Abstract
To identify the intracellular signals which increase the adhesiveness of leukocyte function-associated antigen 1 (LFA-1), we established an assay system for activation-dependent adhesion through LFA-1/intercellular adhesion molecule 1 ICAM-1 using mouse lymphoid cells reconstituted with human LFA-1 and then introduced constitutively active forms of signaling molecules. We found that the phorbol myristate acetate (PMA)-responsive protein kinase C (PKC) isotypes (α, βI, βII, and δ) or phosphatidylinositol-3-OH kinase (PI 3-kinase) itself activated LFA-1 to bind ICAM-1. H-Ras and Rac activated LFA-1 in a PI 3-kinase-dependent manner, whereas Rho and R-Ras had little effect. Unexpectedly, Rap1 was demonstrated to function as the most potent activator of LFA-1. Distinct from H-Ras and Rac, Rap1 increased the adhesiveness independently of PI 3-kinase, indicating that Rap1 is a novel activation signal for the integrins. Rap1 induced changes in the conformation and affinity of LFA-1 and, interestingly, caused marked LFA-1/ICAM-1-mediated cell aggregation. Furthermore, a dominant negative form of Rap1 (Rap1N17) inhibited T-cell receptor-mediated LFA-1 activation in Jurkat T cells and LFA-1/ICAM-1-dependent cell aggregation upon differentiation of HL-60 cells into macrophages, suggesting that Rap1 is critically involved in physiological processes. These unique functions of Rap1 in controlling cellular adhesion through LFA-1 suggest a pivotal role as an immunological regulator.
The leukocyte function-associated antigen 1 (LFA-1; CD11a/CD18) is one of the integrins (β2 integrins) exclusively expressed on leukocytes, and its counterligands are the intercellular adhesion molecules 1, 2, and 3 (ICAM-1, -2, and -3) (13, 35, 59). LFA-1 has been shown to play an important role in leukocyte trafficking. LFA-1/ICAM-1-mediated adhesion is an essential step in the leukocyte-endothelial cell interaction to direct homing or migration from the blood (57). It is also well known that LFA-1/ICAM-1-mediated adhesion establishes and strengthens the T-cell–antigen-presenting cell (APC) contact, which is a critical event for T-cell activation (14, 51, 69).
LFA-1 is not constitutively adhesive, and upregulation of the adhesive activity (avidity) of LFA-1 by external stimuli such as cytokines, chemokines, or antigens is a prerequisite for ligand binding (34, 58). These stimuli are thought to generate intracellular second messengers through cell surface receptors, leading to alteration of the adhesive state of LFA-1 (3, 49, 70). This process is referred to as inside-out signaling (58). The essential role of the integrin cytoplasmic domains in the avidity modulation of integrin was also demonstrated, which leads to the idea that avidity modulation is regulated through integrin cytoplasmic domains by intracellular signals (19, 33, 45). However, the molecular mechanisms of avidity modulation by inside-out signaling have not yet been elucidated.
Since phorbol myristate acetate (PMA) is known as a potent activator of integrins including LFA-1, protein kinase C's (PKCs) are thought to be candidates as activation signals for LFA-1. Although the involvement of PKC in LFA-1 activation was demonstrated using a specific PKC inhibitor (18), there has not been direct evidence that PKC itself can increase the adhesiveness of LFA-1. PKCs are classified into three major subgroups based on their structure and cofactor requirements for activation: conventional PKCs (cPKCs; isoforms α, βI, II, and γ), novel PKCs (nPKCs; isoforms ɛ, δ, ν, θ), and atypical PKCs (aPKCs; isoforms ζ, η, and ι) (20, 36, 41). A particular PKC isotype has been shown to regulate a specific cellular function that reflects its cellular localization and substrate preferences (16, 40, 68). Although leukocytes express multiple isotypes of PKC, little is known about the function of individual PKC isotypes in integrin activation.
Previously we and others reported that the avidity of β1 integrin was regulated by phosphatidylinositol-3-OH kinase (PI 3-kinase) (7, 27, 28, 74). However, it remains to be examined whether PI 3-kinase regulates β2 integrin. Recently, phosphoinositide-dependent protein kinase (PDK-1), which is activated in a manner dependent on phosphatidylinositol 3,4,5-triphosphate, has been shown to mediate the activation of downstream effector molecules such as Akt, PKCζ, and S6 kinase in conjunction with PI 3-kinase (1, 8, 32). The PI 3-kinase/PDK-1/Akt pathway was shown to prevent apoptosis, but the involvement of these molecules in LFA-1 activation is not understood.
The Ras/Rho family of small GTPases regulates the actin cytoskeleton and contributes to the formation of focal adhesion (9, 43). Several members of the Ras/Rho family have been reported to influence integrin-mediated adhesion. H-Ras was demonstrated to suppress the active form of αIIbβ3 chimeras through the mitogen-activated protein kinase pathway (21). However, the H-Ras/mitogen-activated protein kinase pathway was reported to be involved in T-cell receptor (TCR)-activated LFA-1 adhesion (44). A constitutively active R-Ras was found to enhance cellular adhesion to fibronectin by enhancing β1 integrin ligand binding affinity (75). Recently, Rap1 was found to be involved in cell spreading on substratum (67). Rac was also reported to alter integrin-mediated events such as invasion and migration of epithelial cells through the activation of PI 3-kinase (26). Rho was previously shown to be involved in the control of LFA-1-mediated adhesion using C3 exoenzyme (31, 64). However, our previous report showed that C3 exoenzyme had little effect on the adhesive state of LFA-1, although it prevented cell aggregation (24). The ability of the Ras/Rho family members to regulate the avidity of LFA-1 should be reexamined in the same context.
To date, the inside-out signals for integrins have been studied mostly using adherent nonlymphoid cells, in which the avidity modulation of integrins is not clearly distinguished from the enhancement of adhesion by the promotion of postadhesion events such as cell spreading. Studies using inhibitors become difficult to interpret when there are multiple pathways leading to an increased avidity of integrins, because the blocking of one of the inside-out signals does not necessarily lead to an apparent inhibition of adhesion, as is the case for very late antigen 5 (VLA-5) stimulated by receptor tyrosine kinases (28). To circumvent these problems and directly identify molecules that increase the adhesiveness of LFA-1 to ICAM-1, we established an activation-dependent adhesion through human LFA-1/ICAM-1 using a mouse nonadherent lymphoid cell line and determined adhesion-stimulatory activities of constitutively active signaling molecules. Our study revealed that three distinct signaling molecules, PKC, PI 3-kinase, and Rap1, had avidity modulatory activities for LFA-1 with differential effects on the conformation and affinity of LFA-1 and cell-to-cell interaction. Our study further demonstrated that Rap1 played critical roles in upregulation of LFA-1 adhesiveness through the TCR and in the formation of cellular aggregates in PMA-stimulated HL-60 cells.
MATERIALS AND METHODS
Cells and cDNA transfection.
BAF cells, a pro-B-cell line (48), were suspended with RPMI 1640 medium (GibcoBRL, Grand Island, N.Y.) containing 10% fetal calf serum (FCS; Sigma Chemical Co., St. Louis, Mo.), 50 μM β-mercaptoethanol, and 10% WEHI-3 conditioned medium as a source of interleukin-3 (IL-3). For the stable expression of human LFA-1 in BAF cells, 10-μg aliquots of human αL and β2 integrin cDNAs in CDM8 (Invitrogen, Carlsbad, Calif.) were cotransfected with 2 μg of pTK-Hyg (Clontech Laboratories, Palo Alto, Calif.) by electroporation (Bio-Rad Laboratories, Hercules, Calif.) at 370 V and 960 μF. Transfected cells were selected with hygromycin at 0.5 mg/ml (Wako Pure Chemical Industries, Ltd., Osaka, Japan) and expanded for the analysis of LFA-1 expression with a FACScan (Becton Dickinson, Oxnard, Calif.). Several LFA-1-expressing BAF (BAF/hLFA-1) clones were isolated by a limiting dilution and subjected to adhesion assays. BAF/hLFA-1, which shows activation-dependent adhesion to ICAM-1, was further introduced by electroporation using the genes encoding signaling molecules as described below and selected using G418 (1 mg/ml; Gibco) by the procedure described above. G418-resistant clones were isolated and propagated in the presence of G418 and hygromycin.
The T-cell leukemia cell line Jurkat and promyelocyte-like leukemia cell line HL-60 were maintained in RPMI medium containing 10% FCS. They were introduced with the indicated genes by the same procedure as outlined above.
Mutant plasmids of PKCs and Ras/Rho family GTPases.
The constitutively active PKCα, -βI, and -βII were made by replacing glutamic acid with alanine at position 25 (PKCαE25, PKCβIE25, and PKCβIIE25) as described elsewhere (36, 41). PCR was used to insert a Myc epitope tag (EQKLISEEDL) at the carboxyl terminus of PKCαE25, PKCβIE25, or PKCβIIE25. The mutations were verified by sequencing both strands of the plasmids. Similar mutants of PKCδ and PKCζ that have a glutamic acid replacement in the pseudosubstrate sequences (PKCδE144/145 and PKCζE119) were obtained from S. Ohno (Yokohama City University) and P. J. Parker (Imperial Cancer Research Fund, United Kingdom). Constitutively active H-Ras, R-Ras, Rap1, Rac, and Rho mutants were produced from their cDNAs by a single point replacement of glycine with valine at positions 12 (H-RasV12, RacV12, and Rap1V12), 38 (R-RasV38), and 14 (RhoV14). Constitutively active RalA has a single point mutation created by replacement of glutamic acid with leucine at position 72 (RalL72) and was provided by H. Koide (Tokyo Institute of Technology University). A dominant negative form of Rap1 has a point mutation created by substitution of serine for asparagine at position 17 (Rap1N17). Epitope tags were introduced at the amino-terminal end of the mutant small GTPases (Myc epitope tag for RacV12, R-RasV38, and RhoV14; T7 epitope tag for Rap1). Membrane-targeted PI 3-kinase catalytic p110α subunit (p110-CAAX), PDK-1, and Akt/PKB were obtained from J. Downward (Ludwig Institute for Cancer Research, United Kingdom), K. E. Anderson (The Babraham Institute, United Kingdom), and P. N. Tsichlis (Fox Chase Cancer Center), respectively. All constructs were subcloned in pcDNA3 (Invitrogen, Carlsbad, Calif.) for transfection.
Antibodies.
Anti-Myc epitope monoclonal antibody (MAb) 9E10, rabbit polyclonal anti-PKCδ and -ζ antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, Calif.), anti-T7 epitope MAb (Novagen, Madison, Wis.), rabbit polyclonal anti-H-Ras and anti-RalA antibodies (Transduction Laboratory, Lexington, Ky.), antihemagglutinin (anti-HA) MAb 12CA5 (Boehringer Mannheim, Indianapolis, Ind.), anti-phospho-Akt antibody (New England Biolabs Inc., Beverly, Mass.), and peroxidase-linked anti-mouse or rabbit antibody (Amersham Co., Arlington Height, Ill.) were used for immunoprecipitation and immunoblotting as described below. To identify the integrins involved in cell aggregation, the inhibitory monoclonal anti-human LFA-1 (TS1/22), anti-mouse ICAM-1 (KAT-1) (63), anti-mouse LFA-1 (FD441.8 and KBA2), anti-mouse VLA-4 (PS/2) (37), and anti-mouse VCAM-1 (MVCAM-A,429) (Pharmingen, San Diego, Calif.) antibodies were used. Activating MAb against human CD3ɛ (OKT3) was kindly provided by T. Katagiri (National Institute of Neuroscience).
Immunoprecipitation.
BAF cells (5 × 106) were collected by centrifugation and suspended at 0°C for 30 min with 1 ml of lysis buffer (1% Triton X-100, 10 mM Tris-HCl, 150 mM NaCl, 2 mM EDTA, 2 μg of aprotinin per ml, 2 μg of leupeptin per ml, 1 mM phenylmethylsulfonyl fluoride [pH 7.6]) (24). The supernatant was incubated with the indicated antibodies and protein G-Sepharose 4B (Pharmacia Biotech AB, Uppsala, Sweden). The immunoprecipitates were washed with lysis buffer three times and subjected to immunoblotting.
Immunoblotting.
Cell lysates (10 μl per 5 × 104 cells) or immunoprecipitated proteins were subjected to electrophoresis on a sodium dodecyl sulfate 9 or 12% polyacrylamide gel (SDS-PAGE). Proteins were transferred to a polyvinylidene difluoride membrane (Pharmacia Biotech) and blotted with peroxidase-conjugated antibody as described elsewhere (24). The Amersham ECL (enhanced chemiluminescence) system was applied for detection.
Coating plates with ICAM-1.
ICAM-1 was purified by immunoaffinity chromatography using anti-human ICAM-1 antibody (RR1/1)-conjugated Sepharose 4B from cell lysates prepared from 109 JY cells as described before (24). Each well of a polystyrene microtiter plate (96-well plate; Linbro-Flow, Chantilly, Va.) was incubated with 2 μg of the purified ICAM-1 for 90 min at room temperature and then further incubated in phosphate-buffered saline containing 1% bovine serum albumin (BSA) for 30 min at room temperature (24). The amount of ICAM-1 used for coating was chosen to give the maximum cell binding. In this coating condition, site density was about 1,200 sites/mm2 quantified using 125I-labeled RR1/1 (2 μCi/mg) at a final concentration of 20 μg/ml (24).
Cell adhesion assays.
Assays of adhesion using ICAM-1-coating plates were performed as described elsewhere (24). Cells were labeled with 2′,7′-bis-(2-carboxyethyl)-5 (and -6) carboxyfluorescein (Molecular Probes, Inc., Eugene, Oreg.) and suspended with RPMI 1640 containing 10 mM HEPES (pH 7.4) and 5% FCS. For inhibition, coated wells or labeled cells were incubated with 20 μg of anti-human ICAM-1 antibody (RR1/1) or anti-human LFA-1 antibody (TS1/22) per ml for 30 min at room temperature before the assay. Cells were transferred into coated wells at 5 × 104/well and then incubated at 37°C for 30 min. Nonadherent cells were removed with four 21-gauge needle aspirations. Input and bound cells were quantitated in the 96-well plate using a fluorescence concentration analyzer (IDEXX Corp., Westbrook, Maine). For the experiment with wortmannin (Wako Pure Chemical Ltd., Tokyo, Japan), cells were treated with wortmannin at room temperature for 10 min as described (46).
Flow cytometric analysis.
Monoclonal anti-human LFA-1 (TS1/22), anti-activation-dependent epitope of LFA-1 (NKI-L16), anti-human CD18 (TS1/18), anti-mouse ICAM-1 (KAT-1), anti-mouse LFA-1 (FD441.8), and anti-CD3 (OKT3) were used for flow cytometric analysis. Hanks' balanced salt solution containing 3% FBS, 0.1% sodium azide, and 10 mM HEPES (pH 7.4) was used as the staining medium. Cells (106) were incubated with staining buffer containing 10 μg of antibodies per ml on ice for 30 min. They were then washed twice with staining buffer, further incubated with 1 μg/ml of fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse immunoglobulin G (IgG) F(ab′)2 fragments (Cappel, Durham, N.C.), and subjected to flow cytometric analysis with a FACScan (Becton Dickinson, San Jose, Calif.).
Measurement of soluble ICAM-1 binding.
A fusion protein (D1D2-IgG) consisting of the first and second domains of human ICAM-1 fused to the Fc fragment of human IgG1 was kindly provided by T. Takashi (Daiichi Pharmaceutical, Tokyo, Japan) (65, 66). Measurement of the binding of D1D2-IgG to BAF cells was performed as described elsewhere (61). BAF cells were suspended in 50 μl of RPMI 1640 containing 10 mM HEPES (pH 7.4) and 5% FCS and incubated at 2 × 105 cells per 50 μl with D1D2-IgG (1 mg/ml). After 30 min of incubation at 37°C, cells were washed twice and then incubated with FITC-conjugated goat anti-human IgG Fc-specific antibody (10 μg/ml; Cappel, Durham, N.C.) for 20 min on ice. Unbound secondary antibody was removed by washing twice, and then fluorescence of live cells detected using a FACScan flow cytometer.
RESULTS
Establishment of activation-dependent adhesion to ICAM-1 through LFA-1.
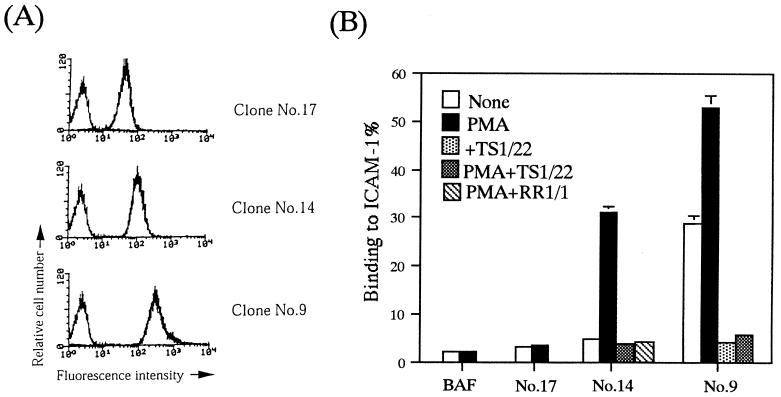
To analyze the avidity modulation of LFA-1, we tried to establish a system in which cells can adhere to ICAM-1 via LFA-1 in an activation-dependent manner, by introducing human αL and β2 in BA/F3, a nonadherent IL-3-dependent mouse pro-B-cell line. We used this cell line because it was nontransformed with a high transfection efficiency. We isolated the stable transfectants with various expression levels of LFA-1 and examined ICAM-1 binding with or without PMA stimulation. The results from the representative clones are shown (Fig. 1). The clone expressing relatively low levels of LFA-1 (clone 17) did not adhere to ICAM-1 even when it was stimulated with PMA for 30 min (Fig. 1). The clone expressing intermediate levels of LFA-1 (clone 14) did not adhere to ICAM-1 by itself but became adherent upon stimulation with PMA (Fig. 1). PMA stimulation did not change the expression levels of LFA-1 (data not shown). The clone expressing relatively high levels of LFA-1 (clone 9) constitutively adhered to ICAM-1 (Fig. 1). Adhesion to ICAM-1 was inhibited completely by anti-human LFA-1 or anti-human ICAM-1 MAbs, confirming a specific LFA-1/ICAM-1-mediated adhesion in the reconstituted system (Fig. 1B). These results suggest that expression levels of LFA-1 in leukocytes are strictly regulated within narrow limits to allow ICAM-1 binding in an activation-dependent manner. To investigate LFA-1 activation signals, we chose clone 14 (BAF/hLFA-1), which showed activation-dependent adhesion, and transfected it with mutationally active signaling molecules.
FIG. 1.
Establishment of activation-dependent adhesion of LFA-1 to ICAM-1. (A) BAF clones transfected with the expression plasmids encoding human αL and β2 were analyzed for LFA-1 expression by flow cytometry. Cells were stained with anti-human LFA-1 antibody (TS1/22) followed by FITC-labeled anti-mouse IgG F(ab′)2 fragments. Data for three clones which represent the different levels of LFA-1 (low [clone 17], intermediate [clone 14], and high [clone 9]) are shown. (B) Adhesion of BAF clones expressing different levels of LFA-1 to ICAM-1. Binding to ICAM-1 or BSA coated onto plates was measured with and without PMA as described in Materials and Methods. The level of adhesion to BSA was less than 1%. Adhesion to ICAM-1 in the presence of anti-human LFA-1 (TS1/22) or anti-human ICAM-1 (RR1/1) MAbs is also shown for BAF clones 14 and 9. Means and standard errors of triplicate determinations are shown.
Active cPKC and nPKC, but not aPKC, increased LFA-1 avidity for ICAM-1.
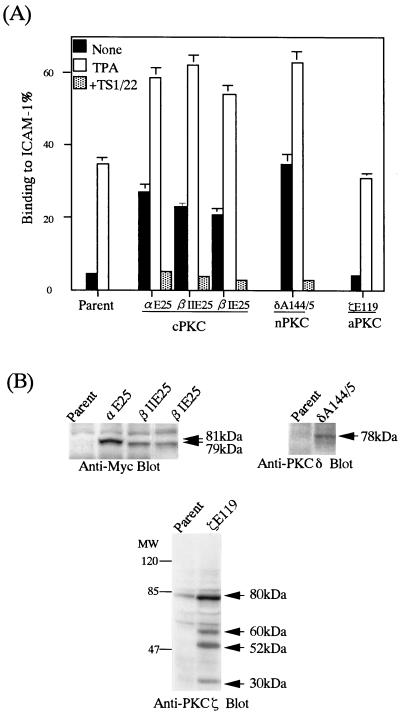
We first investigated the adhesion-stimulatory activities of PKCs which were representative of each isotype, including PKCα, -βI, and -βII for cPKC, PKCδ for nPKC, and PKCζ for aPKC, because they are major isotypes of PKC expressed in leukocytes (20). A mutation in the pseudosubstrate region of PKCs can transform the inactive PKC to the constitutively active form (41). The constitutively active mutants of PKCα (PKCαE25), PKCβI (PKCβIE25), PKCβII (PKCβIIE25), nPKCδ (PKCδE144/45), and PKCζ (PKCζE119) were introduced into BAF/hLFA-1. Several stable clones expressing different amounts of each active PKC isotype were isolated and subjected to adhesion assays. The BAF/hLFA-1 clones expressing either active PKCα, PKCβI, PKCβII, or PKCδ constitutively adhered to ICAM-1; the adhesion levels were comparable to those for the ICAM-1 binding of parental cells stimulated with PMA (Fig. 2). The levels of binding to ICAM-1 correlated with the levels of expression of introduced PKCs (data not shown). The expression levels of LFA-1 in these transfectants were identical to that of parental BAF/hLFA-1 (see Fig. 6), and PMA stimulation did not alter the LFA-1 expression (data not shown). PMA stimulation of the transfectants further enhanced adhesion levels (Fig. 2). This could be due to further activation of the transfected and endogenous PKCs by PMA. In contrast, the enforced expression of constitutively active PKCζE119 did not increase the adhesiveness of LFA-1 to ICAM-1 in the absence of PMA (Fig. 2). As previously reported (72), forced expression of activated PKCζ caused expression of proteins with molecular masses of 60, 52, and 30 kDa in addition to intact 80 kDa, which were the carboxyl-terminal fragments generated by proteolytic degradation (Fig. 2B). These results indicated that the conventional PKCα, -βI, and -βII and novel PKCδ, but not atypical PKCζ itself, were all able to activate LFA-1 binding to ICAM-1.
FIG. 2.
cPKC and nPKC increase adhesiveness of LFA-1. (A) LFA-1/ICAM-1 adhesion for BAF/hLFA-1 clones expressing constitutively active cPKCαE25, -βIE25, and -βIIE25, nPKCδA144/5, and aPKCζE119. Binding to ICAM-1 or BSA was measured with and without PMA. The level of adhesion to BSA was less than 1%. The constitutive adhesion of clones expressing cPKCαE25, -βIE25, and -βIIE25, and nPKC δA144/5 was inhibited by TS1/22. The data are shown as in Fig. 1. (B) Expression levels of PKC isotypes. Mutant cPKCαE25, -βIE25, and -βIIE25 were tagged with the Myc epitope. Lysates from cells expressing each type of PKC were subjected to SDS-PAGE, transferred to a polyvinylidene difluoride filter, and immunoblotted with anti-Myc (9E10), anti-PKCδ, and anti-PKCζ antibodies. Arrows indicate molecular masses of the specific bands corresponding to the introduced PKCs.
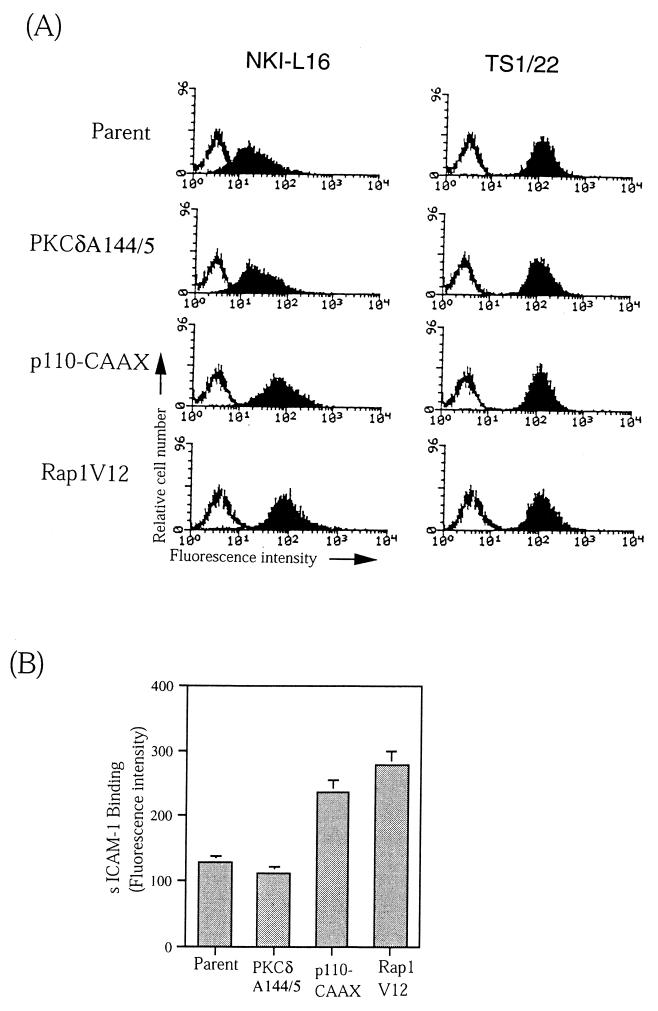
FIG. 6.
PI 3-kinase and Rap1, but not PKC, induce conformational change of LFA-1. (A) Expression of an activation-associated epitope NKI-L16 in parental, PKCδA144/5-, p110-CAAX-, and Rap1V12-expressing BAF/hLFA-1 cells. Cells were stained with NKI-L16 or TS1/22 and FITC-labeled anti-mouse IgG F(ab′)2 fragments and subjected to flow cytometric analysis. (B) Binding of soluble ICAM-1 (D1D2-IgG) to parental, PKCδA144/5-, p110-CAAX-, and Rap1V12-expressing BAF/hLFA-1 cells. Cells were incubated with D1D2-IgG at 1 mg/ml for 30 min at 37°C. Bound sICAM-1 was detected with FITC-conjugated goat anti-human (Fc-specific) antibody and analyzed by flow cytometry. The results are expressed as median fluorescence intensity. This experiment represents three independent assays.
Membrane-targeted PI 3-kinase activated LFA-1, which was not dependent on PDK-1 and Akt/PBK.
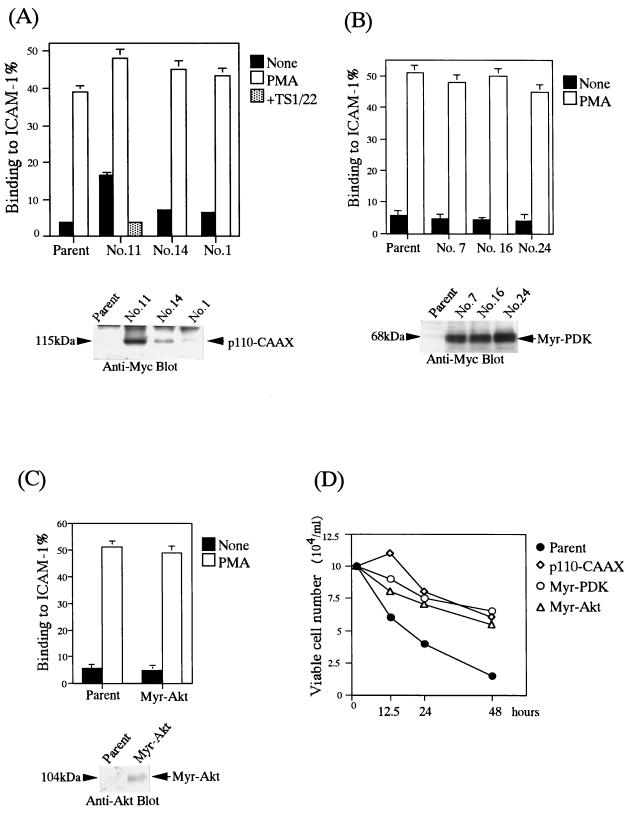
Next, to examine the modulation of LFA-1 avidity by PI 3-kinase, we introduced a constitutively active p110 subunit from PI 3-kinase (p110-CAAX). p110-CAAX alone was found to induce LFA-1/ICAM-1-mediated adhesion in an expression level-dependent fashion (Fig. 3A).
FIG. 3.
PI 3-kinase, but not PDK-1 and Akt, increases LFA-1 avidity. (A) Top, LFA-1/ICAM-1 adhesion of BAF/hLFA-1 clones expressing different levels of constitutively active PI 3-kinase (p110-CAAX). Binding to ICAM-1 or BSA was measured with and without PMA. The level of adhesion to BSA was less than 1%. The constitutive adhesion of clone 11 was inhibited by TS1/22. Results are shown as in Fig. 1. Bottom, expression levels of p110-CAAX. Myc-tagged p110-CAAX was detected by immunoprecipitation and immunoblotting with anti-Myc MAb 9E10 (arrow). (B) Top, LFA-1/ICAM-1 adhesion of BAF/hLFA-1 clones expressing constitutively active PDK-1 (Myr-PDK-1). Binding to ICAM-1 or BSA was measured with and without PMA. The level of adhesion to BSA was less than 1%. The result is shown as in Fig. 1. Bottom, expression levels of Myr-PDK-1. Myc-tagged Myr-PDK-1 was detected by immunoprecipitation and immunoblotting with anti-Myc MAb 9E10 (arrow). (C) Top, LFA-1/ICAM-1 adhesion of BAF/hLFA-1 clones expressing constitutively active Akt (Myr-Akt). Binding to ICAM-1 or BSA was measured with or without PMA. The level of adhesion to BSA was less than 1%. Results are shown as in Fig. 1. Bottom, expression level of myr.Akt. HA-tagged myr.Akt was immunoprecipitated with anti-HA antibody 2CA11 and immunoblotted with anti-Akt antibody (arrow). (D) Effects of active PI 3-kinase, PDK-1, and Akt on cell survival upon withdrawal of IL-3. Parental cells and cells activated by membrane-targeted versions of PI 3-kinase, PDK-1, and Akt were cultured in the IL-3-free medium, and viable cells were counted by the trypan blue exclusion assay.
Recently, it was demonstrated that the sequential activation of PDK-1 and Akt-1 by PI 3-kinase was critical for the antiapoptotic effect of growth factors (11). Membrane localization of PDK-1 was reported to be sufficient to activate downstream targets of PI 3-kinase such as Akt (1, 8, 32). To investigate whether PDK-1 can substitute PI 3-kinase with regard to activation of LFA-1, a membrane-targeted PDK-1 was introduced into BAF/hLFA-1. As shown in Fig. 3B, active PDK-1 did not induce the adhesion to ICAM-1. Constitutively active Akt, Myr-Akt, also failed to induce the adhesion to ICAM-1 (Fig. 3C).
It was reported that the PI 3-kinase/Akt pathway promoted cell survival of BA/F3 cells in the absence of IL-3 (42). To confirm whether activities of PI 3-kinase, PDK-1, and Akt were elevated in these transfectants, we examined cell survival upon withdrawal of IL-3. As shown in Fig. 3D, the viable cell number of parental cells rapidly decreased in IL-3-free medium, but the survival of all these transfectants was prolonged.
These results suggest that activation of LFA-1 is mediated by an effector molecule distinct from PDK-1 and Akt-1.
Activation of LFA-1 by small GTPases of the Ras/Rho family.
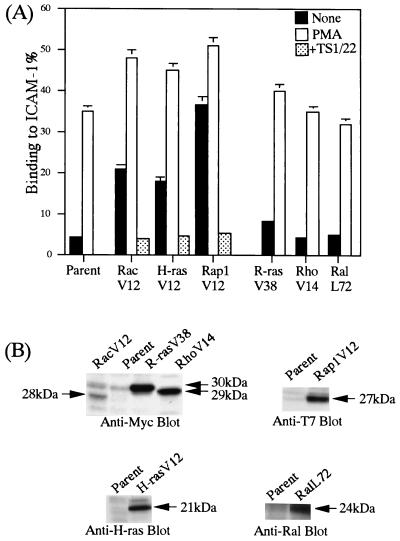
The effects of small GTPases of the Ras/Rho family on the adhesiveness of LFA-1 were examined by introducing the active forms of H-Ras, R-Ras, Rap1, RalA, Rac, and Rho into BAF/hLFA-1. Several clones expressing different amounts of active small GTPases were isolated to confirm expression dependency. The BAF/hLFA-1 clones expressing RacV12, H-RasV12, and Rap1V12 constitutively adhered to ICAM-1 without stimulation (Fig. 4A). The expression levels of RacV12, H-RasV12 and Rap1V12 correlated with the adhesion levels (data not shown). In contrast, RhoV14, R-RasV38, and RalL72 failed to stimulate adhesion to ICAM-1 (Fig. 4A), although the expression levels of R-RasV38 and RhoV14 were higher than those of RacV12 (Fig. 4B).
FIG. 4.
Effects of Ras/Rho family members on LFA-1-mediated adhesion to ICAM-1. (A) LFA-1/ICAM-1 adhesion of BAF/hLFA-1 clones expressing constitutively active RacV12, H-RasV12, Rap1V12, R-RasV38, RhoV14, or RalL72. Binding to ICAM-1 or BSA was measured with and without PMA as indicated. The level of adhesion to BSA was less than 1%. The constitutive adhesion of RacV12-, H-RasV12-, Rap1V12-expressing clones was inhibited by TS1/22. Results are shown as in Fig. 1. (B) Expression levels of introduced small GTPases were detected using anti-Myc antibody for RacV12, R-RasV38, and RhoV14, anti-H-ras antibody for H-rasV12, anti-T7 antibody for Rap1V12, and anti-RalA antibody for RalL72 (arrows).
PI 3-kinase dependency of the activation of LFA-1 by Ras/Rho family members.
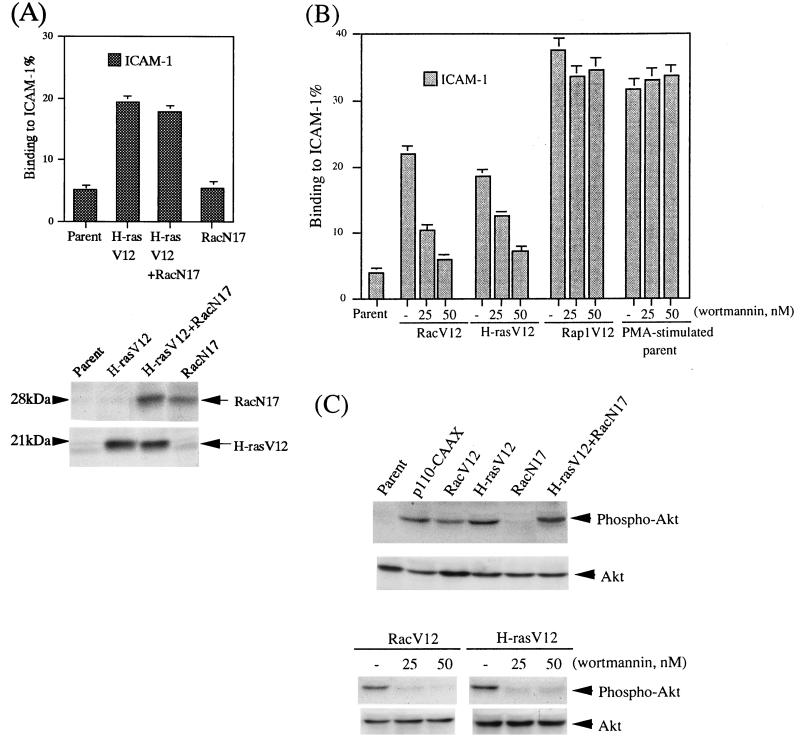
Since H-Ras controls the cytoskeletal organization through Rac (52), we introduced the dominant negative RacN17 together with H-RasV12. As shown in Fig. 5A, the expression of RacN17 did not reduce the induction of LFA-1/ICAM-1-mediated adhesion by H-RasV12, suggesting that the effect of H-RasV12 is not mediated by Rac.
FIG. 5.
Rac and H-Ras activate LFA-1 through PI 3-kinase, but Rap1 has a distinct activation signal for LFA-1. (A) Top, LFA-1/ICAM-1 adhesion in BAF/hLFA-1 clones transfected with either H-RasV12 or a dominant negative mutant of Rac (RacN17) or with both H-RasV12 and RacN17. Binding to ICAM-1 or BSA was measured without stimulation. The level of adhesion to BSA was less than 1%. Results are shown as in Fig. 1. Bottom, expression of RacN17 and H-RasV12 detected by immunoblotting with anti-Myc antibody for RacN17 and anti-H-Ras antibody for H-RasV12 (arrows). (B) Effects of wortmannin on LFA-1/ICAM-1 adhesion of BAF/hLFA-1 clones expressing RacV12, H-RasV12, Rap1V12, and PMA-stimulated parental BAF/hLFA-1 cells. Cells were pretreated with 25 and 50 nM wortmannin for 10 min before adhesion assays. Binding to ICAM-1 or BSA was measured. The level of adhesion to BSA was less than 1%. Results are shown as above. (C) Akt phosphorylation in RacV12- and H-RasV12-transfected BAF/hLFA-1 cells and its inhibition by wortmannin. Parental cells or cells expressing active PI 3-kinase (p110-CAAX), RacV12, H-RasV12, RacN17, and H-rasV12+RacN17 were analyzed for Akt phosphorylation (Phospho-Akt) by immunoblotting using antibodies specific for phospho-Ser473 of Akt. The same membrane was stripped and reprobed with an antibody that recognizes both phosphorylated and unphosphorylated Akt (Akt). BAF/hLFA-1 cells expressing RacV12 and H-RasV12 were pretreated with the indicated doses of wortmannin for 10 min before analysis for Akt phosphorylation as above.
PI 3-kinase was reported to be a downstream effector of both Rac and H-ras (26, 54). To examine the involvement of PI 3-kinase in LFA-1 activation by H-Ras and Rac, the cells were treated with inhibitors for PI 3-kinase before the adhesion assay. LFA-1/ICAM-1-mediated adhesion in both RacV12- and H-RasV12-expressing cells was reduced to background levels following pretreatment with low doses of wortmannin or LY294002 for 10 min before the assay (Fig. 5B and data not shown). To further confirm the involvement of PI 3-kinase, we examined the phosphorylation status of Akt in these transfectants as Akt is phosphorylated upon activation of PI 3-kinase. Consistent with the above result, Akt was phosphorylated in RacV12 and H-RasV12-expressing cells as well as cells expressing p110-CAAX. Wortmannin inhibited the phosphorylation of Akt, in parallel with the LFA-1-mediated adhesion (Fig. 5C).
In contrast, the LFA-1/ICAM-1 adhesion induced by Rap1V12, and also PMA, was not affected by pretreatment with wortmannin or LY294002 (Fig. 5B and data not shown). Thus, H-RasV12 and RacV12 activated LFA-1 through PI 3-kinase, while the Rap1V12-induced activation of LFA-1 was independent of PI 3-kinase.
The above results showed that PKC, PI 3-kinase, and Rap1 upregulate the adhesiveness of LFA-1 to ICAM-1. To obtain further insights into the characteristics of the avidity modulation by these molecules, we examined whether they differ in the ability to cause a conformational change of LFA-1 and morphological changes in cells upon binding to ICAM-1.
Active PI 3-kinase and Rap1 but not PKC induced conformational changes of LFA-1.
The NKI-L16 epitope is a unique Ca2+-dependent activation epitope of LFA-1, which is absent on resting T cells but appears upon in vitro culturing, whose expression is correlated with the capacity of cells to aggregate through LFA-1 and ICAM-1 (71). The expression of NKI-L16 epitope is independent of ligand binding and reflects the conformational change in αL before ligand binding (4). BAF/hLFA-1 expresses low levels of the NKI-L16 epitope, and these expression levels were unchanged by the introduction of active PKC isotypes which increased the adhesiveness of LFA-1 (Fig. 6A and data not shown). In contrast, the levels of NKI-L16 epitope in BAF/hLFA-1 expressing p110-CAAX and Rap1V12 were four- to fivefold higher than in parental cells, while the expression levels of LFA-1 detected by TS1/22 MAb were comparable in all transfectants (Fig. 6A).
Next, we examined whether these signaling molecules can increase the ligand binding affinity of LFA-1 by using soluble ICAM-1 (sICAM-1) (D1D2-IgG). Mn2+-stimulated BAF/hLFA-1 cells were used as a positive control and increased sICAM-1 binding 10-fold (data not shown) compared to nontreated cells. As shown in Fig. 6B, introduction of the active PKC isotype failed to increase the affinity of LFA-1 binding to sICAM-1. However, p110-CAAX and Rap1V12 transfectants increased the binding to D1D2-IgG between two- and threefold compared to parental cells (Fig. 6B). These bindings were inhibited by anti-ICAM-1 antibody (data not shown). Examination of membrane distribution of LFA-1 by immunofluorescence microscopy revealed no significant difference between the parental cells and these transfectants (data not shown).
Taken together, these results demonstrated that the activation of LFA-1 by PI 3-kinase and Rap1, but not PKC, accompanied changes in the conformation and ligand binding affinity of the LFA-1 molecule, suggesting that the activation mechanisms of PI 3-kinase and Rap1 are different from those by PKC.
Rap1V12, but not active PI 3-kinase and PKC, induced cytochalasin D-sensitive cell aggregation.
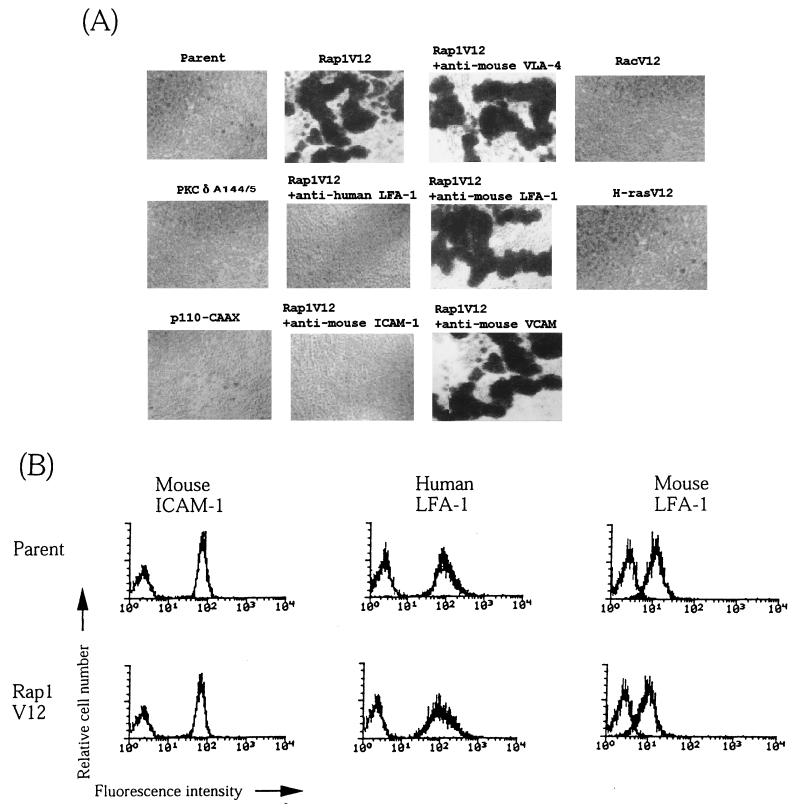
Rap1V12-expressing cells were found to strongly aggregate during culture (Fig. 7A). Cell aggregation was consistently observed in all independent clones expressing Rap1V12. Cell aggregation was not found in BAF/hLFA-1 or cells expressing active PKC isotypes and p110-CAAX (Fig. 7A). The cell aggregation was completely inhibited either by anti-human LFA-1 MAb (TS1/22) or anti-mouse ICAM-1 antibody MAb (KAT-1) but not by MAbs against mouse LFA-1, VLA-4, and VCAM-1 (Fig. 7A). This indicates that cell aggregation was mediated by human LFA-1 and mouse ICAM-1. Cellular aggregation was not observed in cells expressing RacV12 and H-RasV12 (Fig. 7A). Rap1V12 did not change the expression levels of introduced human LFA-1 or endogenous mouse LFA-1 and ICAM-1 (Fig. 7B).
FIG. 7.
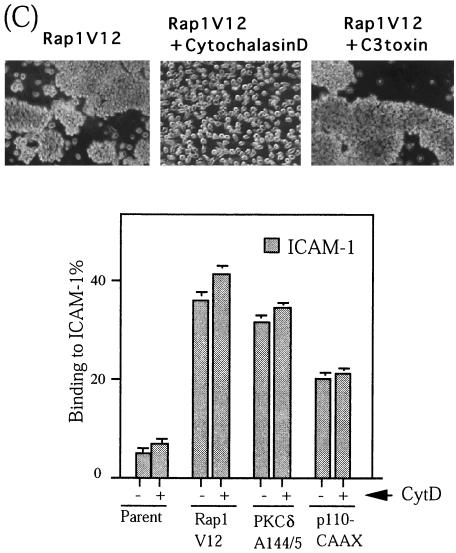
Rap1V12 induces LFA-1/ICAM-1-mediated cellular aggregation. (A) Rap1V12-expressing, but not parental, active PKCδA144/5- or p110-CAAX-expressing BAF/hLFA-1 cells exhibited cellular aggregation. The single cell suspension (105 cells/ml) was incubated in 24-well plates for 1 h with or without antibodies (20 μg/ml) as indicated. Rap1V12-induced aggregation was inhibited by anti-human LFA-1 antibody (TS1/22) or anti-mouse ICAM-1 antibody (KAT-1) but not by anti-mouse LFA-1(FD441.8), VLA-4(PS/2), and anti-VCAM(429) antibodies. RacV12 and H-RasV12-expressing BAF/hLFA-1 cells did not exhibit cellular aggregation. Original magnification is 40-fold. (B) Expression levels of mouse ICAM-1, human LFA-1, and mouse LFA-1 in parental or Rap1V12-expressing BAF/hLFA-1 cells. Cells were stained with anti-mouse ICAM-1, anti-human LFA-1, or anti-mouse LFA-1 antibodies followed by FITC-labeled anti-mouse IgG F(ab′)2 fragments. (C) Top, inhibition of Rap1V12-induced cell aggregation by cytochalasin D but not by C3 exoenzyme. Rap1V12-expressing BAF/hLFA-1 cells were treated with 2 μM cytochalasin D or 20 μM C3 exoenzyme for 1 or 24 h, respectively, and cultured as for panel A. (A). Original magnification is 100-fold. Bottom, effects of cytochalasin D on LFA-1/ICAM-1 adhesion. The parental, Rap1V12-, PKCδA144/5-, p110-CAAX-expressing BAF/hLFA-1 cells were untreated (−) or pretreated (+) with 2 μM cytochalasin D for 30 min before the adhesion assay. Binding to ICAM-1 or BSA was measured without stimulation. The level of adhesion to BSA was less than 1%. Results are shown as in Fig. 1.
To determine whether the aggregation induced by Rap1V12 is a direct consequence of the marked increase of the adhesiveness of LFA-1 or caused by postadhesion events accompanying the actin-based cytoskeletal reorganization (62), we treated aggregated cells with cytochalasin D. The treatment completely inhibited the Rap1V12-induced LFA-1/ICAM-1-dependent cell aggregation, showing that the cell aggregation required polymerization of the actin cytoskeleton (Fig. 7C). In contrast, cell aggregation was not prevented with C3 exoenzyme, a specific inhibitor of Rho (Fig. 7C), suggesting that Rho is not involved in Rap1V12-induced cell aggregation. On the other hand, the treatment with cytochalasin D failed to inhibit ICAM-1 binding of the cells expressing Rap1V12 as well as active PKCδ or p110-CAAX (Fig. 7C), demonstrating that the increase in the avidity of LFA-1 does not require actin polymerization.
Taken together, these results indicate that Rap1 not only upregulates LFA-1 avidity but also promotes cytoskeletal rearrangement to induce stable cell-cell interactions and morphological changes.
Rap1 was involved in TCR-mediated LFA-1 activation and LFA-1/ICAM-1-dependent cell aggregation of PMA-stimulated HL-60 cells.
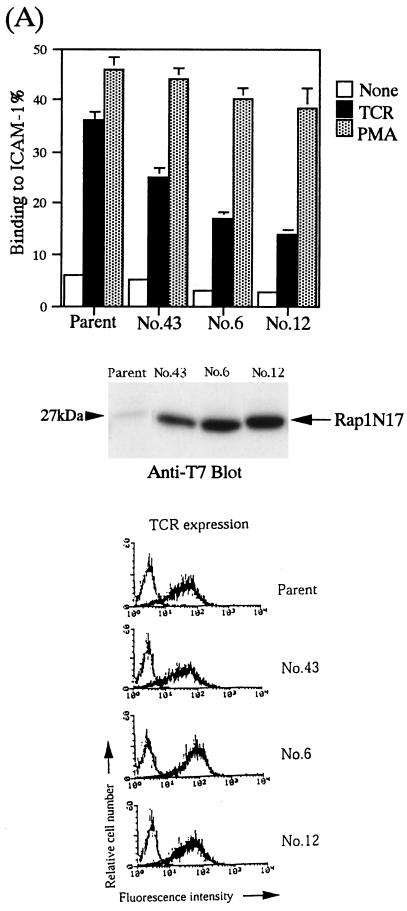
Since Rap1V12 has notable functions in stimulating cellular adhesion through LFA-1, we investigated whether Rap1 was physiologically involved in LFA-1/ICAM-1 adhesion using a dominant negative form of Rap1, Rap1N17. We examined LFA-1 activation by TCR cross-linking in Jurkat T cells and LFA-1/ICAM-1-mediated cell aggregation upon PMA-induced monocytic differentiation of HL-60 cells, because Rap1 was reported to be activated in both of these systems (references 53 and 73 and unpublished data).
As shown in Fig. 8A, the enforced expression of Rap1N17 in Jurkat T cells reduced TCR-induced LFA-1/ICAM-1 adhesion to less than half of that in TCR-stimulated parental cells. Cell surface expression levels of both the TCR-CD3 complex and LFA-1 were found to be similar in clones expressing the dominant negative Rap1 and in parental cells (Fig. 8A and data not shown). In contrast, the expression of Rap1N17 was little effect on PMA-induced LFA-1/ICAM-1 adhesion (Fig. 8A). We also examined whether the residual adhesion activity that was not inhibited by Rap1N17 was due to the insufficient expression of Rap1N17 or other signaling pathways. The high concentration of a PKC-specific inhibitor, calphostin (more than 500 nM) or wortmannin (more than 100 nM), had only marginal effects on TCR-induced LFA-1/ICAM-1 adhesion (data not shown). These results demonstrated that Rap1 played an essential role in regulating the adhesiveness of LFA-1 by TCR-mediated signaling in Jurkat T cells.
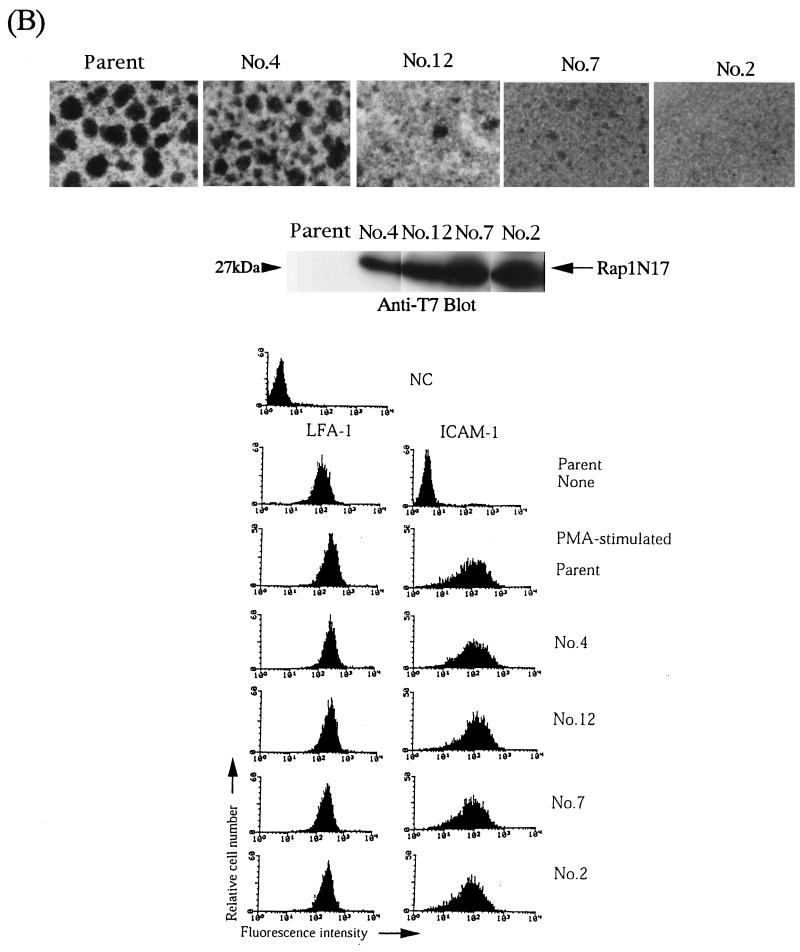
FIG. 8.
Inhibition of LFA-1/ICAM-1 adhesion by expression of dominant negative Rap1N17. (A) Top, reduction of TCR-activated LFA-1 adhesion to ICAM-1 by Rap1N17 in Jurkat T cells. Adhesion of parental or Rap1N17-expressing Jurkat T-cell clones 43, 6, and 12 was triggered by anti-CD3ɛ antibody (OKT3; 1 μg/ml) or PMA (10 ng/ml). Binding to ICAM-1 or BSA coated onto plates was measured with and without anti-CD3 antibody or with PMA. The level of adhesion to BSA was less than 1%. Means and standard errors of triplicate determinations are shown. Middle, expression of Rap1N17 in Jurkat T-cell clones 43, 6, and 12 detected by immunoblotting anti-T7 antibody (arrow). Bottom, expression of CD3 in parental cells and Rap1N17 transfectants. Cells were stained with OKT3 followed by FITC-labeled anti-mouse IgG F(ab′)2 fragments. (B) Top, prevention of PMA-induced LFA-1/ICAM-1-dependent aggregation of HL-60 cells by Rap1N17. Clones 4, 12, 7, and 2, expressing different levels of Rap1N17, were isolated. Parental cells and transfectants (2 × 105 cells/ml) were stimulated for 24 h with 10 ng of PMA per ml (PMA-stimulated) under agitation. Original magnification is 40-fold. Middle, expression of Rap1N17 detected by immunoblotting with anti-T7 antibody (arrow). Bottom, expression of LFA-1 and ICAM-1 in nonstimulated parental cells (None) or in PMA-stimulated parental or Rap1N17-expressing clones. Cells were stained with anti-human LFA-1 (TS1/22) or anti-human ICAM-1 (RR1/1) antibodies followed by FITC-labeled anti-mouse IgG F(ab′)2 fragments. NC, negative control.
Our previous papers reported that the cell aggregation observed in PMA-stimulated HL-60 cells was completely inhibited by anti-αL or anti-ICAM-1 antibodies (23, 25). As shown in Fig. 8B, the expression of Rap1N17 in HL-60 cells prevented such LFA-1/ICAM-1-mediated cell aggregation in a dose-dependent manner. Cell surface expression of LFA-1 and ICAM-1 was almost identical in transfectants and parental cells (Fig. 8B). We also examined the effects of dominant negative forms of H-Ras, Rac, R-Ras, and Rho on the adhesion of PMA-stimulated HL-60 cells. Our previous report showed that H-RasN17 inhibited the induction of monocytic differentiation markers, including the expression of ICAM-1 (22). RacN17, RhoN19, and R-RasN43 did not affect LFA-1/ICAM-1-dependent cell aggregation in PMA-stimulated HL-60 cells (unpublished data). Thus, Rap1 was critically involved in stable LFA-1/ICAM-1-dependent cellular interactions for PMA-induced differentiation of HL-60 cells into macrophages.
DISCUSSION
In this study, we have established a reconstitution system for activation-dependent adhesion by LFA-1, using a nonadherent, lymphoid cell line, and examined the effects of a series of active signaling molecules on adhesion to ICAM-1. We have demonstrated that active forms of PMA-responsive PKC isotypes, PI 3-kinase, H-Ras, Rac, and Rap1 activate LFA-1 to allow ICAM-1 binding. The effects of H-Ras and Rac converge on PI 3-kinase. However, Rap1-induced adhesion was not mediated by PI 3-kinase, suggesting that PKC, PI 3-kinase, and Rap1 are distinct signaling pathways which increase the adhesiveness of LFA-1. Furthermore, our study revealed that PKC, PI 3-kinase, and Rap1 had differential effects on the conformation and ligand binding affinity of LFA-1 and adhesion-dependent cytoskeletal changes, such as aggregation. Therefore, cellular adhesion mediated by LFA-1 and ICAM-1 can be regulated by at least three signaling pathways. This makes it possible to generate diverse adhesive interactions between immune cells, such as transendothelial migration and the formation of T-cell–APC conjugates.
It has been suggested, but not demonstrated directly, that PKC is involved in the inside-out signals because of potent integrin activation by PMA. We directly examined the ability of an individual PKC isotype to activate LFA-1, using mutant PKCs that unlock the inhibition of catalytic activity but retain the regulatory domain that helps determine substrate specificity (41, 68). We have shown that cPKCαE25, -βIE25, and -βIIE25 and nPKCδE144/5 had comparable effects on the activation of LFA-1 (Fig. 2). In contrast, aPKCζE119 had little effect on LFA-1 activation, while this mutant was previously shown to activate human atrial natriuretic factor-promoting activity (12). aPKCζ has been reported not to respond to phorbol esters in vivo and in vitro (36). Therefore, our results demonstrate that the PMA-responsive isotypes of PKC such as α, βI, βII, and δ can activate LFA-1 to bind ICAM-1. The fact that multiple isotypes of PKC can activate LFA-1 is consistent with a previous report (20) suggesting functional redundancy in a member of the PKC family. Specificity within the PKC family is more likely to be affected through regulatory inputs, with PKC isotypes responding to different activation and localization signals (20, 40). PKC isotypes, which are physiologically involved in LFA-1 activation, are most likely determined by regulated expression of PKC isotypes and activation signals.
We have recently shown that PI 3-kinase is an affinity modulator of VLA-5 and is critically involved in affinity modulation of VLA-5 in mast cells stimulated with FcɛRI and steel factor (27). PI 3-kinase has also been reported to activate VLA-4 upon stimulation by cross-linking of CD28 (74). The present study demonstrated that LFA-1 was also activated by PI 3-kinase. Thus, PI 3-kinase can activate both β1 and β2 integrin family members. Moreover PI 3-kinase, but not PKC, is capable of inducing conformational changes and increasing the ligand binding affinity of LFA-1, suggesting that the mechanism of LFA-1 activation by PI 3-kinase are different from those of PKC.
Active PKCs could not induce the conformational change of LFA-1 in this system. However, since it was reported that PMA treatment could modestly induce the expression of NKI-L16 (56), we could not exclude the possibility that PKC might induce conformational changes of LFA-1 in other cells. We showed that PKC did not increase the binding affinity for ICAM-1. Integrin adhesiveness is thought to be regulated through alternations of integrin affinity for ligand or cell surface distribution and clustering (4, 34, 60). PKC might induce ligand-dependent clustering of LFA-1 by increasing the ability of lateral mobility.
The effector molecules downstream of PI 3-kinase are unknown. We confirmed that both constitutively active PDK-1 and PKB/Akt failed to induce an adhesive state of LFA-1, consistent with our previous finding for VLA-5 (27). Cytohesin-1, which has a pleckstrin homology domain at the carboxyl terminus and is a guanine nucleotide exchange factor for a small GTPase, ADP-ribosylation factor (5, 47), has been reported to associate with LFA-1, and its overexpression induces a constitutive activation state in LFA-1 (30, 39). However, we could not demonstrate the association of cytohesin-1 with LFA-1 and the increase of LFA-1-mediated adhesion by cytohesin-1 in our system (data not shown).
Our study has shown that Rap1 was the most potent stimulator of LFA-1 adhesiveness among the Ras/Rho family members. Unlike RacV12 and H-RasV12, Rap1V12-induced activation of LFA-1 was not affected by inhibitors of PI 3-kinase, suggesting that Rap1 is a distinct signaling pathway for the activation of LFA-1. Moreover, only Rap-1V12-expressing cells showed stable cell aggregation, indicating that a marked cytoskeletal reorganization was induced. Rap1 was originally identified as the molecule able to revert the phenotype of Ki-Ras transformed fibroblasts to a flat morphology (29). Rap1 was also reported to function as an inducer of T-cell anergy under certain circumstances (6). Rap1 was thought to exert Ras-antagonistic effects by a nonproductive interaction between Rap1 and Ras effector molecules (10, 29). However, recent reports have shown that Rap1 has unique functions in cell migration, localization, and spreading on the substratum (2, 15, 38, 67), which are closely related to cellular adhesion. Here we have shown that Rap1N17 inhibits TCR-stimulated adhesion mediated by LFA-1 and ICAM-1. Inhibitors of PI-3 kinase failed to inhibit TCR-activated adhesion to ICAM-1 (data not shown). Thus, Rap1 plays a major role in inside-out signaling of LFA-1 through TCR. We propose that avidity modulation of LFA-1 is one of the key functions of Rap1 as an immunological regulator.
The ability of Rap1V12 to induce stable cell-cell contact through LFA-1/ICAM-1 implies a possible role for Rap1 in the formation of the “immunological synapse” (17) between T cells and APC. This occurs through LFA-1/ICAM-1 binding, which is cytochalasin D sensitive and requires actin polymerization (51). We noticed that the inhibitory effect of Rap1N17 on cell aggregation of HL-60 cells tended to be stronger than that on adhesion to coated ICAM-1 (data not shown). It is, therefore likely that activated Rap1 plays an essential role in the formation of stable cell-to-cell interactions by regulating cytoskeletal reorganization as well as the adhesive activity of LFA-1. It should be noted that adherence of HL-60 cells to the plastic surface of the culture dish was not affected, suggesting that the effect of Rap1N17 was not general to cell attachment and spreading but is rather specific to LFA-1/ICAM-1-mediated cell aggregation. However, we could find no significant difference in F-actin staining patterns between parental cells and Rap1V12 transfectants. Rap1 may not increase the levels of actin polymerization but regulate the association of cytoplasmic regions of LFA-1 with actin cytoskeleton and accumulation of cytoskeletal components at contact sites (50, 55).
Our study has shown that distinct members of the small GTPase Ras/Rho family preferentially activate integrin subfamily members. The effect of Rap1 on β1 integrins is much lower than that of β2 integrins, whereas R-Ras selectively activated β1 integrins such as VLA-4 and VLA-5 (reference 75 and unpublished data). Selective activation of the integrin subfamily by inside-out signals is critical for cell migration and localization, which require differential utilization of a group of adhesion molecules. For instance, transendothelial migration of leukocytes into peripheral tissues involves successive adhesive interactions with endothelial cells and the extracellular matrix through selectin and β2 and β1 integrins (57). Further studies are required to establish whether or not the selective activation of Rap1 and R-Ras contributes to the differential activation of integrin family members under physiological conditions.
In summary, our study clearly and for the first time demonstrates that three distinct signaling molecules, PKC, PI 3-kinase, and Rap1, have avidity modulatory activities and can induce differential adhesive states in LFA-1 molecules. In particular, Rap1 plays a critical role in TCR-stimulated inside-out signals and cell-to-cell contact formations by LFA-1 and ICAM-1. The selective activation of LFA-1 by inside-out signaling molecules following external stimuli is likely to lead to distinct cell adhesion behavior and hence influence growth, differentiation, and effector functions in immune cells. Further studies are required to identify the specific roles of inside-out signal molecules in LFA-1-mediated adhesion induced by physiological stimulation. Our study has yielded important insights into these processes.
ACKNOWLEDGMENTS
We thank S. Narumiya for RhoV14 and C3 exoenzyme, Y. van Kooyk and C. G. Figdor for NKI-L16, J. Downward for p110α-CAAX, P. N. Tsichlis for pSR-Akt, Y. Nishizuka for PKCα, -βI, -βII, and -ζ, S. Ohno for PKCδA144/5, P. J. Parker for PKCζE119, K. E. Anderson for myr.PDK-1, and H. Koide for RalAL72, and T. Takashi for soluble ICAM-1.
This work was supported in part by a grant-in-aid from the Ministry of Education, Science, Sport, and Culture of Japan.
REFERENCES
- 1.Anderson K E, Coadwell J, Stephens L R, Hawkins P T. Translocation of PDK-1 to the plasma membrane is important in allowing PDK-1 to activate protein kinase B. Curr Biol. 1998;8:684–691. doi: 10.1016/s0960-9822(98)70274-x. [DOI] [PubMed] [Google Scholar]
- 2.Asha H, de Reuter N D, Wang M G, Hariharan I K. The Rap1 GTPase functions as a regulator of morphogenesis in vivo. EMBO J. 1999;18:605–615. doi: 10.1093/emboj/18.3.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Axelsson B, Youseffi E R, Hammarstrom S, Perlmann P. Induction of aggregation and enhancement of proliferation and IL-2 secretion in human T cells by antibodies to CD43. J Immunol. 1988;141:2912–2917. [PubMed] [Google Scholar]
- 4.Bazzoni G, Hemler M E. Are changes in integrin affinity and conformation overemphasized? Trends Biochem Sci. 1998;23:30–34. doi: 10.1016/s0968-0004(97)01141-9. [DOI] [PubMed] [Google Scholar]
- 5.Betz S F, Schnuchel A, Wang H, Olejniczak E T, Meadows R P, Lipsky B P, Harris E, Staunton D E, Fesik S W. Solution structure of the cytohesin-1 (B2-1) Sec7 domain and its interaction with the GTPase ADP ribosylation factor 1. Proc Natl Acad Sci USA. 1998;95:7909–7914. doi: 10.1073/pnas.95.14.7909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boussiotis V A, Freeman G J, Berezovskaya A, Barber D L, Nadler L M. Maintenance of human T cell anergy: blocking of IL-2 gene transcription by activated Rap1. Science. 1997;278:124–128. doi: 10.1126/science.278.5335.124. [DOI] [PubMed] [Google Scholar]
- 7.Chan A S H, Mobley J L, Fields G B, Shimizu Y. CD7-mediated regulation of integrin adhesiveness on human T cells involves tyrosine phosphorylation-dependent activation of phosphatidylinositol 3-kinase. J Immunol. 1997;159:934–942. [PubMed] [Google Scholar]
- 8.Chou M M, Hou W, Johnson J, Graham L K, Lee M H, Chen C S, Newton A C, Schaffhausen B S, Toker A. Regulation of protein kinase Cζ by PI 3-kinase and PDK-1. Curr Biol. 1998;8:1069–1077. doi: 10.1016/s0960-9822(98)70444-0. [DOI] [PubMed] [Google Scholar]
- 9.Clark E A, King W G, Brugge J S, Symons M, Hynes R O. Integrin-mediated signals regulated by members of the rho family of GTPases. J Biol Chem. 1998;142:573–586. doi: 10.1083/jcb.142.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cook S J, Rubinfeld B, Albert I, McCormick F. RapV12 antagonizes Ras-dependent activation of ERK1 and ERK2 by LPA and EGF in Rat-1 fibroblasts. EMBO J. 1993;12:3475–3485. doi: 10.1002/j.1460-2075.1993.tb06022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Datta S R, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg M E. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 12.Decock J B, Gillespie B J, Parker P J, Sugden P H, Fuller S J. Classical, novel and atypical isoforms of PKC stimulate ANF- and TRE/AP-1-regulated-promoter activity in ventricular cardiomyocytes. FEBS Lett. 1994;356:275–278. doi: 10.1016/0014-5793(94)01283-0. [DOI] [PubMed] [Google Scholar]
- 13.De Fougerolles A R, Springer T A. Intercellular adhesion molecule 3, a third adhesion counter-receptor for lymphocyte function-associated molecule 1 on resting lymphocytes. J Exp Med. 1992;175:185–190. doi: 10.1084/jem.175.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dustin M L, Springer T A. T-cell receptor cross-linking transiently stimulates adhesiveness through LFA-1. Nature. 1989;341:619–624. doi: 10.1038/341619a0. [DOI] [PubMed] [Google Scholar]
- 15.Franke B, Akkerman J W, Bos J L. Rapid Ca2+-mediated activation of Rap1 in human platelets. EMBO J. 1997;16:252–259. doi: 10.1093/emboj/16.2.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Genot E M, Parker P J, Cantrell D A. Analysis of the role of protein kinase C-α, -ɛ, and -ζ in T cell activation. J Biol Chem. 1995;270:9833–9839. doi: 10.1074/jbc.270.17.9833. [DOI] [PubMed] [Google Scholar]
- 17.Grakoui A, Bromley S, Sumen C, Davis M, Shaw A, Allen P, Dustin M. The immunological synapse: a molecular machine controlling T cell activation. Science. 1999;285:221–227. doi: 10.1126/science.285.5425.221. [DOI] [PubMed] [Google Scholar]
- 18.Hauss P, Mazerolles F, Hivroz C, Lecomte O, Barbat C, Fischer A. GF109203X, a specific PKC inhibitor, abrogates anti-CD3 antibody-induced upregulation of CD4+ T cell adhesion to B cells. Cell Immunol. 1993;150:439–446. doi: 10.1006/cimm.1993.1211. [DOI] [PubMed] [Google Scholar]
- 19.Hibbs M L, Xu H, Stacker S A, Springer T A. Regulation of adhesion to ICAM-1 by the cytoplasmic domain of LFA-1 integrin β subunit. Science. 1991;251:1611–1613. doi: 10.1126/science.1672776. [DOI] [PubMed] [Google Scholar]
- 20.Hug H, Sarre T F. Protein kinase C isoenzymes: divergence in signal transduction? Biochem J. 1993;291:329–343. doi: 10.1042/bj2910329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hughes P E, Renshaw M W, Pfaff M, Forsyth J, Keivens V M, Schwartz M A, Ginsberg M H. Suppression of integrin activation: a novel function of a Ras/Raf-initiated MAP kinase pathway. Cell. 1997;88:521–530. doi: 10.1016/s0092-8674(00)81892-9. [DOI] [PubMed] [Google Scholar]
- 22.Katagiri K, Hattori S, Nakamura S, Yamamoto T, Yoshida T, Katagiri T. Activation of Ras and formation of GAP complex during TPA-induced monocytic differentiation of HL-60 cells. Blood. 1994;84:1780–1789. [PubMed] [Google Scholar]
- 23.Katagiri K, Katagiri T, Koyama Y, Morikawa M, Yamamoto T, Yoshida T. Expression of src family genes during monocytic differentiation of HL-60 cells. J Immunol. 1991;146:701–707. [PubMed] [Google Scholar]
- 24.Katagiri K, Kinashi T, Irie S, Katagiri T. Differential regulation of leukocyte function-associated antigen-1/intercellular adhesion molecules-1-dependent adhesion and aggregation in HL-60 cells. Blood. 1996;87:4276–4285. [PubMed] [Google Scholar]
- 25.Katagiri K, Yokosawa H, Kinashi T, Kawashima S, Irie S, Tanaka K, Katagiri T. Ubiquitin-proteasome system is involved in induction of LFA-1/ICAM-1-dependent adhesion of HL-60 cells. J Leukoc Biol. 1999;65:778–785. doi: 10.1002/jlb.65.6.778. [DOI] [PubMed] [Google Scholar]
- 26.Keely P J, Westwick J K, Whitehead I P, Der C J, Parise L V. Cdc42 and Rac1 induce integrin-mediated cell motility and invasivness through PI(3)K. Nature. 1997;390:632–635. doi: 10.1038/37656. [DOI] [PubMed] [Google Scholar]
- 27.Kinashi T, Asaoka T, Setoguchi R, Takatsu K. Affinity modulation of very late antigen-5 through phosphatidylinositol 3-kinase in mast cells. J Immunol. 1999;162:2850–2857. [PubMed] [Google Scholar]
- 28.Kinashi T, Escobedo J A, Williams L T, Takatsu K, Springer T A. Receptor tyrosine kinase stimulates cell-matrix adhesion by phosphatidylinositol 3 kinase and phospholipase C-γ1 pathways. Blood. 1995;86:2086–2090. [PubMed] [Google Scholar]
- 29.Kitayama H, Sugimoto Y, Matsuzaki T, Ikawa Y, Noda M. A ras-related gene with transformation suppressor activity. Cell. 1989;56:77–84. doi: 10.1016/0092-8674(89)90985-9. [DOI] [PubMed] [Google Scholar]
- 30.Kolanus W, Nagel W, Schiller B, Zeitlmann L, Godar S, Stockinger H, Seed B. αLβ2 integrin/LFA-1 binding to ICAM-1 induced by cytohesin-1, a cytoplasmic regulatory molecule. Cell. 1996;86:233–242. doi: 10.1016/s0092-8674(00)80095-1. [DOI] [PubMed] [Google Scholar]
- 31.Laudanna C, Campbell J J, Butcher E C. Role of Rho in chemoattractant-activated leukocyte adhesion through integrins. Science. 1996;271:981–983. doi: 10.1126/science.271.5251.981. [DOI] [PubMed] [Google Scholar]
- 32.Le Good J A, Ziegler W H, Parekh D B, Alessi D R, Cohen P, Parker P J. Protein kinase C isotypes controlled by phosphoinositide 3-kinase through the protein kinase PDK1. Science. 1998;281:2042–2045. doi: 10.1126/science.281.5385.2042. [DOI] [PubMed] [Google Scholar]
- 33.Lu C-H, Springer T A. The a subunit cytoplasmic domain regulates the assembly and adhesiveness of integrin lymphocyte-function-associated antigen-1. J Immunol. 1997;159:268–278. [PubMed] [Google Scholar]
- 34.Lub M, van Kooyk Y, Figdor C G. Ins and outs of LFA-1. Immunol Today. 1995;16:479–483. doi: 10.1016/0167-5699(95)80031-x. [DOI] [PubMed] [Google Scholar]
- 35.Marlin S D, Springer T A. Purified intercellular adhesion molecule-1 (ICAM-1) is a ligand for lymphocyte function-associated antigen 1 (LFA-1) Cell. 1987;51:813–819. doi: 10.1016/0092-8674(87)90104-8. [DOI] [PubMed] [Google Scholar]
- 36.Mellor H, Parker P J. The extended protein kinase C superfamily. Biochem J. 1998;332:281–292. doi: 10.1042/bj3320281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miyake K, Hasunuma Y, Yagita H, Kimoto M. Requirement for VLA-4 and VLA-5 integrins in lymphoma cells binding to and migration beneath stromal cells in culture. J Cell Biol. 1992;119:653–662. doi: 10.1083/jcb.119.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.M'Rabet L, Coffer P, Zwartkruis F, Franke B, Segal A W, Koenderman L, Bos J L. Activation of the small GTPase Rap1 in human neutrophils. Blood. 1998;92:2133–2140. [PubMed] [Google Scholar]
- 39.Nagel W, Zeitlmann L, Schilcher P, Geiger C, Kolanus J, Kolanus W. Phosphoinositide 3-OH kinase activates the beta2 integrin adhesion pathway and induces membrane recruitment of cytohesin-1. J Biol Chem. 1998;273:14853–14861. doi: 10.1074/jbc.273.24.14853. [DOI] [PubMed] [Google Scholar]
- 40.Newton A C. Protein kinase C: ports of anchor in the cell. Curr Biol. 1996;6:806–809. doi: 10.1016/s0960-9822(02)00600-0. [DOI] [PubMed] [Google Scholar]
- 41.Newton A C. Protein kinase C: structure, function, and regulation. J Biol Chem. 1995;270:28495–28498. doi: 10.1074/jbc.270.48.28495. [DOI] [PubMed] [Google Scholar]
- 42.Nishida K, Kaziro Y, Sato T. Anti-apoptotic function of Rac in hematopoietic cells. Oncogene. 1999;18:407–415. doi: 10.1038/sj.onc.1202301. [DOI] [PubMed] [Google Scholar]
- 43.Nobes C D, Hall A. Rho, Rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- 44.O'Rourke A M, Shao H, Kaye J. A role for p21ras/MAP kinase in TCR-mediated activation of LFA-1. J Immunol. 1998;161:5800–5803. [PubMed] [Google Scholar]
- 45.O'Toole T E, Katagiri Y, Faull R J, Peter K, Tamura R, Quaranta V, Loftus J C, Shattil S J, Ginsberg M H. Integrin cytoplasmic domains mediate inside-out signal transduction. J Cell Biol. 1994;124:1047–1059. doi: 10.1083/jcb.124.6.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Okada T, Sakuma L, Fukui Y, Hazeki O, Ui M. Blockage of chemotactic peptide-induced stimulation of neutrophils by wortmannin as a result of selective inhibition of phophatidylinositol 3-kinase. J Biol Chem. 1994;269:3563–3567. [PubMed] [Google Scholar]
- 47.Pacheco-Rodriguez G, Meacci E, Vitale N, Moss J, Vaughan M. Guanine nucleotide exchange on ADP-ribosylation factors catalyzed by cytohesin-1 and its Sec7 domain. J Biol Chem. 1998;273:26543–26548. doi: 10.1074/jbc.273.41.26543. [DOI] [PubMed] [Google Scholar]
- 48.Palacios R, Steinmetz M. IL-3-dependent mouse clones that express B-220 surface antigen, contain Ig genes in germ-line configuration, and generate B lymphocytes in vivo. Cell. 1985;41:727–734. doi: 10.1016/s0092-8674(85)80053-2. [DOI] [PubMed] [Google Scholar]
- 49.Pardi R, Inverardi L, Bender J R. Regulatory mechanisms in leukocyte adhesion: flexible receptors for sophisticated travelers. Immunol Today. 1992;13:224–230. doi: 10.1016/0167-5699(92)90159-5. [DOI] [PubMed] [Google Scholar]
- 50.Pavalko F, LaRoche S. Activation of human neutrophils induces an interaction between the integrin β2-subunit (CD18) and the actin binding protein α-actinin. J Immunol. 1993;151:3795–3807. [PubMed] [Google Scholar]
- 51.Penninger J M, Crabtree G R. The actin cytoskeleton and lymphocyte activation. Cell. 1999;96:9–12. doi: 10.1016/s0092-8674(00)80954-x. [DOI] [PubMed] [Google Scholar]
- 52.Qiu R G, Chen J, Kirn D, McCormick F, Symons M. An essential role for Rac in Ras transformation. Nature. 1995;374:457–459. doi: 10.1038/374457a0. [DOI] [PubMed] [Google Scholar]
- 53.Reedquist K, Bos J. Costimulation through CD28 suppresses T cell receptor-dependent activation of the ras-like small GTPase Rap1 in human T lymphocytes. J Biol Chem. 1998;273:4944–4949. doi: 10.1074/jbc.273.9.4944. [DOI] [PubMed] [Google Scholar]
- 54.Rodriguez-Viciana P, Warne P H, Khwaja A, Marte B M, Papppin D, Das P, Waterfield M D, Ridley A, Downward J. Role of phosphoinositide 3-OH kinase in cell transformation and control of the actin cytoskeleton by Ras. Cell. 1997;89:457–467. doi: 10.1016/s0092-8674(00)80226-3. [DOI] [PubMed] [Google Scholar]
- 55.Sampath R, Gallagher P, Pavalko F. Cytoskeletal interactions with the leukocyte integrin β2 cytoplasmic tail. J Biol Chem. 1998;273:33588–33594. doi: 10.1074/jbc.273.50.33588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shames R, Goetzl E J. Activation of human neutrophil LFA-1(CD11a) by leukotriene B4. Inflammation. 1993;17:371–382. doi: 10.1007/BF00918998. [DOI] [PubMed] [Google Scholar]
- 57.Springer T A. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 58.Springer T A, Dustin M L, Kishimoto T K, Marlin S D. The lymphocyte function-associated LFA-1, CD2, and LFA-3 molecules: cell adhesion receptors of the immune system. Annu Rev Immunol. 1987;5:223–252. doi: 10.1146/annurev.iy.05.040187.001255. [DOI] [PubMed] [Google Scholar]
- 59.Staunton D E, Dustin M L, Springer T A. Functional cloning of ICAM-2, a cell adhesion ligand for LFA-1 homologous to ICAM-1. Nature. 1989;339:61–64. doi: 10.1038/339061a0. [DOI] [PubMed] [Google Scholar]
- 60.Stewart M, Hogg N. Regulation of leukocyte integrin function: affinity vs. avidity. J Cell Biochem. 1996;61:554–561. doi: 10.1002/(sici)1097-4644(19960616)61:4<554::aid-jcb8>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 61.Stewart M, McDowall A, Hogg N. LFA-1-mediated adhesion is regulated by cytoskeletal restraint and by a Ca2+-dependent protease, calpain. J Cell Biol. 1998;140:699–707. doi: 10.1083/jcb.140.3.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stossel T P. On the crawling of animal cells. Science. 1993;260:1086–1094. doi: 10.1126/science.8493552. [DOI] [PubMed] [Google Scholar]
- 63.Sun R, Hojo H, Kato K, Hashimoto Y. Effects of anti-intercellular adhesion molecule-1 and anti-lymphocyte-function associated antigen-1 monoclonal antibodies on the metastasis of murine tumors. Invasion Metastasis. 1996;16:39–48. [PubMed] [Google Scholar]
- 64.Tominaga T, Sugie K, Hirata M, Morii N, Fukata J, Uchida A, Imura H, Narumiya S. Inhibition of PMA-induced, LFA-1 dependent lymphocyte aggregation by ADP ribosylation of the small molecular weight GTP binding protein, rho. J Cell Biol. 1993;120:1529–1537. doi: 10.1083/jcb.120.6.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tominaga Y, Kita Y, Satoh A, Asai S, Kato K, Ishikawa K, Horiuchi T, Takashi T. Affinity and kinetic analysis of the molecular interaction of ICAM-1 and leukocyte function-associated antigen-1. J Immunol. 1998;161:4016–4022. [PubMed] [Google Scholar]
- 66.Tominaga Y, Kita Y, Uchiyama T, Sato K, Sato K, Takashi T, Horiuchi T. Expression of a soluble form of LFA-1 and demonstration of its binding activity with ICAM-1. J Immunol Methods. 1998;212:61–68. doi: 10.1016/s0022-1759(97)00207-x. [DOI] [PubMed] [Google Scholar]
- 67.Tsukamoto N, Hattori M, Yang H, Bos J L, Minato N. Spa-1 negatively regulates cell adhesion through GAP activity for Rap1. J Biol Chem. 1999;274:18463–18469. doi: 10.1074/jbc.274.26.18463. [DOI] [PubMed] [Google Scholar]
- 68.Uberall F, Giselbrecht S, Hellbert K, Fresser F, Bauer B, Gschwendt M, Grunicke H H, Baier G. Conventional PKC-α, novel PKC-ɛ and PKC-θ, but not atypical PKC-λ are MARCKS kinases in intact NIH 3T3 fibroblasts. J Biol Chem. 1997;272:4072–4078. doi: 10.1074/jbc.272.7.4072. [DOI] [PubMed] [Google Scholar]
- 69.van Kooyk Y, van de Wiel-van Kemenade P, Weder P, Kuijpers T W, Figdor C G. Enhancement of LFA-1-mediated cell adhesion by triggering through CD2 or CD3 on T lymphocytes. Nature. 1989;342:811–813. doi: 10.1038/342811a0. [DOI] [PubMed] [Google Scholar]
- 70.van Kooyk Y, Weder P, Heije K, de Waal Malefijt R, Figdor C G. Role of intracellular Ca2+ levels in the regulation of CD11a/CD18 mediated cell adhesion. Cell Adhes Commun. 1993;1:21–32. doi: 10.3109/15419069309095679. [DOI] [PubMed] [Google Scholar]
- 71.van Kooyk Y, Weder P, Heije K, Figdor C G. Extracellular Ca2+ modulates leukocyte function-associated antigen-1 cell surface distribution on T lymphocytes and consequently affects cell adhesion. J Biol Chem. 1994;124:1061–1070. doi: 10.1083/jcb.124.6.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ways D K, Cook P P, Webster C, Parker P J. Effect of phorbol esters on protein kinase C-ζ. J Biol Chem. 1992;267:4799–4805. [PubMed] [Google Scholar]
- 73.Yamashiro S, Takayama K, Shiku H, Furukawa K. Up-regulation of small GTP-binding proteins smg P21A and rasP21S during TPA-induced differentiation of human leukemia cell lines. Leuk Res. 1993;17:129–136. doi: 10.1016/0145-2126(93)90057-r. [DOI] [PubMed] [Google Scholar]
- 74.Zell T, Hunt S W 3, Mobley J L, Finkelstein L D, Shimizu Y. CD28-mediated up-regulation of β1-integrin adhesion involves phosphatidylinositol 3-kinase. J Immunol. 1996;156:883–886. [PubMed] [Google Scholar]
- 75.Zhang Z, Vuori K, Wang H, Reed J C, Ruoslahti E. Integrin activation by R-ras. Cell. 1996;85:61–69. doi: 10.1016/s0092-8674(00)81082-x. [DOI] [PubMed] [Google Scholar]