPG ≥16 mmHg is associated with an increased risk of short-term mortality after surgery. (C.1) (New)
PPG in the setting of TIPS
-
1.20.
Portal pressure gradient (PPG) should be measured before and after transjugular intrahepatic portosystemic shunt (TIPS) insertion. (A.1) (New)
-
1.21.
Anatomic locations for post-TIPS PPG measurement should include the main portal vein and the IVC (at the shunt outflow). (B.1) (New)
-
1.22.
The immediate post-TIPS PPG may be influenced by various factors, such as general anaesthesia, use of vasoactive agents or haemodynamic instability and therefore immediate post-TIPS PPG may not represent long-term PPG.(B.1) PPG measurements in haemodynamically stable, non-sedated patients better reflect post-TIPS PPG values and are recommended.(B.1) (New)
-
1.23.
In patients with variceal bleeding undergoing TIPS, reduction of absolute PPG to <12 mmHg is associated with near complete protection from portal hypertensive bleeding and is the preferred target for haemodynamic success. (A.1) A relative reduction of PPG, by at least 50% from pre-TIPS baseline, may also be useful. (B.2) (New)
-
1.24.
PPG re-measurement is indicated to evaluate the need for TIPS revision if there is clinical or Doppler-ultrasonographic suspicion of TIPS dysfunction. (B.1) (New)
Research agenda
Further evaluate the usefulness, safety, and accuracy of direct portal pressure measurement by endoscopic ultrasound.
Further investigate the prognostic role of HVPG and define specific cut-offs in patients with NASH-cirrhosis.
Confirm the utility of HVPG-guided therapy in randomised clinical trials.
Further investigate the prognostic role of HVPG in patients undergoing extrahepatic surgery in prospective cohorts that should compare HVPG with non-invasive tests.
Evaluate test-retest HVPG reliability at an individual level and examine factors that determine variability.
Evaluate portocaval- vs. porto-atrial-measured PPG and clinical outcomes after TIPS (e.g., rebleeding).
Determine the optimal PPG decrease required to medically control recurrent/refractory ascites. Further investigate the correlation between the haemodynamic outcomes of TIPS and the clinical response of ascites.
Investigate the optimal PPG increase (in the context of TIPS reduction) needed to ameliorate adverse events related to over-shunting.
2). Non-invasive tools for cACLD and portal hypertension
Definition of cACLD
-
2.1
The use of elastography in clinical practice has enabled the early identification of patients with untreated/active chronic liver disease at risk of having CSPH and consequently, at risk of decompensation and liver-related death. (A.1) (Changed)
-
2.2
The term “compensated advanced chronic liver disease (cACLD)” had been proposed to reflect the continuum of severe fibrosis and cirrhosis in patients with ongoing chronic liver disease. A pragmatic definition of cACLD based on liver stiffness measurement (LSM) is aimed at stratifying the risk of CSPH and decompensation at point of care, irrespective of histological stage or the ability of LSM to identify these stages. (B.1) (Changed)
-
2.3
Currently, both terms “cACLD” and “compensated cirrhosis” are acceptable, but not interchangeable. (B.1) (Changed)
Criteria to identify cACLD
-
2.4
LSM values by transient elastography (TE) <10 kPa in the absence of other known clinical/imaging signs rule out cACLD; values between 10 and 15 kPa are suggestive of cACLD; values >15 kPa are highly suggestive of cACLD. (B.1) (Changed)
-
2.5
Patients with chronic liver disease and an LSM <10 kPa by TE have a negligible 3-year risk (≤1%) of decompensation and liver-related death. (A.1) (New)
-
2.6
Patients with cACLD should be referred to a liver disease specialist for further work-up. (B.1) (Changed)
-
2.7
Invasive methods (liver biopsy, HVPG) can be used for further work-up in an individualised manner at referral centres. (B.1) (Changed)
Outcome and prognosis
-
2.8
LSM (irrespective of the technique used for its measurement) holds prognostic information in cACLD, both at index investigation and during follow-up. (A.1) (New)
-
2.9
A rule of 5 for LSM by TE (10-15-20-25 kPa) should be used to denote progressively higher relative risks of decompensation and liver-related death independently of the aetiology of chronic liver disease. (B.1) (New)
How to monitor
-
2.10
Patients with LSM values 7–10 kPa and ongoing liver injury should be monitored on a case-by-case basis for changes indicating progression to cACLD. (C.2) (New)
-
2.11
TE can lead to false positive results, therefore an index LSM ≥10 kPa should be repeated in fasting conditions as soon as feasible or complemented with an established serum marker of fibrosis (fibrosis-4 ≥2.67, enhance liver fibrosis test ≥9.8, FibroTest ≥0.58 for alcohol-related/viral liver disease, FibroTest ≥0.48 for non-alcoholic fatty liver disease). (B.2) (New)
-
2.12
In patients with cACLD, LSM could be repeated every 12 months to monitor changes. (B.2) (New)
-
2.13
A clinically significant decrease in LSM, which is associated with substantially reduced risk of decompensation and liver-related death, can be defined as a decrease in LSM of ≥20% associated with LSM <20 kPa or any decrease to a LSM <10 kPa. (C.2) (New)
Diagnosis of CSPH in patients with cACLD
-
2.14
Although the concept of CSPH is HVPG-driven, non-invasive tests are sufficiently accurate to identify CSPH in clinical practice. (A.1) (New)
-
2.15
LSM by TE ≤15 kPa plus platelet count ≥150×109/L rules out CSPH (sensitivity and negative predictive value >90%) in patients with cACLD. (B.2) (New)
-
2.16
In patients with virus- and/or alcohol-related cACLD and non-obese (BMI <30 kg/m2) NASH-related cACLD, a LSM value by TE of ≥25 kPa is sufficient to rule in CSPH (specificity and positive predictive value >90%), defining the group of patients at risk of endoscopic signs of portal hypertension and at higher risk of decompensation. (B.1) (Changed)
-
2.17
In patients with virus- and/or alcohol-related and non-obese NASH-related cACLD with LSM values <25 kPa, the ANTICIPATE model can be used to predict the risk of CSPH. Based on this model, patients with LSM values between 20–25 kPa and platelet count <150×109/L or LSM values between 15–20 kPa and platelet count <110×109/L have a CSPH risk of at least 60%. (B.2) (New)
-
2.18
In patients with NASH-related cACLD, the ANTICIPATE-NASH model (including LSM, platelet count and BMI) may be used to predict the risk of CSPH, but further validation is needed. (C.2) (New)
Varices and screening endoscopy in patients that cannot be treated with NSSBs
-
2.19
Patients with compensated cirrhosis who are not candidates for initiating NSBBs (contraindication/intolerance) for the prevention of decompensation should undergo an endoscopy for variceal screening if LSM by TE is ≥20 kPa or platelet count is ≤150×109L. (A.1) (New)
-
2.20
Patients avoiding screening endoscopy can be followed up by yearly repetition of TE and platelet count. If LSM increases (≥20 kPa) or platelet count declines (≤150×109L), these patients should undergo screening endoscopy (Fig. 1). (D.1) (Unchanged)
Fig. 1. Algorithm for the non-invasive determination of cACLD and CSPH.
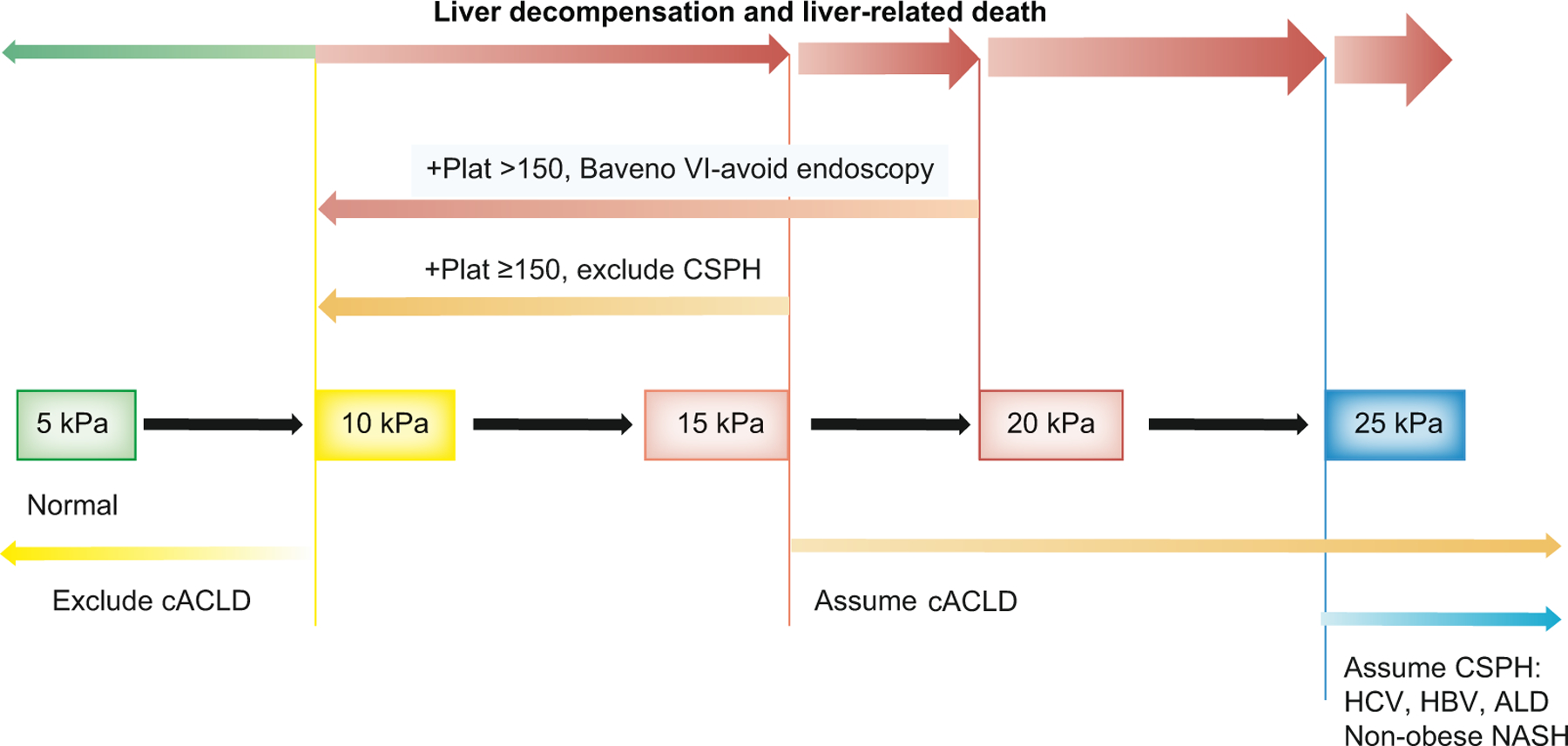
ALD, alcohol-related liver disease; cACLD, compensated advanced chronic liver disease; CSPH, clinically significant portal hypertension; NASH, non-alcoholic steatohepatitis.
Spleen stiffness
-
2.21
Spleen stiffness measurement (SSM) by TE can be used in cACLD due to viral hepatitis (untreated HCV; untreated and treated HBV) to rule out and rule in CSPH (SSM <21 kPa and SSM >50 kPa, respectively). Validation of the best cut-off using a 100 Hz specific TE-probe, as well as using point-shear wave elastography and 2D-shear wave elastography is needed. (B.2) (New)
-
2.22
In patients who are not candidates for NSBBs (contraindication/intolerance) and in whom endoscopy would be required according to the Baveno VI criteria (LSM by TE ≥20 kPa or platelet count ≤150×109L), SSM ≤40 kPa by TE can be used to identify those at low probability of high-risk varices, in whom endoscopy can be avoided. (C.2) (New)
Research agenda
Define risk of decompensation associated with different LSM cut-offs in different aetiologies of cACLD.
Validate and refine non-invasive tools for CSPH in patients with NASH.
Evaluate the diagnostic value of LSM for CSPH in aetiologies other than viral/alcohol/NASH.
Establish whether sex and age require specific calibration of NITs for CSPH.
Validate circulating biomarkers for prediction of decompensation in all aetiologies.
Validate LSM thresholds for CSPH, high-risk varices and decompensation obtained from devices other than TE.
Validate what constitutes a clinically significant improvement or worsening of LSM in all aetiologies.
Validate SSM in non-viral aetiologies.
Evaluate emerging methods to diagnose CSPH and determine response to NSBBs, such as contrast-enhanced ultrasound-based methods (SHAPE), MRI methods, and the combination of elastography, novel imaging methods and tests addressing liver function.
3). Management of ACLD after removal/suppression of the primary aetiological factor
-
3.1
Removal/suppression of the primary aetiological factor includes sustained virological response (SVR) in patients with HCV infection, HBV suppression in the absence of HDV co-infection in patients with chronic HBV infection, and long-term abstinence from alcohol in patients with alcohol-related liver disease. (A.1) (New)
-
3.2
The definition and impact of the removal/suppression of the primary aetiological factor in other ACLDs is less well established. (A.1) (New)
-
3.3
Overweight/obesity, diabetes, and alcohol consumption are important contributors to liver disease progression even after removal/suppression of the primary aetiological factor and should be addressed. (A.1) (Changed)
-
3.4
Removal/suppression of the primary aetiological factor leads to potentially meaningful decreases in HVPG in most patients and substantially reduces the risk of hepatic decompensation. (A.1) (Changed)
-
3.5
Absence/resolution of CSPH following removal/suppression of the primary aetiological factor prevents hepatic decompensation. (B.1) (Changed)
-
3.6
The optimal percent/absolute decrease in HVPG associated with a reduction in hepatic decompensation following the removal/suppression of the primary aetiological factor in patients with cACLD and CSPH has yet to be established. (B.1) (New)
-
3.7
In the absence of co-factors, patients with HCV-induced cACLD who achieve SVR and show consistent post-treatment improvements with LSM values of <12 kPa and PLT >150×109/L can be discharged from portal hypertension surveillance (LSM and endoscopy), as they do not have CSPH and are at negligible risk of hepatic decompensation. In these patients, HCC surveillance should continue until further data is available. (B.1) (New)
-
3.8
The Baveno VI criteria (i.e., LSM <20 kPa and PLT >150×109/L) can be used to rule out high-risk varices in patients with HCV- and HBV-induced cACLD who achieved SVR and viral suppression, respectively. (B.1) (New)
-
3.9
Patients with cACLD on NSBB therapy with no evident CSPH (LSM <25 kPa) after removal/suppression of the primary aetiological factor, should be considered for repeat endoscopy, preferably after 1–2 years. In the absence of varices, NSBB therapy can be discontinued. (C.2) (New)
Research agenda
Define the impact of the removal/suppression of primary aetiological factors (particularly non-alcoholic fatty liver disease) other than HCV/HBV infection and alcohol-related liver disease in cACLD.
Identify factors responsible for liver disease progression despite removal/suppression of the primary aetiological factor.
Establish the optimal percent/absolute decrease in HVPG associated with a reduction in hepatic decompensation following the removal/suppression of the primary aetiological factor in patients with cACLD and CSPH.
Evaluate the diagnostic ability of NITs for monitoring disease regression and determining the presence of CSPH after removal/suppression of a non-viral primary aetiological factor.
Evaluate and validate other non-invasive risk stratification algorithms (e.g., LSM/VITRO [von Willebrand factor antigen to platelet ratio] and SSM) in patients in whom the primary aetiological factor has been removed/suppressed.
Establish estimates for the regression of varices after removal/suppression of the primary aetiological factor and collect long-term data on the risk of hepatic decompensation (and more specifically, variceal bleeding) and its evolution over time in patients with cACLD.
4). Impact of non-aetiological therapies
-
4.1
The use of statins should be encouraged in patients with cirrhosis and an approved indication for statins since these agents may decrease portal pressure (A.1) and improve overall survival. (B.1) (Changed)
-
4.2
In patients with Child-Pugh B and C cirrhosis, statins should be used at a lower dose (simvastatin at max. 20 mg/d) and patients should be followed closely for muscle and liver toxicity.(A.1) In Child-Pugh C cirrhosis the benefit of statins has not been proven yet and their use should be more restrictive. (D.1) (Changed)
-
4.3
The use of aspirin should not be discouraged in patients with cirrhosis and an approved indication for aspirin, since it may reduce the risk of HCC, liver-related complications, and death. (B.2) (New)
-
4.4
Long-term albumin administration may reduce complications of cirrhosis and improve transplant-free survival in patients with uncomplicated ascites, but a formal recommendation cannot be given until further data become available. (B.2) (New)
-
4.5
Short-term albumin administration is indicated for spontaneous bacterial peritonitis (SBP) (A.1), acute kidney injury (AKI) >stage 1A (C.1), large-volume paracentesis (A.1) and combined with terlipressin for hepatorenal syndrome (HRS)-AKI. (B.1) (New)
-
4.6
Primary antibiotic prophylaxis is recommended in selected patients (i.e., gastrointestinal [GI] haemorrhage, Child-Pugh C cirrhosis with low protein ascites) at high risk of SBP. (B.1) (New)
-
4.7
Secondary antibiotic prophylaxis is indicated in patients with previous SBP. (A.1) (New)
-
4.8
Rifaximin is indicated for the secondary prophylaxis of hepatic encephalopathy. (A.1) (New)
-
4.9
Rifaximin should be considered for prophylaxis of overt hepatic encephalopathy in patients with previous overt hepatic encephalopathy undergoing elective TIPS. (B.2) (New)
-
4.10
Rifaximin is not indicated beyond these indications, including primary or secondary prophylaxis of SBP. (C.1) (New)
-
4.11
Anticoagulation should not be discouraged in patients with cirrhosis and an approved indication for anticoagulation, since anticoagulation may reduce liver-related outcomes in patients with and without portal vein thrombosis (PVT) and may improve overall survival. (B.1) (Changed)
-
4.12
Direct-acting oral anticoagulants (DOACs) are as safe and effective for the prevention of cardiovascular events in patients with Child-Pugh A/B cirrhosis as in those without cirrhosis (B.2) DOACs are not recommended in patients with Child-Pugh C cirrhosis outside study protocols. (B.2) (New)
Research agenda
The gut microbiome can be targeted by several means including pre-, pro-, syn- and post-biotics, diet, faecal microbiota transplantation, phage therapy, drugs, bioengineered bacteria, and antibiotics. Interventional trials are needed to assess the functional mechanisms and clinical outcomes associated with such therapies.
The composition of the gut microbiome (e.g., high relative abundance of Enterobacteriaceae) in various body fluids (stool, saliva, blood, bile, intestinal mucosa, skin) is associated with severity of cirrhosis, complications, and presence of organ failures and acute-on-chronic liver failure (ACLF). Components of the gut microbiome should be explored for biomarkers to inform stage of disease (diagnostic), and to predict the risk of progression (prognostic), the likelihood of benefitting from an intervention (predictive) and the efficacy of an intervention.
Faecal microbiota transplant (by enema or by the oral route) seems to be safe in patients with cirrhosis and hepatic encephalopathy but efficacy studies are pending.
Antifibrotic strategies including targeting the farnesoid X receptor pathway, the renin-angiotensin system, and angiogenesis should be further explored in cirrhosis and portal hypertension.
5). Prevention of (first) decompensation
-
5.1
Compensated cirrhosis is defined by the absence of present or past complications of cirrhosis. The transition from compensated to decompensated cirrhosis leads to an increased mortality risk. (A.1) (New)
-
5.2
Compensated cirrhosis can be divided into 2 stages, based on the absence or presence of CSPH. Patients with CSPH are at increased risk of decompensation. The goal of treatment in compensated cirrhosis is to prevent complications that define decompensation. (A.1) (Changed)
-
5.3
Prevention of decompensation is especially relevant in compensated patients with CSPH and/or oesophageal or gastric varices due to their higher risk of developing decompensation. (B.1) (New)
-
5.4
The events that define decompensation in a compensated patient are overt ascites (or pleural effusion with increased serum ascites albumin gradient [>1.1 g/dl]), overt hepatic encephalopathy (West Haven grade ≥II) and variceal bleeding. (B.1) (New)
-
5.5
Other relevant liver-related events in compensated cirrhosis are the development of superimposed liver injury (see statement 5.12)/ACLF and HCC. (B1) (New)
-
5.6
Insufficient data are available regarding whether a minimal amount of ascites only detected in imaging procedures, minimal hepatic encephalopathy, and occult bleeding from portal hypertensive gastroenteropathy (PHG) can be considered as decompensation. (D.1) (New)
-
5.7
Limited data suggest that jaundice alone (in non-cholestatic aetiologies) may be the first manifestation of cirrhosis in a minority of patients; however, its definition, whether it should be considered true first decompensation or if it reflects superimposed liver injury/ACLF in compensated cirrhosis requires further research. (D.1) (New)
-
5.8
Non-hepatic comorbidities are frequent in patients with compensated cirrhosis, can adversely impact prognosis, and should be specifically dealt with. (A.1) (Changed)
-
5.9
There is insufficient data to draw definitive conclusions on the impact of sarcopenia and frailty on the natural history of compensated cirrhosis. (D.1) (New)
-
5.10
Bacterial infections are frequent in compensated patients with CSPH, can lead to decompensation (ascites, variceal bleeding, hepatic encephalopathy) and, consequently, adversely affect natural history. (B.1) (New)
-
5.11
There is insufficient data as to whether infections are frequent in compensated cirrhosis without CSPH and whether they may impact prognosis per se. (D.1) (New)
-
5.12
Superimposed liver damage, such as (acute) alcoholic hepatitis, acute viral hepatitis (HEV, HAV), HBV flares or drug-induced liver injury can precipitate decompensation. (A.1) (New)
-
5.13
Other factors such as HCC and major surgery can precipitate decompensation of cirrhosis in patients with CSPH. (B.1) (New)
-
5.14
Treatment with NSBBs (propranolol, nadolol or carvedilol*) should be considered for the prevention of decompensation in patients with CSPH. (B.1) (New) *In contrast with the traditional NSBBs (i.e. propranolol and nadolol), carvedilol has intrinsic anti-alpha adrenergic vasodilatory effects that contribute to its greater portal pressure reducing effect.
-
5.15
Carvedilol is the preferred NSBB in compensated cirrhosis, since it is more effective at reducing HVPG (A.1), has a tendency towards greater benefit in preventing decompensation and towards better tolerance than traditional NSBBs and has been demonstrated to improve survival (B.1) compared to no active therapy in compensated patients with CSPH. (Changed)
-
5.16
The decision to treat with NSBBs should be taken when clinically indicated, independent of the possibility of measuring HVPG. (B.2) (Unchanged)
-
5.17
Patients with compensated cirrhosis who are on NSBBs for the prevention of decompensation do not need a screening endoscopy for the detection of varices since endoscopy will not change management. (B.2) (New)
-
5.18
There is no evidence that endoscopic therapies such as endoscopic band ligation or glue might prevent ascites or hepatic encephalopathy. (D.1) (New)
-
5.19
In compensated patients with high-risk varices who have contraindications or intolerance to NSBBs, endoscopic band ligation is recommended to prevent first variceal bleeding. (A.1) (Changed)
-
5.20
There is no indication at present to use NSBBs in patients without CSPH. (A.1) (Unchanged)
-
5.21
Although a single study suggested that cyanoacrylate injection is more effective than propranolol in preventing first bleeding in patients with large type 2 gastro-oesophageal varices or isolated type 1 gastric varices, there were no differences in survival. However, NSBBs are indicated in these patients to prevent decompensation.(B.1) Further studies are required in these patients using new therapeutic approaches in addition to NSBBs. (D.1) (Changed)
-
5.22
There is no indication at present for balloon-occluded retrograde (antegrade) transvenous obliteration (BRTO or BATO) or TIPS in primary prophylaxis of gastric variceal bleeding in compensated patients. (D.1) (New)
Research agenda
Competing risks from comorbidities should be considered in future studies in compensated cirrhosis.
Determine the impact of early detection and treatment of comorbidities.
Determine the impact of sarcopenia and frailty (and of its treatment) on prognosis and mortality of patients with compensated cirrhosis.
Determine the prognostic significance of the sole presence of minimal ascites only detected in imaging procedures, minimal hepatic encephalopathy, and chronic bleeding from PHG.
Determine the prognostic significance of the sole presence of jaundice in compensated cirrhosis, and its definition.
Evaluate the role of statins in preventing decompensation.
Determine the impact of sole bacterial infection and non-bacterial infections on the natural history of compensated cirrhosis.
Determine the impact of vaccination (pneumococcal, haemophilus, influenza, coronavirus) on the natural history of compensated cirrhosis.
Evaluate the prevention of bacterial infections in patients with CSPH and its impact on the incidence of decompensation.
Identify factors predicting which infections will give rise to decompensation and/or worsen prognosis.
6). Acute variceal bleeding
-
6.1
The goal of resuscitation is to preserve tissue perfusion. Volume restitution should be initiated to restore and maintain haemodynamic stability. (D.2) (Unchanged)
-
6.2
Packed red blood cell transfusions should be performed conservatively, with a target haemoglobin level between 7–8 g/dl, although transfusion policy in individual patients should also consider other factors such as cardiovascular disorders, age, haemodynamic status and ongoing bleeding. (A.1) (Unchanged)
-
6.3
Intubation is recommended before endoscopy in patients with altered consciousness and those actively vomiting blood. (D.1) (New)
-
6.4
Extubation should be performed as quickly as possible after endoscopy. (D.2) (New)
-
6.5
In suspected variceal bleeding, vasoactive drugs (terlipressin, somatostatin, octreotide) should be started as soon as possible and continued for 2–5 days. (A.1) (Changed)
-
6.6
Hyponatremia has been described in patients on terlipressin, especially in patients with preserved liver function. Therefore, sodium levels should be monitored. (B.1) (Unchanged)
-
6.7
Antibiotic prophylaxis is an integral part of therapy for patients with cirrhosis presenting with upper gastrointestinal bleeding and should be instituted from admission. (A.1) (Unchanged)
-
6.8
The risk of bacterial infection and mortality are very low in patients with Child-Pugh A cirrhosis, but more prospective studies are still needed to assess whether antibiotic prophylaxis can be avoided in this subgroup of patients. (B.2) (Unchanged)
-
6.9
Intravenous ceftriaxone 1 g/24 h should be considered in patients with advanced cirrhosis (A.1) in hospital settings with high prevalence of quinolone-resistant bacterial infections and in patients on previous quinolone prophylaxis, and should always be in accordance with local resistance patterns and antimicrobial policies. (D.2) (Changed)
-
6.10
Malnutrition increases the risk of adverse outcomes in patients with cirrhosis and acute variceal bleeding (AVB) and oral nutrition should be started as soon as possible. (D.2) (New)
-
6.11
Airway manipulation, including use of a nasogastric tube, should be performed with caution because of the risk of pulmonary infection. (D.2) (New)
-
6.12
Proton pump inhibitors, when started before endoscopy, should be stopped immediately after the procedure unless there is a strict indication to continue them. (D.2) (New)
-
6.13
Six-week mortality should be the primary endpoint for studies on the treatment of AVB. (D.1) (Unchanged)
-
6.14
Five-day treatment failure is defined either by absence of control of bleeding or by rebleeding within the first 5 days. (D.1) (Changed)
-
6.15
Child-Pugh class C, the updated model for end-stage liver disease (MELD) score, and failure to achieve primary haemostasis are the variables most consistently found to predict 6-week mortality. (B.2) (Unchanged)
-
6.16
Child-Pugh and MELD scores are currently the most utilised severity scoring systems. (D.2) (Unchanged)
-
6.17
Following haemodynamic resuscitation, patients with suspected AVB should undergo upper endoscopy within 12 h of presentation (B.1). If the patient is unstable, endoscopy should be performed as soon as safely possible. (D.1) (Changed)
-
6.18
The availability of an on-call GI endoscopist proficient in endoscopic haemostasis and on-call support staff with technical expertise in the usage of endoscopic devices, enabling performance of endoscopy on a 24/7 basis, is recommended. Trainees performing the procedure must always be closely supervised by the GI endoscopist. (D.1) (Changed)
-
6.19
In the absence of contraindications (QT prolongation), pre-endoscopy infusion of erythromycin (250 mg IV 30–120 minutes before endoscopy) should be considered. (B.1) (Unchanged)
-
6.20
Patients with AVB should be managed in intensive or intermediate care units. (D.1) (Unchanged)
-
6.21
Ligation is the recommended form of endoscopic therapy for acute oesophageal variceal bleeding. (A.1) (Unchanged)
-
6.22
Endoscopic therapy with tissue adhesives (e.g. N-butyl-cyanoacrylate/thrombin) is recommended for acute bleeding from isolated gastric varices (A.1) and type 2 gastro-oesophageal varices that extend beyond the cardia. (D.2) (Unchanged)
-
6.23
Endoscopic variceal ligation (EVL) or tissue adhesive can be used in bleeding from type 1 gastro-oesophageal varices. (D.1) (Unchanged)
-
6.24
Based on current evidence, haemostatic powder cannot be recommended as first-line endoscopic therapy for AVB. (D.1) (New)
-
6.25
Endoscopic therapy (argon plasma coagulation, radio-frequency ablation or band ligation for PHG and gastric antral vascular ectasia) may be used for local treatment of PHG bleeding. (C.2) (New)
-
6.26
All patients with AVB should undergo abdominal imaging, preferably contrast-enhanced cross-sectional imaging (CT or MRI) to exclude splanchnic vein thrombosis, HCC and to map portosystemic collaterals in order to guide treatment. (D.1) (New)
-
6.27
Pre-emptive TIPS with polytetrafluoroethylene (PTFE)-covered stents within 72 h (ideally <24 h) is indicated in patients bleeding from oesophageal varices and type 1/2 gastro-oesophageal varices who meet any of the following criteria: Child-Pugh class C <14 points or Child-Pugh class B >7 with active bleeding at initial endoscopy or HVPG >20 mmHg at the time of haemorrhage. (A.1) (Changed)
-
6.28
In patients fulfilling the criteria for pre-emptive TIPS, ACLF, hepatic encephalopathy at admission and hyperbilirubinemia at admission should not be considered contraindications. (B.1) (New)
-
6.29
In refractory variceal bleeding, balloon tamponade or self-expandable metal stents (SEMS) should be used as a bridge therapy to a more definite treatment such as PTFE-covered TIPS. SEMS are as efficacious as balloon tamponade and are a safer option. (B.1) (Changed)
-
6.30
Failure to control variceal bleeding despite combined pharmacological and endoscopic therapy is best managed by salvage PTFE-covered TIPS. (B.1) (Changed)
-
6.31
TIPS may be futile in patients with Child-Pugh ≥14 cirrhosis, or with a MELD score >30 and lactate >12 mmol/L, unless liver transplantation is envisioned in the short-term.(B.1) The decision to perform TIPS in such patients should be taken on a case-by-case basis. (D.1) (New)
-
6.32
In patients with AVB and hepatic encephalopathy, bouts of hepatic encephalopathy should be treated with lactulose (oral or enemas). (D.1) (New)
-
6.33
In patients presenting with AVB, rapid removal of blood from the gastrointestinal tract (lactulose oral or enemas) should be used to prevent hepatic encephalopathy. (B.1) (New)
-
6.34
Variceal bleeding is due to portal hypertension, and the aim of the treatment should be focused on lowering portal pressure rather than correcting coagulation abnormalities. (B.1) (New)
-
6.35
Conventional coagulation tests, namely, prothrombin time/international normalised ratio (PT/INR) and activated partial thromboplastin time, do not accurately reflect the haemostatic status of patients with advanced liver diseases. (B.1) (Changed)
-
6.36
In the AVB episode, transfusion of fresh frozen plasma is not recommended as it will not correct coagulopathy and may lead to volume overload and worsening of portal hypertension. (B.1) (New)
-
6.37
In the setting of AVB, there is no evidence that platelet count and fibrinogen levels are correlated with the risk of failure to control bleeding or rebleeding. However, in case of failure to control bleeding, the decision to correct the haemostatic abnormalities should be considered on a case-by-case basis. (D.2) (New)
-
6.38
Recombinant factor VIIa and tranexamic acid are not recommended in AVB. (A.1) (New)
-
6.39
In patients with AVB who are on anticoagulants, these should be temporarily discontinued until the haemorrhage is under control. Length of discontinuation should be individualised based on the strength of the indication for anticoagulation. (D.2) (New)
-
6.40
In patients with GOV2, type 1 isolated gastric varices, and ectopic varices, BRTO could be considered as an alternative to endoscopic treatment or TIPS, provided it is feasible (type and diameter of shunt) and local expertise is available, as it has been shown to be safe and effective. (D.2) (New)
-
6.41
Either endovascular or endoscopic treatment should be considered in patients with ectopic varices. (D.1) (New)
-
6.42
TIPS may be combined with embolisation to control bleeding or to reduce the risk of recurrent variceal bleeding from gastric or ectopic varices, particularly in cases when, despite a decrease in portosystemic pressure gradient, portal flow remains diverted to collaterals. (D.2) (New)
-
6.43
In patients with cirrhosis and PVT, management of AVB should be performed according to the guidelines for patients without PVT, when possible. (D.1) (New)
Research agenda
Determine the role of vasoactive drugs and antibiotics in Child-Pugh A patients.
Identify an optimal shorter time frame limit for vasoactive drug therapy?
Define active bleeding at endoscopy, and assess its subjectivity and prognostic value.
Identify the clinical role of non-invasive markers of portal pressure.
Determine the role of haemostatic powder in acute and refractory variceal bleeding.
Determine the role of thrombin in gastric variceal bleeding.
Assess pre-emptive TIPS in patients with gastric varices.
Define the optimal management of patients not fulfilling the high-risk criteria used for pre-emptive TIPS.
Determine the cost-effectiveness of SEMS.
Develop alternatives to Blakemore/Linton as they are in short supply.
Determine the role of global haemostasis tests, such as viscoelastic tests and thrombin generation assays, to assess and correct haemostasis abnormalities in decompensated cirrhosis and AVB (using clinical endpoints).
Determine the potential role of prothrombin complex concentrates, fibrinogen, or cryoprecipitate in bleeding patients with cirrhosis.
Evaluate whether there is any relation between low platelet count (up to which level?) or fibrinogen and the risk of variceal bleeding, failure to control bleeding, or bleeding after endoscopic band ligation.
Identify patients that will benefit from variceal embolisation during TIPS.
Determine the role of endoscopic ultrasound-guided therapy with tissue adhesive with or without coils.
Determine the impact of PVT on the prognosis of cirrhotic patients with AVB.
Identify the optimal duration of vasoactive therapy in cirrhotic patients with PVT and AVB.
Determine the role of pre-emptive TIPS in cirrhotic patients with PVT presenting with AVB.
Establish the optimal management of AVB in patients with cirrhosis and PVT, including management of anticoagulation and timing of endoscopic/invasive procedures.
7). Prevention of further decompensation
Definition of “further decompensation”
-
7.1Further decompensation in cirrhosis represents a prognostic stage associated with an even higher mortality than that associated with first decompensation. Specific events that define further decompensation are any of the following: (B.1) (New)
- Development of a second portal hypertension-driven decompensating event (ascites, variceal haemorrhage or hepatic encephalopathy) and/or jaundice;
- Development of recurrent variceal bleeding, recurrent ascites (requirement of ≥3 large-volume paracenteses within 1 year), recurrent encephalopathy, development of SBP and/or HRS-AKI;
- In patients presenting with bleeding alone, development of ascites, encephalopathy, or jaundice after recovery from bleeding but not if these events occur around the time of bleeding.
Preventing further decompensation in patients with ascites
-
7.2
Patients with decompensated cirrhosis should be considered for liver transplantation. (A.1) (New)
-
7.3
Patients with ascites who are not on traditional NSBBs (i.e., propranolol or nadolol) or carvedilol should undergo screening endoscopy. (B.1) (New)
-
7.4
TIPS should be considered in patients with recurrent ascites (requirement of ≥3 large-volume paracenteses within 1 year) irrespective of the presence or absence of varices or history of variceal haemorrhage. (A.1) (New)
-
7.5
In patients with ascites and low-risk varices (small [<5 mm], no red signs, not Child-Pugh C), traditional NSBBs or carvedilol may be used to prevent first variceal haemorrhage. (B.2) (Changed)
-
7.6
In patients with ascites and high-risk varices (large varices [≥5 mm]), or red spot signs, or Child-Pugh C), prevention of first variceal haemorrhage is indicated, with traditional NSBBs or carvedilol being preferred over EVL. (B.1) (Changed)
-
7.7
In patients with ascites, traditional NSBBs or carvedilol should be dose-reduced or discontinued in case of persistently low blood pressure (systolic blood pressure <90 mmHg or mean arterial pressure <65 mmHg) and/or HRS-AKI.(B.1) Once blood pressure returns to baseline and/or HRS-AKI resolves, NSBBs can be re-initiated or re-titrated.(B.1) If a patient remains intolerant to NSBBs, EVL is then recommended to prevent variceal haemorrhage. (B.1) (Changed)
Preventing recurrent variceal haemorrhage (secondary prophylaxis)
-
7.8
First-line therapy for the prevention of recurrent variceal haemorrhage is the combination of traditional NSBBs or carvedilol and EVL. (A.1) (Changed)
-
7.9
TIPS is the treatment of choice in patients who rebleed despite traditional NSBBs or carvedilol and EVL. (B.1) (Unchanged)
-
7.10
In patients who cannot get/tolerate EVL or carvedilol or traditional NSBBs, any of these therapies can be maintained alone (A1) and TIPS should be considered in patients with recurrent ascites. (B.1) (Changed)
-
7.11
In patients who bleed despite adherence to traditional NSBBs or carvedilol as primary prophylaxis, the combination of traditional NSBBs or carvedilol and EVL is recommended, and TIPS should be considered in those with recurrent ascites. (B.1) (New)
Preventing recurrent bleeding from PHG
-
7.12
PHG and portal hypertension-associated gastric or small intestinal polypoid lesions have to be distinguished from gastric antral vascular ectasia because treatments are different. (B.1) (Changed)
-
7.13
NSBBs are the first-line therapy for preventing recurrent bleeding from PHG. (A.1) (Unchanged)
-
7.14
Endoscopic therapy (e.g., argon plasma coagulation or hemospray) may be used to treat recurrent bleeding from PHG. (D.1) (New)
-
7.15
TIPS should be considered for transfusion-dependent PHG despite traditional NSBBs or carvedilol and endoscopic therapy. (C.1) (Changed)
Role of infections in decompensated cirrhosis
-
7.16
Bacterial infections are common in patients with decompensated cirrhosis and may cause further decompensation. (A.1) (New)
-
7.17
In all patients hospitalised with decompensation, bacterial infections should be ruled out. The minimal work-up for infections should include diagnostic paracentesis, chest X-ray, cultures of blood, ascites and urine, and skin examination. (A.1) (New)
-
7.18
Patients with bacterial infections should be promptly treated with antibiotics. The empirical antibiotic treatment should be tailored to local epidemiology, risk factors for multidrug-resistant bacteria and severity of infection.(A.1) If no response to antibiotics is observed, consider viral and fungal infections. (C.1) (Changed)
The role of sarcopenia and frailty in further decompensation
-
7.19
Frailty, malnutrition, and sarcopenia have an impact on survival in patients with decompensated cirrhosis. They should be evaluated with available standardised tools. (B.1) (New)
-
7.20
All patients with decompensated cirrhosis should receive nutrition consultation and be advised regarding the benefits of regular exercise. (B.1) (New)
-
7.21
While sarcopenia improves in some patients after TIPS, pre-procedural sarcopenia has also been associated with poor outcomes (e.g., encephalopathy, slower resolution of ascites) and a higher mortality. Therefore, sarcopenia by itself should not be an indication for TIPS. (C.2) (New)
Definition of cirrhosis recompensation
-
7.22
The concept of recompensation implies that there is at least partial regression of the structural and functional changes of cirrhosis after removal of the aetiology of cirrhosis. (A.1) (New)
-
7.23Clinically, the definition of “recompensation” is based on expert consensus and requires fulfilment of all the following criteria: (C.2) (New)
- Removal/suppression/cure of the primary aetiology of cirrhosis (viral elimination for hepatitis C, sustained viral suppression for hepatitis B, sustained alcohol abstinence for alcohol-induced cirrhosis);
- Resolution of ascites (off diuretics), encephalopathy (off lactulose/rifaximin) and absence of recurrent variceal haemorrhage (for at least 12 months);
- Stable improvement of liver function tests (albumin, INR, bilirubin).
-
7.24
Because CSPH may persist despite recompensation, NSBBs should not be discontinued unless CSPH resolves. (B.1) (New)
-
7.25
Resolution of ascites (while on diuretics or after TIPS) and/or lack of recurrent variceal haemorrhage (while on traditional NSBBs + EVL or carvedilol + EVL or after TIPS) without removal/suppression/cure of the primary aetiologic factor and without improvement in liver synthetic function, is not evidence of recompensation. (B.1) (New)
Research agenda
Further decompensation and recompensation
Investigate the effect of time to further decompensation on prognosis.
Obtain data to support the suggested concept of cirrhosis recompensation, particularly on the timeframe necessary to consider a patient truly recompensated.
Evaluate the association between recompensation and resolution of CSPH.
Determine the impact of aetiological therapy other than alcohol abstinence and antiviral therapy on recompensation.
NSBBs and further decompensation
Prospective studies should assess if NSBB treatment prevents further (non-rebleeding) decompensation in decompensated patients.
Prospective studies should assess if HVPG-guided (traditional NSBB/carvedilol) therapy is more efficient to prevent further decompensation over non-HVPG-guided strategies.
Identify optimal blood pressure cut-offs (mean arterial pressure/systolic arterial pressure) to define safe use of traditional NSBBs/carvedilol therapy and determine whether dose reduction (vs. discontinuation) is safe.
Determine the impact of NSBB discontinuation on the natural history of decompensated cirrhosis.
Assess the benefit of carvedilol over traditional NSBBs in secondary prophylaxis of variceal haemorrhage.
TIPS and further decompensation
Assess the benefit of TIPS for secondary prophylaxis in patients with NSBB intolerance/non-response and ascites that do not meet the strict criteria for recurrent ascites.
Establish whether TIPS placement past the 72 h pre-emptive TIPS window is still beneficial.
Evaluate the haemodynamic and non-haemodynamic effects of NSBBs in patients after TIPS.
Sarcopenia, frailty and nutrition and further decompensation
Determine the impact of nutritional interventions on the natural history of decompensation.
Determine the impact of therapies targeting sarcopenia and frailty on the natural history of decompensation.
Define the role of sarcopenia in the selection of patients for TIPS.
8). Splanchnic vein thrombosis
Aetiological work-up in primary thrombosis of the portal venous system or hepatic venous outflow tract
-
8.1
For patients with primary thrombosis of the splanchnic veins in the absence of cirrhosis, close collaboration with subspecialists is recommended for a complete work-up that considers prothrombotic factors and systemic diseases. (A.1) (Changed)
-
8.2
Various combinations of risk factors for thrombosis can be present, so that identification of 1 risk factor does not deter from a complete work-up. (A.1) (New)
-
8.3
In all adult patients, myeloproliferative neoplasia (MPN) should be searched for by testing for the V617F JAK2 mutation in peripheral blood. (A.1) (Unchanged)
-
8.4
In patients with no detectable JAK2 V617F mutation, consider additional investigations for MPN, including somatic calreticulin and JAK2-exon12 mutations, and next-generation sequencing. (A.1) (Changed)
-
8.5
In all adult patients with primary thrombosis of the splanchnic veins without an MPN driver mutation, bone marrow biopsy should be discussed in collaboration with haematologists to rule out MPN, irrespective of blood cell counts. Bone marrow biopsy should be considered particularly in patients without major risk factors for thrombosis. (B.2) (Changed)
Budd-Chiari syndrome – definition
-
8.6
Budd-Chiari syndrome (BCS) is the consequence of an obstruction to the hepatic venous outflow. Obstruction can be located from the level of the small hepatic veins to the level of the entrance of the IVC into the right atrium. (A.1) (Unchanged)
-
8.7
BCS is the preferred designation for any primary hepatic venous outflow tract obstruction. (D.1) (New)
-
8.8
BCS is considered secondary when the mechanism for venous obstruction is an extrinsic compression, for example by a benign or malignant tumour. BCS is considered primary otherwise. (A.1) (Changed)
Budd-Chiari syndrome – diagnosis
-
8.9
BCS presentation and manifestations are extremely diverse, so that the diagnosis must be considered in any patient with acute, acute-on-chronic, or chronic liver disease. (A.1) (Changed)
-
8.10
BCS is diagnosed by the demonstration of an obstruction of the venous lumen, or by the presence of hepatic vein collaterals together with the absence of patent hepatic veins. (A.1) (Unchanged)
-
8.11
Liver biopsy should not be performed to diagnose BCS when vascular imaging demonstrates obstruction of the hepatic venous outflow tract. (B.1) (Unchanged)
-
8.12
Liver biopsy is necessary to diagnose BCS if obstruction of the small hepatic veins is not seen on imaging. (B.1) (Changed)
-
8.13
In patients with BCS, hepatic nodules are frequent and most often benign. However, HCC may occur and therefore patients should be monitored with periodic imaging and alpha-fetoprotein measurements. (B.1) (Changed)
-
8.14
A 6-month interval can be proposed for periodic imaging. (C.1) (New)
-
8.15
It is still unclear which of ultrasound or MRI should be used for periodical screening. (C.1) (New)
-
8.16
Patients developing nodules should be referred to centres experienced in managing BCS. (D.1) (Unchanged)
-
8.17
Characterisation of the nodule may first include MRI using hepatobiliary contrast agents.(C.1) Biopsy of the lesion is indicated for a definitive diagnosis of HCC. (C.1) (New)
Budd-Chiari syndrome – management
-
8.18
Management of BCS should be undertaken using a stepwise approach including anticoagulation, angioplasty/stent/thrombectomy/thrombolysis, TIPS and orthotopic liver transplantation, at experienced centres. (B.1) (Unchanged)
-
8.19
Long-term anticoagulation should be given to all patients with primary BCS. (B.1) (Changed)
-
8.20
Because of the increased risk of heparin-induced thrombocytopenia, the use of unfractionated heparin is generally not recommended and may only be reserved for special situations (e.g., glomerular filtration rate <30 ml/min, pending invasive procedures). (D.2) (New)
-
8.21
Stenoses that are amenable to percutaneous angioplasty/stenting (short length stenoses) should be actively looked for and treated accordingly. (B.1) (Unchanged)
-
8.22
TIPS insertion should be attempted by operators with specific experience in BCS when angioplasty/stenting/thrombectomy/thrombolysis is not feasible, and when the patient does not improve on medical therapy including anticoagulants. (B.1) (Unchanged)
-
8.23
Consider improvement as a combination of several of the following outcomes: decreasing rate of ascites formation, decreasing serum bilirubin, serum creatinine and INR when elevated (or increasing factor V in patients receiving vitamin K antagonists). (D.1) (New)
-
8.24
The BCS-TIPS prognostic index score can be used to predict outcome in patients in whom TIPS insertion is being considered. (B.1) (Changed)
-
8.25
Liver transplantation should be considered in patients with uncontrolled clinical manifestation despite a stepwise approach, or in patients with a high BCS-TIPS prognostic index score (>7) before TIPS placement. (C.1) (Changed)
-
8.26
In patients with BCS presenting as acute liver failure, urgent liver transplantation should be considered. Emergency TIPS should be performed, if possible, independently of listing for liver transplantation. (C.1) (New)
PVT and portal cavernoma in the absence of cirrhosis – definition
-
8.27
PVT is characterised by the presence of a thrombus in the portal vein trunk or its branches. Portal cavernoma is a network of porto-portal collaterals which develops as a consequence of prior portal vein obstruction. (D.1) Obstruction leading to cavernoma is mostly related to thrombosis in adults, but less likely so in children and young adults. (B.1) (Changed)
-
8.28
Imaging tools should be used to distinguish PVT from the extravascular compression of the venous lumen by a neighbouring space-occupying formation. (D.1) (New)
-
8.29
Cirrhosis and/or malignancy should be ruled out and other underlying liver diseases (e.g., PSVD or other chronic liver disease) should be investigated. (D.1) (Changed)
PVT and portal cavernoma in the absence of cirrhosis – diagnosis
-
8.30
For diagnosis of PVT or cavernoma, Doppler ultrasound, CT or MR angiography should demonstrate solid intraluminal material not showing enhancement after injection of vascular contrast agents (for PVT) or a network of porto-portal collaterals (for cavernoma). (B.1) If diagnosed by Doppler ultrasound, confirmation with contrast-enhanced CT or MR angiography is needed. (D.1) (Changed)
-
8.31
A standardised documentation (as proposed in Table 1) of initial site, extent/degree of luminal obstruction, and chronicity of clot formation is required to enable subsequent evaluation of the spontaneous course and/or response to treatment. (D.1) (New)
-
8.32
PVT and portal cavernoma in adults are frequently associated with ≥1 risk factor(s) for thrombosis, which may be occult at presentation and should be investigated. (B.1) (Unchanged)
-
8.33
In patients with PVT following abdominal surgery or pancreatitis, invasive procedures (e.g., bone marrow biopsy and liver biopsy) should be discussed on an individual basis considering the expected low diagnostic yield in such populations and the risk of morbidity associated with these procedures. (C.2) (New)
-
8.34
If the liver is dysmorphic on imaging or liver tests are persistently abnormal, liver biopsy and HVPG measurement are recommended to rule out cirrhosis or PSVD.(B.1) Liver stiffness by TE may be useful to exclude cirrhosis although precise cut-offs cannot be proposed yet. (C.2) (Changed)
Table 1.
Recommended standardised nomenclature for the description of portal vein thrombosis and portal cavernoma in both the clinical and research setting.18
| Feature | Definition |
|---|---|
| Time course | |
| Recent | Portal vein thrombosis presumed to be present for <6 months |
| Chronic | Portal vein thrombosis present or persistent for >6 months |
|
| |
| Percent occlusion of main portal vein | |
| Completely occlusive | No persistent lumen |
| Partially occlusive | Clot obstructing >50% of original vessel lumen |
| Minimally occlusive | Clot obstructing <50% of original vessel lumen |
| Cavernous transformation | Gross porto-portal collaterals without original PV seen |
|
| |
| Response to treatment or interval change | |
| Progressive | Thrombus increases in size or progresses to more complete occlusion |
| Stable | No appreciable change in size or occlusion |
| Regressive | Thrombus decreases in size or degree of occlusion |
PVT and portal cavernoma in the absence of cirrhosis - management
-
8.35
In the absence of cirrhosis, recent PVT rarely resolves spontaneously. Therefore, at diagnosis, anticoagulation should be started immediately at a therapeutic dosage. (B.1) (Changed)
-
8.36
Because of the increased risk of heparin-induced thrombocytopenia, the use of unfractionated heparin is not generally recommended and may only be reserved for special situations (e.g. glomerular filtration rate <30 ml/min, pending invasive procedures). (D.2) (New)
-
8.37
As a primary treatment option for recent PVT in the absence of cirrhosis, start with low-molecular-weight heparin (LMWH) and switch to vitamin K antagonists when possible. (B.1) (Changed) DOACs can be considered the primary option in selected cases in the absence of so-called “triple positive” anti-phospholipid syndrome, although data are limited. (C.2) (New)
Recent PVT in the absence of cirrhosis – management
-
8.38
Anticoagulation should be given for at least 6 months in all patients with recent PVT in the absence of cirrhosis. (B.1) (Unchanged)
-
8.39
After 6 months, long-term anticoagulation is recommended in patients with a permanent underlying prothrombotic state (B.1) and should also be considered in patients without an underlying prothrombotic state. (B.2) (New)
-
8.40
If anticoagulation is discontinued, D-dimers <500 ng/ml 1 month after discontinuation may be used to predict a low risk of recurrence. (C.2) (New)
-
8.41
In patients without cirrhosis who do not develop complications of recent PVT despite the absence of portal vein recanalisation, interventions other than anticoagulation are not required. (B.2) (Changed)
-
8.42
A follow-up contrast-enhanced CT scan should be performed 6 months after recent PVT. (C.1) (New)
-
8.43
Because of the risk of recurrence of splanchnic vein thrombosis, patients need to be followed up, irrespective of the discontinuation of anticoagulation. (C.1) (New)
-
8.44
The risk of intestinal infarction and organ failure is increased in patients with recent PVT and (i) persistent severe abdominal pain despite anticoagulation therapy, (ii) bloody diarrhoea, (iii) lactic acidosis, (iv) bowel loop distention, or (v) occlusion of second order radicles of the superior mesenteric vein. Therefore, a multidisciplinary approach with early image-guided intervention, thrombolysis and surgical intervention should be considered in referral centres. (C.2) (New)
Past PVT or cavernoma in the absence of cirrhosis – management
-
8.45
In patients with past PVT or cavernoma, including those with incomplete resolution of recent PVT at 6 months, long-term anticoagulation is recommended in patients with a permanent underlying prothrombotic state (B.1) and should also be considered in patients without an underlying prothrombotic state. (B.2) (New)
-
8.46
No data are available to recommend or discourage anticoagulation in childhood-onset past PVT or cavernoma in the absence of an underlying prothrombotic state. (C.1) (New)
-
8.47
In patients with past PVT or cavernoma not yet receiving anticoagulants, anticoagulation should be started after adequate prophylaxis for portal hypertensive bleeding has been initiated in patients with high-risk varices. (C.2) (Changed)
-
8.48
Mesenteric-left portal vein bypass (Meso-Rex operation) should be considered in all children with complications of portal cavernoma, and these patients should be referred to centres with experience in treating this condition. (B.1) (Unchanged)
-
8.49
Patients with refractory complications of PVT or cavernoma should be referred to expert centres to consider percutaneous recanalisation of the portal vein or other vascular interventional procedures. (C.1) (New)
Treatment of portal hypertension in extrahepatic portal vein obstruction
-
8.50
There is insufficient data on whether beta-blockers or endoscopic therapy should be preferred for primary prophylaxis of portal hypertension-related bleeding in patients with past PVT or cavernoma. Guidelines for cirrhosis should be applied. (C.2) (Changed)
-
8.51
Oesophageal variceal band ligation can be performed safely without withdrawing vitamin K antagonists. (C.2) (New)
-
8.52
All patients in whom thrombosis has not been recanalised should be screened for gastroesophageal varices within 6 months of the acute episode. In the absence of varices, endoscopy should be repeated at 12 months and 2 years thereafter. (B.1) (Unchanged)
-
8.53
In patients with acute portal hypertension-related bleeding, recommendations for patients with cirrhosis may be applied. (D.1) (Changed)
-
8.54
Based on recommendations for cirrhosis, the combination of NSBBs and band ligation is recommended for secondary prophylaxis. (D.1) (New)
Research agenda
Budd-Chiari syndrome:
Determine risk factors for HCC in patients with BCS.
Identify the best approach for the non-invasive diagnosis of HCC in patients with BCS.
Evaluate criteria for short-term (8 days) evolution that predict a good mid/long-term outcome (i.e., criteria for “treatment response”) in patients with BCS.
PVT without cirrhosis
Identify predictors of development, progression, and spontaneous resolution of PVT.
Determine the influence of beta-blockers on the natural history of PVT.
Determine the effect of early recanalisation using interventional radiology or TIPS vs. fibrinolytic agents and/or anticoagulants in patients with recent PVT.
Determine the efficacy of anticoagulation on recanalisation and on prevention of progression of PVT in children/young adults with PVT.
Evaluate the pathophysiology and management of cytopenia in patients with non-cirrhotic portal hypertension.
9). Other issues in vascular liver disorders
Use of anticoagulants in non-cirrhotic vascular liver diseases
-
9.1
LMWH and vitamin K antagonists are widely accepted and used to treat primary thrombosis of the portal venous system or hepatic venous outflow tract. (A.1) (Unchanged)
-
9.2
There are no major concerns regarding the safety of DOACs in patients with non-cirrhotic vascular liver diseases, as long as liver function is preserved. DOACs should be used with caution in patients with impaired liver function (equivalent to Child-Pugh class B), as well as in patients with creatinine clearance below 30 ml/min. The use of DOACs in patients with severe liver dysfunction (equivalent to Child-Pugh C) is not recommended outside study protocols. (C.2) (New)
Anticoagulation and PVT in cirrhosis
-
9.3
Screening for PVT is recommended in all patients who are potential liver transplant candidates, at the time of screening for HCC. (D.2) (Changed)
-
9.4
Occurrence of PVT in the presence of HCC does not directly imply vascular malignant invasion, but further imaging is recommended (CT scan and/or MRI and/or contrast-enhanced ultrasound). (D.2) (Changed)
-
9.5
Anticoagulation is recommended in patients with cirrhosis and (i) recent (<6 months) completely or partially occlusive (>50%) thrombosis of the portal vein trunk with or without extension to the superior mesenteric vein, or (ii) symptomatic PVT, independently of the extension, or (iii) PVT in potential candidates for liver transplantation, independently of the degree of occlusion and extension. (C.2) (New)
-
9.6
In potential liver transplant candidates, the goal of anticoagulation is to prevent re-thrombosis or progression of thrombosis to facilitate adequate portal anastomosis in liver transplantation and reduce post-transplant morbidity and mortality. (C.1) (Changed)
-
9.7
Anticoagulation should be considered in patients with cirrhosis and minimally occlusive (<50%) thrombosis of the portal vein trunk that (i) progresses on short-term follow-up (1–3 months) or (ii) compromises the superior mesenteric vein. (C.2) (New)
-
9.8
Anticoagulation should be (i) maintained until portal vein recanalisation or for a minimum of 6 months, (ii) continued after recanalisation in patients awaiting liver transplantation, and (iii) considered after recanalisation in all others, while balancing the benefits of preventing recurrence and increasing survival with the risk of bleeding. (C.1) (New)
-
9.9
Patients with low platelet count (e.g., <50 ×109/L) are at higher risk of PVT, but also of bleeding complications on anticoagulation, and should be assessed on a case-by-case basis. (C.2) (Changed)
-
9.10
TIPS is recommended in patients with thrombosis of the portal vein trunk without recanalisation on anticoagulation, especially in patients listed for liver transplantation. (C.2) (New)
-
9.11
Anticoagulation is preferably initiated with LMWH and maintained with either LMWH, vitamin K antagonists or DOACs. Advantages of LMWH are that its use is based on solid data. Vitamin K antagonists carry challenges with regard to INR monitoring in patients with cirrhosis. Advantages of DOACs are that they are easier to use but less data are available. (C.1) (Changed)
-
9.12
Currently available data suggest that there are no major safety concerns regarding the use of DOACs in patients with Child-Pugh class A cirrhosis. Due to the possibility of accumulation, DOACs should be used with caution in patients with Child-Pugh class B cirrhosis, as well as in patients with creatinine clearance below 30 ml/min. The use of DOACs in those with Child-Pugh class C cirrhosis is not recommended outside study protocols. (B.2) (New)
-
9.13
DOACs likely have different safety-efficacy profiles in patients with cirrhosis, although at the moment no recommendation can be made in favour of a specific DOAC in this setting. (D.2) (New)
Porto-sinusoidal vascular disorder
-
9.14
PSVD is a broad clinico-pathological entity encompassing non-cirrhotic portal fibrosis, idiopathic portal hypertension or non-cirrhotic intrahepatic portal hypertension, and various overlapping histological patterns including nodular regenerative hyperplasia, obliterative portal venopathy, hepatoportal sclerosis, incomplete septal cirrhosis. (B.1) (New)
-
9.15
The absence of portal hypertension does not rule out PSVD. The presence of common causes of liver disease (e.g., viral hepatitis, excessive alcohol consumption, metabolic syndrome, etc.) does not rule out PSVD, and both can coexist. The presence of PVT does not rule out PSVD, and both can coexist. (B.1) (New)
-
9.16
PSVD should be considered in the following situations: (i) signs of portal hypertension contrasting with atypical features of cirrhosis (e.g., HVPG <10 mmHg; liver stiffness measurement <10 kPa; smooth liver surface and no atrophy of segment IV; hepatic vein-to-vein communications; although none of these features is considered pathognomonic for PSVD); or (ii) liver blood test abnormalities or portal hypertension in a patient with a condition known to be associated with PSVD (Table S1); or (iii) unexplained liver blood test abnormalities even without signs of portal hypertension. (B.1) (New)
Diagnosis of PSVD
-
9.17
PSVD can be observed in the absence of clinical, laboratory or imaging features of portal hypertension. (B.1) (New)
-
9.18
A liver biopsy specimen of adequate size (>20 mm) and of minimal fragmentation – or otherwise considered adequate for interpretation by an expert pathologist – is required for the diagnosis of PSVD. (C.1) (New).
-
9.19
Diagnosis of PSVD requires the exclusion of cirrhosis and of other causes of portal hypertension (B.1), together with 1 of the following 3 criteria (C.2): (i) at least 1 feature specific for portal hypertension; or (ii) at least 1 histologic lesion specific for PSVD; or (iii) at least 1 feature not specific for portal hypertension together with at least 1 histologic lesion compatible although not specific for PSVD (Table 2). (New)
Table 2.
Criteria in the definition of porto-sinusoidal vascular disorder (adapted from19).
| Feature of portal hypertension | Histological lesions suggestive of porto-sinusoidal vascular disorder assessed by an expert pathologist | |
|---|---|---|
| Specific |
|
|
| Not specific |
|
|
Management of PSVD
-
9.20
Once the diagnosis of PSVD is made, patients should be screened for associated immunological diseases, prothrombotic or genetic disorders and exposure to drugs/toxins (Table S1). (D.2) (New)
-
9.21
Endoscopic screening for gastro-oesophageal varices is required at diagnosis of PSVD. (C.1) (New)
-
9.22
The non-invasive Baveno VII criteria for screening of oesophageal varices used in patients with cirrhosis cannot be applied to patients with PSVD. (B.1) (New)
-
9.23
During follow-up, the frequency of endoscopic screening for varices has not yet been defined. Management according to cirrhosis guidelines is recommended, except for stopping rules. (D.2) (New)
-
9.24
There is insufficient data on which therapy should be preferred for portal hypertension prophylaxis in PSVD. Management according to cirrhosis guidelines is recommended. (D.2) (New)
-
9.25
A contrast-enhanced CT scan is suggested at diagnosis of PSVD in order to assess the anatomy/patency of the portal venous system and potential portosystemic collaterals. (D.2) (New)
-
9.26
Screening for PVT in patients with PSVD: there is no data on the best screening method and interval.(D.2) (New) Doppler ultrasound every 6 months is suggested in patients with PSVD and features of portal hypertension.(C.1) (New) In case of abdominal pain, Doppler ultrasound or cross-sectional imaging should be performed to rule out splanchnic vein thrombosis. (B.1) (New)
-
9.27
No recommendation can be made regarding anticoagulation therapy to prevent the development of PVT in PSVD. (D.2) (New)
-
9.28
In those patients developing PVT, anticoagulant therapy should be started according to recommendations for non-cirrhotic PVT. (C.1) (New)
-
9.29
TIPS can be considered to treat severe complications of portal hypertension. Underlying/associated conditions, which negatively impact post-TIPS outcome, must be taken into account in making individual decisions regarding TIPS insertion. (C.2) (New)
-
9.30
Liver transplantation is an option in selected patients with PSVD and severe or refractory complications of portal hypertension or with advanced liver dysfunction. Indications should be discussed in expert centres. (D.2) (New)
Research agenda
Anticoagulation in PVT in cirrhosis
Assess the safety and efficacy of each DOAC in patients with cirrhosis.
Identify indicators associated with a favourable outcome in patients with cirrhosis and PVT treated with anticoagulants.
Develop stopping rules for long-term anticoagulant treatment in patients with cirrhosis and PVT.
Evaluate the advantages and disadvantages of prophylactic vs. full dose anticoagulation in patients with cirrhosis and PVT.
Define response to treatment in patients with cirrhosis and PVT.
PSVD
Evaluate the natural history of PSVD without portal hypertension.
Develop improved non-invasive methods to screen for PSVD (e.g., cross-sectional imaging, SSM).
Evaluate the prophylaxis for PVT in patients with PSVD and signs of portal hypertension.
Evaluate the incidence and predictors of development of PVT in patients with PSVD and assess the efficacy of anticoagulation in this setting.
Other issues
Besides the supporting data and consensus recommendations for the 9 Baveno sessions, 7 lectures were given at Baveno VII. The topics of these lectures were: ‘New concepts of risk stratification’, ‘Clinical stages and ordinal outcomes in portal hypertension’, ‘Lifestyle and genetic modifiers of liver disease progression’, Parenchymal extinction lesions (PELS) in progression. Can they regress?’, ‘Fibrogenesis and regression of fibrosis’, ‘Angiogenesis and progression of advanced chronic liver disease (ACLD)’, and ‘Drugs to modify liver fibrosis progression and regression’. The 9 Baveno sessions and the 7 lectures will be summarised in the Baveno VII proceedings book.17 The Baveno VII consensus workshop was followed by a paediatric satellite meeting entitled ‘Primary prophylaxis of variceal haemorrhage, complexities in the development of evidence-based approaches in paediatrics’.
Readers interested in examining the evolution of the recommendation on cirrhosis and portal hypertension can refer to the Baveno I-VI reports.2–4,7–10,12–14
Use of the definitions and adherence to the recommendations in future studies is encouraged to provide further validation. The topics listed in the research agenda reflect the opinions of the experts about the areas where new information is most needed.
Supplementary Material
Financial support
The Baveno VII Consensus workshop was endorsed and supported with unrestricted grants by the following: a) International Scientific Societies: EASL (European Association for the Study of the Liver), b) national scientific societies: AASLD (American Association for the Study of Liver Disease); AEEH (Spanish Association for the Study of the Liver), AFEF (French Association for the Study of the Liver), AIGO (Italian Association of Hospital Gastroenterologists and Endoscopists); AISF (Italian Association for the Study of the Liver); CIBERehd (Spanish network of biomedical investigation in liver and digestive diseases), ÖGGH (Austrian Society for Gastroenterology and Hepatology), SASL (Swiss Association for the Study of the Liver), SIGE ( Italian Society of Gastroenterology), c) International Research Groups; Decision, Galaxy, Microb-Predict. JB is supported by the Stiftung für Leberkrankheiten, Bern (Switzerland).
Abbreviations
- ACLF
acute-on-chronic liver failure
- AKI
acute kidney injury
- AVB
acute variceal bleeding
- BATO
balloon-occluded antegrade transvenous obliteration
- BCS
Budd-Chiari syndrome
- BRTO
balloon-occluded retrograde transvenous obliteration
- cACLD
compensated advanced chronic liver disease
- CSPH
clinically significant portal hypertension
- DOAC
direct-acting oral anticoagulants
- EVL
endoscopic variceal ligation
- HCC
hepatocellular carcinoma
- HRS
hepatorenal syndrome
- HVPG
hepatic vein pressure gradient
- ICU
intensive care unit
- INR
international normalised ratio
- IVC
inferior vena cava
- LMWH
low-molecular-weight heparin
- LSM
liver stiffness measurement
- MELD
model for end-stage liver disease
- MPN
myeloproliferative neoplasm
- MR
magnetic resonance
- NASH
non-alcoholic steatohepatitis
- NIT(s)
non-invasive test(s)
- NSBB(s)
non-selective beta blocker(s)
- PHG
portal hypertensive gastropathy
- PPG
portal pressure gradient
- PSVD
porto-sinusoidal vascular disorder
- PTFE
polytetrafluoroethylene
- PVT
portal vein thrombosis
- SBP
spontaneous bacterial peritonitis
- SEMS
self-expanding metal stent
- SSM
spleen stiffness measurement
- SVR
sustained virological response
- TE
transient elastography
- TIPS
transjugular intrahepatic portosystemic shunt
- VNT
varices needing treatment
- WHVP
wedged hepatic venous pressure
Baveno VII Faculty
The following were members of the Baveno VI Scientific Committee:
Roberto de Franchis [Milan, Italy (Honorary President)], Jaime Bosch [Bern, Switzerland (Chair)] Guadalupe Garcia-Tsao [West Haven, USA (Vice-Chair)], Thomas Reiberger [Vienna, Austria (Scientific Secretary)], Cristina Ripoll [Jena, Germany (Scientific Secretary)], Juan G Abraldes (Edmonton, Canada), Agustin Albillos (Madrid, Spain), Annalisa Berzigotti (Bern, Switzerland), Gennaro D’Amico (Palermo, Italy), Andrea De Gottardi (Lugano, Switzerland), Alessandra Dell’Era (Milan, Italy), Juan Carlos Garcia-Pagàn (Barcelona, Spain), Joan Genescà (Barcelona, Spain), Aleksander Krag (Odense, Denmark), Wim Laleman (Leuven, Belgium), Vincenzo La Mura (Milan, Italy), Dominique Thabut (Paris, France), Jonel Trebicka (Frankfurt, Germany), Emmanouil Tsochatzis (London, UK), Dominique Valla (Paris, France), Candid Villanueva (Barcelona Spain).
The following chaired sessions or lectures:
Agustin Albillos (Madrid, Spain), Annalisa Berzigotti (Bern, Switzerland), Jaime Bosch (Barcelona, Spain), Roberto de Franchis (Milan, Italy), Andrea De Gottardi (Lugano, Switzerland), Hector Ferral (Evanston, USA),Juan Carlos Garcia-Pagàn (Barcelona, Spain), Guadalupe Garcia-Tsao (West Haven, USA), Joan Genescà (Barcelona, Spain), Virginia Hernandez-Gea (Barcelona, Spain), Wim Laleman (Leuven, Belgium), Mattias Mandorfer (Vienna, Austria), David Patch (London, UK), Pierre Emmanuel Rautou (Paris, France), Thomas Reiberger (Vienna, Austria), Cristina Ripoll (Jena, Germany), Shiv K Sarin (New Delhi, India), Dominique Thabut (Paris, France), Jonel Trebicka (Frankfurt, Germany), Emmanouil Tsochatzis (London, UK), Dominique Valla (Paris, France).
The following participated in the presentations and the discussions as panellists in the consensus sessions:
Anna Baiges (Barcelona, Spain), Jasmohan Bajaj (Richmond, USA), Rafael Bañares (Madrid, Spain), Marta Barrufet (Barcelona, Spain), Lina Benajiba (Paris, France), Christophe Bureau (Toulouse, France), Vincenza Calvaruso (Palermo, Italy), Andres Cardenas (Barcelona, Spain), Alessandra Dell’Era (Milan, Italy), Angels Escorsell (Barcelona, Spain), Jonathan Fallowfield (Edinburgh, UK), Sven Francque (Antwerp, Belgium), Ron Gaba (Chicago, USA), Susana Gomes Rodrigues (Bern, Switzerland), Guogong Han (Xi’an, China), Jidong Jia (Beijing, China), Jean Jacques Kiladjian (Paris, France), Aleksander Krag (Odense, Denmark), Vincenzo La Mura (Milan, Italy), Sabela Lens (Barcelona, Spain), Xuefeng Luo (Chengdu, China), Sarwa Darwish Murad (Rotterdam, The Netherlands), Valerie Paradis (Clichy, France), Salvatore Piano (Padua, Italy), Aurelie Plessier (Clichy, France), Massimo Primignani (Milan, Italy), Bogdan Procopet (Cluj-Napoca, Romania), Marika Rudler (Paris, France), Filippo Schepis, (Modena, Italy), Marco Senzolo (Padua, Italy), Akash Shukla (Mumbai, India), Puneeta Tandon (Edmonton, Canada), Luis Tellez (Madrid, Spain), Maja Thiele (Odense, Denmark), Dhiraj Tripathi (Birmingham, UK), Laura Turco (Bologna, Italy), Fanny Turon (Barcelona, Spain), Candid Villanueva (Barcelona Spain), Hitoshi Yoshiji (Nara, Japan).
The following gave review lectures:
Juan G Abraldes (Edmonton, Canada), Annalisa Berzigotti (Bern, Switzerland), Gennaro D’Amico (Palermo, Italy), Jordi Gracia-Sancho (Barcelona, Spain), Massimo Pinzani (London, UK), Vijay Shah (Rochester, USA), Ian Wanless (Halifax, Canada).
Footnotes
Conflict of interest
None relating to this manuscript. JB has been a consultant for Zydus, Surrozen and Actelion. TR is a consultant for and/or receives research support from Abbvie, Bayer, Boehringer-Ingelheim, Gilead, Intercept, MSD, Philips, Pliant Pharmaceuticals, Roche, and Siemens. RdF, CR and GGT have no disclosures to report.
Please refer to the accompanying ICMJE disclosure forms for further details.
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhep.2021.12.022.
References
- 1.Burroughs AK, editor. Methodology and review of clinical trials in portal hypertension. Amsterdam, New York, Oxford: Excerpta Medical Congress Service N◦; 763; 1987. [Google Scholar]
- 2.de Franchis R, Pascal JP, Ancona E, Burroughs AK, Henderson JM, Fleig W, et al. Definitions, methodology and therapeutic strategies in portal hypertension. A consensus development workshop. J Hepatol 1992;15:256–261. [DOI] [PubMed] [Google Scholar]
- 3.de Franchis R Developing consensus in portal hypertension. J Hepatol 1996;25:390–394. [DOI] [PubMed] [Google Scholar]
- 4.de Franchis R, editor. Portal Hypertension II. Proceedings of the second Baveno international consensus workshop on definitions, methodology and therapeutic strategies. Oxford: Blackwell Science; 1996. [Google Scholar]
- 5.Spina GP, Arcidiacono R, Bosch J, Pagliaro L, Burroughs AK, Santambrogio R, et al. Gastric endoscopic features in portal hypertension: final report of a consensus conference. J Hepatol 1994;21:461–467. [DOI] [PubMed] [Google Scholar]
- 6.Grace ND, Groszmann RJ, Garcia-Tsao G, Burroughs AK, Pagliaro L, Makuch RW, et al. Portal hypertension and variceal bleeding: an AASLD single topic symposium. Hepatology 1998;28:868–880. [DOI] [PubMed] [Google Scholar]
- 7.de Franchis R Updating consensus in portal hypertension: report of the Baveno III consensus workshop on definitions, methodology and therapeutic strategies in portal hypertension. J Hepatol 2000;33:846–852. [DOI] [PubMed] [Google Scholar]
- 8.de Franchis R, editor. Portal Hypertension III. Proceedings of the IIIrd Baveno International Consensus Workshop on Definitions, Methodology and Therapeutic Strategies. Oxford, UK: Blackwell Science; 2001. [Google Scholar]
- 9.de Franchis R Evolving consensus in portal hypertension. Report of the Baveno IV consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol 2005;43:167–176. [DOI] [PubMed] [Google Scholar]
- 10.de Franchis R, editor. Portal Hypertension IV. Proceedings of the IVth Baveno international consensus workshop on methodology of diagnosis and treatment. Oxford, UK: Blackwell publishing; 2006. [Google Scholar]
- 11.Garcia-Tsao G, Bosch J, Groszmann R. Portal hypertension and variceal bleeding, unresolved issues. Summary of an American Association for the study of liver disease and of the European Association for the Study of the Liver single-topic conference. Hepatology 2008;47:1764–1772. [DOI] [PubMed] [Google Scholar]
- 12.de Franchis R, on behalf of the Baveno V Faculty. Revising consensus in portal hypertension: report of the Baveno V consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol 2010;53:762–768. [DOI] [PubMed] [Google Scholar]
- 13.de Franchis R, editor. Portal Hypertension V. Proceedings of the Vth Baveno international consensus workshop. Oxford, UK: Wiley-Blackwell; 2011. [Google Scholar]
- 14.de Franchis R, on behalf of the Baveno VI Faculty. Expanding consensus in portal hypertension: report of the Baveno VI consensus workshop: stratifying risk and individualizing care for portal hypertension. J Hepatol 2015;63:743–752. [DOI] [PubMed] [Google Scholar]
- 15.de Franchis R, editor. Portal Hypertension VI. Proceedings of the VIth Baveno consensus workshop: stratifying risk and individualizing care. New York, USA: Springer; 2016. [Google Scholar]
- 16.GRADE working group. Grading quality of evidence and strength of recommendations. BMJ 2004;328:1490–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Franchis R, editor. Portal Hypertension VII. Proceedings of the VIIth Baveno consensus workshop: Personalized care in portal hypertension. New York, USA: Springer; 2022. [Google Scholar]
- 18.Northup PG, Garcia-Pagan JC, Garcia-Tsao G, Intagliata NM, Superina RA, Roberts LN, et al. Vascular liver disorders, portal vein thrombosis, and procedural bleeding in patients with liver disease: 2020 practice guidance by the American association for the study of liver diseases. Hepatology 2021;73:366–413. [DOI] [PubMed] [Google Scholar]
- 19.De Gottardi A, Rautou PE, Schouten J, Rubbia-Brandt L, Leebeek F, Trebicka J, VALDIG group. Porto-sinusoidal vascular disease: proposal and description of a novel entity. Lancet Gastroenterol Hepatol 2019;4:399–411. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


