Abstract
Investigations of the efficiency and safety of human adenovirus vector (AdV)-mediated gene transfer in the airways of patients with cystic fibrosis (CF) in vivo have demonstrated little success in correcting the CF bioelectrical functional defect, reflecting the inefficiency of AdV-mediated gene transfer to the epithelial cells that line the airway luminal surface. In this study, we demonstrate that low AdV-mediated gene transfer efficiency to well-differentiated (WD) cultured airway epithelial cells is due to three distinct steps in the apical membrane of the airway epithelial cells: (i) the absence of specific adenovirus fiber-knob protein attachment receptors; (ii) the absence of αvβ3/5 integrins, reported to partially mediate the internalization of AdV into the cell cytoplasm; and (iii) the low rate of apical plasma membrane uptake pathways of WD airway epithelial cells. Attempts to increase gene transfer efficiency by increasing nonspecific attachment of AdV were unsuccessful, reflecting the inability of the attached vector to enter (penetrate) WD cells via nonspecific entry paths. Strategies to improve the efficiency of AdV for the treatment of CF lung disease will require methods to increase the attachment of AdV to and promote its internalization into the WD respiratory epithelium.
Successful gene therapy for cystic fibrosis lung disease requires efficient in vivo gene transfer to airway epithelia (2). We have previously reported that the efficiency of adenovirus vector (AdV)-mediated gene transfer to poorly differentiated (PD) airway epithelial cells in vitro is high whereas the efficiency of gene transfer to well-differentiated (WD) ciliated airway epithelium in vivo is low (18, 25). We have speculated that the lower efficiency observed in vivo is due to the absence of an early step in the vector-cell interaction (25). Ad is thought to enter cells by a two-step process: (i) initial attachment of the viral fiber-knob protein to high-affinity receptors, for which a candidate has recently been identified (the human coxsackievirus B and Ad2 and Ad5 receptor [hCAR]) (1, 29), and (ii) translocation of the virus into the cell cytoplasm via coated-pit internalization processes, mediated in part by an interaction of the viral penton base with αvβ3/5 integrins (32).
The target cell type for cystic fibrosis lung gene therapy is the WD ciliated columnar airway epithelial cell, but the interactions of AdV with this cell type in vivo have not been comprehensively studied. Inefficient AdV-mediated gene transfer to a bronchial xenograft model of human ciliated airway epithelia has been reported to reflect the absence of the αvβ3/5 integrins from the luminal membrane of the epithelium (14). Similarly, luminal αvβ3/5 integrin expression was reported to correlate with the efficiency of AdV-mediated gene transfer to murine airway epithelium (13). However, the exact role of αvβ3/5 integrins in determining AdV-mediated gene transfer efficiency to the respiratory epithelium is not known and may not alone account for decrements in efficacy. Mutants of AdV that lack penton base RGD sequences (normally required for αvβ3/5 integrin interactions) are able to efficiently transduce human epithelial cells, although the rate of internalization is reduced (12). In a β5-knockout mouse model, airway epithelial cells lacking the β5 integrin subunit had the same susceptibility to AdV-mediated gene transfer as did wild-type airway cells (17), again suggesting that αvβ3/5 integrins may be facilitative rather than necessary for efficient vector entry into the cell cytoplasm.
In this study, we set out to identify the rate-limiting steps responsible for limiting the uptake of AdV into the respiratory airway epithelium to determine which step(s) is responsible for the inefficiency of AdV-mediated gene transfer to this tissue. For these studies, we have used human and rat airway epithelial cell culture models that can generate cultures with PD and WD phenotypes from common progenitor cells. With this system, we systematically compared AdV entry into and attachment to PD and WD cells and related the findings to the differences in gene transfer efficiencies.
MATERIALS AND METHODS
Virus preparations.
Human Ad5 vector (AdV), with deletions of the E1a, E1b, and E3 genes, contained the Escherichia coli lacZ gene under the control of the cytomegalovirus promoter (9). [35S]methionine-radiolabeled AdVCMVlacZ (35S-AdV) was prepared as described previously (16) with modification for 293 cells by harvesting at 48 h postinfection. The adenovirus particle-to-infectious-unit ratio was routinely 100:1 and was determined by plaque assay on either 293 or 911 cells (similar values were obtained for the two cell lines). Serial dilutions of virus in Dulbecco’s modified Eagle medium (DMEM-H) were incubated with 50% confluent cell cultures in six-well plates at a volume of 1 ml per well (fluid height, 0.1 cm). The cultures were incubated for 1 h at 37°C without agitation. The medium was removed and replaced with 3 ml of agar overlay (1× DMEM-H, 10% fetal bovine serum [FBS], and 1% SeaPlaque LGT agarose). The cultures were fed with 1 ml of overlay (containing 2% FBS) every 3 days until the plaques were counted at 6 days postinfection for 911 cells or 10 to 12 days postinfection for 293 cells. The specific activity of the radiolabeled vector was approximately 4 × 10−5 cpm per particle. To directly visualize AdV, Cy3 FluoroLink (Amersham Life Science Inc., Arlington Heights, Ill.) was conjugated to the AdV capsid coat by incubation of 1012 particles of AdVlacZ as described previously to form CyAdV (23). This process reduced the AdV titer by less than 10-fold (23). CyAdV was characterized by assessment of fluorescent attachment and transgene expression with HeLa and CHO K1 cell lines in the absence and presence of competing purified fiber-knob protein. The Ad5 fiber-knob protein was produced by expressing pBEVαfibre (a gift from Robert Gerard, Katholieke Universtiet Leuven, Louvain, Belgium) in E. coli TG-1 cells, and purified fiber-knob protein was obtained exactly as described previously (19).
Cell culture.
Human tracheobronchial epithelial cells were derived from non-CF airway specimens and were cultured by procedures similar to those described by Gray et al. (15). Portions of the lower trachea and mainstem bronchi representing excess donor tissue were obtained at the time of lung transplantation under institutional review board-approved protocols. Epithelial cells were removed from the specimens by protease XIV digestion as described previously (34), and 106 cells were plated per 100-mm tissue culture dish in modified LHC9 medium (22). The modifications were increased epidermal growth factor concentration to 25 ng/ml, adjustment of the retinoic acid concentration to 5× 10−8 M, and supplementation with 0.5 mg of bovine serum albumin per ml and 0.8% bovine pituitary extract. At approximately 75% confluence, the cells were harvested with trypsin and passage 1 cells were plated at a density of 3.33 × 105 cells on Transwell-Col inserts (diameter, 24 mm; pore size, 0.4 μm; Corning-Costar, Cambridge, Mass.) in modified medium. The medium is similar to the supplemented LHC9, except that a 50:50 mixture of LHC Basal (Biofluids Inc., Rockville, Md.) and DMEM-H was used as the base, amphotericin and gentamicin were omitted, and the epidermal growth factor concentration was reduced to 0.5 ng/ml. After 4 to 6 days, the cells became confluent and were used as PD cultures. For the production of WD cultures, confluent cultures were maintained with an air/liquid interface for another 25 to 30 days.
Rat tracheal epithelial cells were isolated from pathogen-free male F344 rats (200 g), and 2 × 105 cells were plated on permeable Transwell-Col matrix supports (as above) by the method of Kaartinen et al. (21). After 5 days of culture, the cells became confluent and were used as PD cultures. For the production of rat WD cultures, after the cells became confluent the apical surfaces of the cultures were given an air/liquid interface for at least 19 days.
HeLa cells (American Type Culture Collection) were plated on Transwell-Col supports (as above), grown to confluence, and maintained in Eagle’s minimum essential medium supplemented with nonessential amino acids and 10% FBS. HeLa cells expressing the tetracycline-sensitive wild-type and K44a mutant form of dynamin were kind gifts from Sandra Schmid (Scripps Research Institute, La Jolla, Calif.) (7) and were initially maintained in DMEM–10% FBS–400 μg of gentamicin per ml–200 ng of puromycin per ml–1 μg of tetracycline per ml. To induce dynamin overexpression, the cells were cultured in the absence of tetracycline for 2 days before being exposed to AdV.
Expression, entry, and attachment studies.
Analyses of expression, entry, and attachment were performed within a single batch of cells, on the same day, with identical reagents. For the lacZ expression studies, the luminal surfaces of cultures were exposed to 1010 particles of AdV (multiplicity of infection, ∼100) at 37°C for 6 h, unbound virus was removed from the cells by three washes in ice-cold medium, and the cells were returned to 37°C before gene expression analyses 48 h after the initial exposure to AdV. β-Galactosidase (β-gal) enzyme activity was assessed as described previously (18).
Internalization of radiolabeled AdV was assessed as follows. After exposure of cultures to AdV (1010 particles) for 6 h at 37°C, the cultures were transferred to 4°C and washed three times in ice-cold medium. The cells were then rinsed with an acid-salt wash (0.2 N acetic acid, 0.5 M NaCl [pH 2.5]) at 4°C and exposed for 1 h to protease (0.25% pronase XIV with 0.0025% DNase in serum-free culture medium at 4°C) to remove extracellular bound vector. This method effectively removed more than 95% of the vector attached to the cell surface at 4°C, as determined by assessing removal-resistant counts after this treatment. After further washing in ice-cold medium, the cells were solubilized in 1% sodium dodecyl sulfate (SDS)–0.3 N NaOH and the counts were assessed by liquid scintillation counting. For attachment studies, cultures maintained at 4°C were exposed to 35S-AdV (1010 particles) for 6 h. The cultures were then washed three times in ice-cold medium, and the cells were solubilized and subjected to liquid scintillation counting. For the studies with purified fiber-knob protein and RGD peptides, the cultures were preexposed to fiber-knob protein (10 μg/ml), RGD peptide (4.0 mg/ml; Gibco BRL, Bethesda, Md.) or cyclical RGD peptide (0.4 mg/ml; Immunodynamics, La Jolla, Calif.) for 2 h at 4°C before the addition of AdV for 6 h at 4°C. The cultures were then washed three times in ice-cold medium and either immediately solubilized as above for scintillation counting or maintained at 37°C for a further 48 h before being subjected to expression analyses.
For both species, only fully confluent cultures were exposed to AdV to ensure a standard surface area and to avoid nonspecific binding of AdV to the matrix support. The transepithelial resistance (Rt) values of the cultures at the time of AdV exposure were as follows: human PD and WD cultures, 1,076 ± 95 (n = 48) and 1,342 ± 53 (n = 36) Ω · cm2, respectively; rat PD and WD cultures, 223 ± 5 and 2,653 ± 75 Ω · cm2, respectively (n = 12 for both). Radioactive counts per minute and β-gal activity were standardized with respect to the nominal surface area of the culture surface, since we consider the apical surface area of cells exposed to vector to be the most appropriate denominator, allowing direct comparison to the epithelium in vivo. The β-gal activity was measured as microunits per square centimeter of epithelium, where 1 U of enzyme will hydrolyze 1 μmol of o-nitrophenyl-β-d-galactopyranoside per min at pH 7.3 and 37°C.
For attachment and expression studies with HeLa cell mutants, cells were plated onto six-well plates in tetracycline-deficient medium to induce wild-type or mutant dynamin expression. After 2 days in culture, the cells were cooled to 4°C and exposed to 1010 particles of AdVlacZ per ml for 2 h. After three washes with ice-cold medium the cells were prepared for liquid scintillation counting as above or placed at 37°C for 48 h until used for expression analyses. For each stage of the experiment, parallel cultures were used to determine cell numbers.
Generation of hCAR-expressing cell lines.
To generate stable expression of hCAR in cell lines, hCAR cDNA was placed in a Moloney murine leukemia virus-based retroviral vector. The hCAR cDNA was a gift from Jeffrey Bergelson (Dana-Farber Cancer Institute, Boston, Mass.). Briefly, hCAR cDNA was cloned into the LxPin retroviral vector (24a) containing a poliovirus internal ribosome entry site and a neomycin resistance gene. The resulting plasmid was packaged by transient transfection of PA317 cells and pseudotyped with vesicular stomatitis virus glycoprotein G.
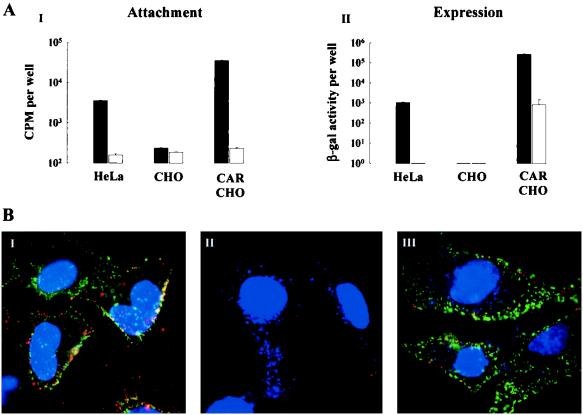
Functional analyses of the retroviral construct was performed by introducing hCAR into a cell line that exhibits reduced susceptibility to AdV transduction. Chinese hamster ovary (CHO K1; American Type Culture Collection) cells were infected with retroviral vectors containing hCAR-Neo (CAR-CHO) or Neo alone (CHO) as a control, and stably expressing cell lines were selected by standard methods (4). To test the functional expression of hCAR, cells were grown in 12-well culture dishes until confluent, 35S-AdVlacZ was exposed to the cultures (1010 particles/ml for 2 h at 4°C) in the absence or presence of excess purified fiber-knob protein (10 μg/ml), and attachment and expression were assessed as above.
HeLa, CHO, and CAR-CHO cells were exposed to monoclonal antibody (MAb) RmcB (a hybridoma cell culture supernatant generated against hCAR [20], a gift from Jeffrey Bergelson) as follows. Cells grown on glass coverslips were cooled to 4°C and blocked with 3% bovine serum albumin. After incubation with RmcB, the cells were washed and exposed to fluorescein isothiocyanate-conjugated goat anti-mouse immunoglobulin G1 (IgG1) (Jackson ImmunoResearch Labs Inc., West Grove, Pa.) in the presence of CyAdV (1010 particles). The cells were washed in phosphate-buffered saline and fixed in paraformaldehyde (4%), mounted with Vectashield containing 4′,6-diamidino-2-phenylindole (DAPI) (Vector Laboratories Inc.), and viewed by conventional fluorescence microscopy. Controls consisted of untransfected CHO cells or CHO cells expressing neo alone. In addition, the absence of primary antibody or the use of irrelevant isotyped MAb (anti-bromodeoxyuridine; Boehringer-Mannheim Corp., Indianapolis, Ind.) were used as controls. Localization of hCAR in human airway cultures was performed as follows. Human PD and WD cultures were fixed and permeabilized in paraformaldehyde (4%) and Triton X-100 (0.2%), respectively. The cultures were then exposed to RmcB (or anti-bromodeoxyuridine as a control) and, after being washed, exposed to Texas Red-conjugated goat anti-mouse IgG1 (Jackson ImmunoResearch Labs Inc.). After being washed, the cultures were fixed in paraformaldehyde and viewed by confocal fluorescence microscopy, and XZ sections were generated.
Electron and confocal microscopy.
For the transmission electron microscopy studies, the luminal surfaces of human cultures were exposed to AdV (1010 particles) for 6 h at 4°C, washed as above, and fixed in 1.5% glutaraldehyde overnight before being processed for electron microscopy by standard methods (28). For experiments with fluorescent microspheres, the luminal surfaces of human cultures were exposed to 100-nm fluorescent microspheres (0.02% in medium; Molecular Probes) for 6 h at 37°C and then washed three times with medium. En face fluorescent images were taken with a conventional inverted fluorescence microscope (Leica DM IRB). Images perpendicular to the cell layer were captured by XZ sectioning with a confocal microscope (Leica TCS/4D), and images were generated with the Metamorph image analysis system (Universal Image Co.).
Immunoprecipitation and Western analyses.
Apical or basolateral membranes of human and rat PD and WD cultures were exposed to sulfosuccinimidobiotin (0.5 mg/ml; Pierce Chemical Co.) for 30 min at 4°C and then the cells were washed in ice-cold phosphate-buffered saline containing protease inhibitors (2 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, and 1 mM [each] leupeptin-pepstatin) and solubilized in lysis buffer (150 mM NaCl, 1% Nonidet P-40, 0.5% deoxycholate, 0.1% SDS, 50 mM Tris-HCl [pH 7.5] with protease inhibitors). Before immunoprecipitation of integrins, the cell lysates were precleared with normal rabbit serum and protein G beads (Pierce Chemical Co.). The αvβ3/5 integrins were immunoprecipitated by rabbit anti-human/rat αvβ3/5 integrin polyclonal antibody (838 [3], a kind gift of S. Albelda, University of Pennsylvania, Philadelphia) or a rabbit anti-human vitronectin receptor polyclonal antibody (AB1904; Chemicon International Inc.). Precipitates were subjected to SDS-polyacrylamide gel electrophoresis (14% Tris-glycine gel under reduced conditions) and transferred to polyvinylidene difluoride, and biotinylated membrane proteins were detected with streptavidin-conjugated peroxidase and visualized by enhanced chemiluminescence.
Inulin uptake studies.
To measure the nonspecific uptake capacity of cells, confluent PD human primary airway epithelial cells were generated on tissue culture plastic and WD cultures were generated as described above. The luminal surfaces of the cultures were exposed to freshly prepared [3H]inulin (35 μg/ml, 4 cpm/nl; Amersham Life Sciences Inc.) at either 4 or 37°C for 6 h. The cultures were then returned to 4°C, washed five times with ice-cold medium containing excess inulin (1 mg/ml), and solubilized for liquid scintillation counting. To measure cell uptake of [3H]inulin, cell-associated counts at 37°C were subtracted from those at 4°C as previously described (31). The volume of fluid uptake into the cells was calculated based on the known counts per minute of the applied solution.
Statistics.
Statistical analysis was performed by Student’s t test, and P < 0.05 was considered significant.
RESULTS
Analyses of gene transfer, vector entry, and attachment with models of respiratory epithelium. (i) Human cultures.
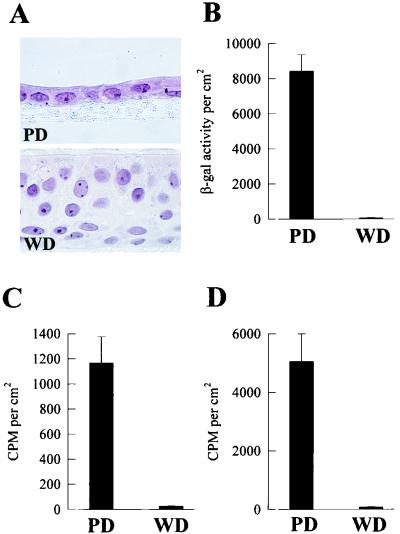
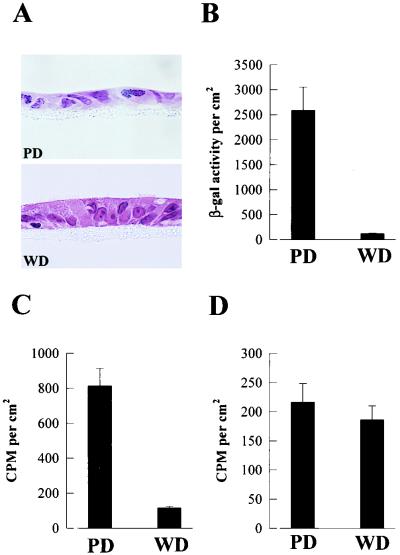
Modifications of a cell culture system reported by Gray et al. (15) generate from human tracheobronchial airway epithelial cells polarized cultures with PD and WD cellular phenotypes from the same patient (Fig. 1A). The morphology of WD cultures resembles the pseudostratified ciliated epithelium exhibited by human cartilaginous airway in vivo. Exposure of the apical surfaces of human WD and PD cultures to AdVlacZ resulted in significantly less β-gal expression in WD cultures than in PD cultures (Fig. 1B). These results demonstrate that human WD and PD airway epithelial cells are resistant and susceptible, respectively, to AdV-mediated gene transfer, recapitulating the phenomenon observed in vivo for human and rodent cartilaginous airway epithelia (18).
FIG. 1.
Interaction of AdV with human airway epithelial cell cultures. (A) Representative histological cross-sections of human airway cells after 5 days in culture showing PD epithelial cells and after >25 days in culture showing WD pseudostratified ciliated epithelial cells. Hematoxylin and eosin counterstain; magnification, ×215. (B to D) Comparative analyses of lacZ gene expression in PD and WD cultures 48 h after exposure to AdVlacZ (6 h at 37°C) (B), internalization of AdV into PD and WD cultures after exposure to 35S-AdVlacZ (6 h at 37°C) (C), and attachment of AdV to PD and WD cultures after exposure to 35S-AdVlacZ (6 h at 4°C) (D). Only the apical surfaces of cultures were exposed to AdV (1010 particles/ml). β-Gal activity and counts per minute (CPM) were measured per square centimeter of epithelial surface area. Values shown are mean ± standard error (SE) (n = 3). The results shown are representative of a total of three different experiments.
We have previously shown that the reduced efficiency of gene transfer to WD cells compared to PD cells is not due to a specific interaction of the transgene promoter with these cell types or to the proliferative status of the cells at the time of transduction (25). In the present study, we tested the hypothesis that early steps in the vector-cell interaction determine the gene transfer efficiency. Using radiolabeled AdV, we measured the penetration of AdV into cells and determined which step(s) is rate limiting for efficient AdV-mediated gene transfer to human airway epithelial cells.
To determine if WD and PD cultures internalized different amounts of AdV, both culture types were exposed to 35S-AdV and the quantity of internalized AdV was determined by measuring the cell-associated counts that were resistant to removal from the external cell surface by acid and protease treatment. Figure 1C shows that the internalization of AdV into WD cultures was markedly reduced compared to the amount internalized into PD cultures. To investigate whether the differences in entry reflect the degree of attachment of AdV, the apical surfaces of human WD and PD cultures were exposed to 35S-AdV at 4°C to measure cellular attachment of vector in the absence of internalization. These studies showed that the surface of WD cultures bound markedly less AdV than the PD cultures (Fig. 1D). Collectively, these results suggest that the reduction in gene transfer to WD cultures results from a reduced internalization of AdV into this cell type, which may be related to the absolute amount of AdV attached to the luminal surface of the cultures.
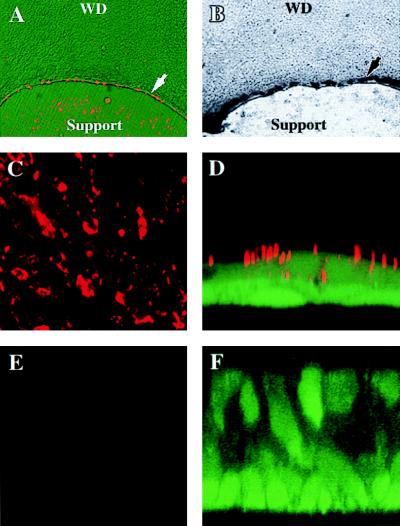
We confirmed this conclusion with a second human culture system, containing cellular islands that exhibit a WD phenotype in the center and a PD phenotype at the edges (24). These cultures were exposed to fluorescence-labeled AdV (CyAdV) at 37°C and viewed by fluorescence microscopy after being washed. At 6 h after exposure, CyAdV was routinely associated with PD cells at the periphery and only rarely associated with individual cells within the WD regions (Fig. 2A). This cellular distribution of CyAdV localization is paralleled by the cellular distribution of lacZ expression in the same cultures 24 h later, assayed by 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) histochemistry (Fig. 2B). Therefore, by direct visualization of vector, AdV entry into PD cells far exceeds that into WD cells.
FIG. 2.
Direct visualization of specific and nonspecific cellular uptake pathways with human cultures expressing both PD and WD cellular phenotypes. (A) Exposure of cellular islands to CyAdV (1010 particles/ml for 6 h at 37°C) (red) resulted in association of CyAdV with PD cells at the periphery of the islands (arrow) and only rarely with individual cells within the WD regions. (B) The cellular distribution of CyAdV is paralleled by the cellular distribution of lacZ expression (arrow) in the same cultures 24 h later. (C and E) To assess whether uptake into PD cultures was restricted to specific AdV uptake, the apical surfaces of confluent cultures were exposed to fluorescent microspheres (∼1010 spheres/ml for 6 h at 37°C), resulting in a large quantity of microspheres (red) associated with PD (C) but not WD (E) cultures viewed en face. (D and F) To determine the cellular localization of microspheres, confocal microscopy-generated XZ sections revealed that microspheres (red) were both attached to and internalized into PD cultures (D) but not WD cultures (F). For panels D and F, the cells were counterstained with calcein. Magnifications, ×48 (A and B), ×24 (C and E), and ×240 (D and F).
To investigate whether the differences in attachment and internalization with WD and PD culture types are specific to AdV, the uptake of fluorescent microspheres that approximate the size of the vector (100 nm) into PD and WD cultures was examined. Incubation of cultures with microspheres for 6 h at 37°C resulted in a large quantity of microspheres associated with PD but not WD cultures, as shown by conventional fluorescence microscopy (Fig. 2C and E, respectively). To determine the cellular localization of the microspheres, confocal microscopy-generated XZ sections revealed that they were both attached and internalized only into PD cultures (Fig. 2D). These data suggest that PD cultures can internalize nonspecifically attached particles and that the apical membrane of PD cultures can undergo nonspecific uptake processes. In contrast, microspheres did not enter WD cultures (Fig. 2F), due to reduced nonspecific attachment and possibly to reduced uptake into the cells.
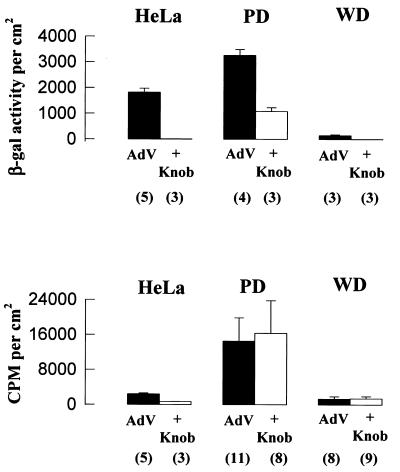
To determine whether AdV attachment to PD and WD cultures is a specific fiber-knob-mediated interaction, transgene expression and AdV attachment were measured in the presence of excess purified fiber-knob protein. Experiments with HeLa cells showed that purified fiber-knob protein was capable of inhibiting transgene expression by more than 99% and AdV attachment by ∼75% (Fig. 3), indicating that specific fiber-knob binding to HeLa cells accounted for the subsequent transgene expression. Excess fiber-knob protein inhibited ∼70% of transgene expression in PD cultures and completely inhibited the low level of expression in WD cultures, suggesting that the predominant route of AdV entry into both culture types is via fiber-knob receptor-mediated endocytosis. In the PD but not the WD cultures, however, a significant portion of transgene expression was not blocked by excess fiber-knob protein. This non-fiber-knob-protein-mediated expression may be due to nonspecific uptake processes, as described above (Fig. 2). In contrast to transgene expression, AdV attachment to both PD and WD cultures was not inhibited by fiber-knob protein, suggesting that we cannot detect fiber receptor-specific attachment given the large component of “nonspecific” attachment in both culture types.
FIG. 3.
Specificity of AdV interactions with human PD and WD airway cultures. Inhibition of AdV-mediated gene transfer (top) and AdV attachment (bottom) to cells preincubated with purified fiber-knob protein (10 μg/ml) (+knob). Fiber-knob protein produced only a partial inhibition of AdV-mediated gene transfer to PD cultures but inhibited the small amount of gene transfer to WD cultures. In contrast to HeLa cells, fiber-knob protein did not inhibit AdV attachment to either PD or WD cultures. AdV represents cultures exposed to AdV but not preincubated with fiber-knob protein. Values shown represent mean ± SE of n determinations, where n is shown in parentheses.
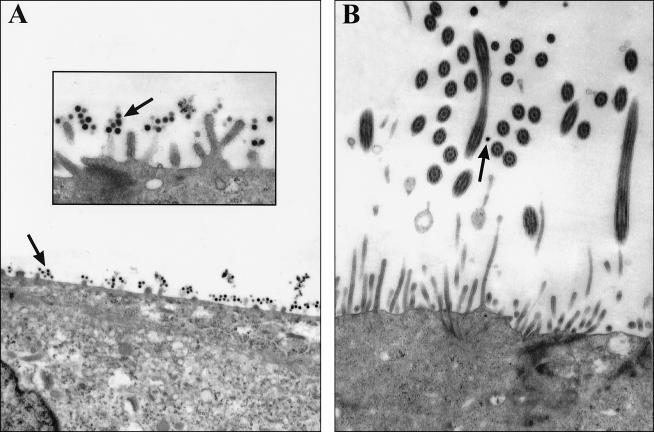
To investigate the mechanism of nonspecific attachment of AdV, we visually assessed the binding of AdV to PD and WD cultures by transmission electron microscopy (TEM). The majority of AdV attached to the human PD culture surface was associated with the abundant glycocalyx present on the microvilli (Fig. 4A), suggesting that the glycocalyx itself was binding AdV by a fiber-knob-independent mechanism. Assessment of the binding of AdV to human WD cultures by TEM (Fig. 4B) showed reduced levels of glycocalyx on the WD cultures and little AdV associated with the cell surface. Therefore, we speculate that the great nonspecific attachment of AdV to PD cultures reflects the relative mass of glycocalyx on these cells.
FIG. 4.
Investigation of nonspecific attachment of AdV to human PD and WD airway cells. The apical surfaces of human cultures were exposed to AdV (1010 particles/ml for 6 h at 4°C), and tissues were processed for TEM to assess AdV attachment. (A) With human PD cultures, AdV (arrows) was associated with cellular glycocalyx-like structures on the apical membrane (inset). (B) With WD cultures, in agreement with the attachment studies, little AdV was associated with the apical surface. Magnifications, ×7,000 (A and B) and 20,000 (inset).
The recent identification of hCAR as a putative specific AdV attachment receptor (1, 29) prompted investigation of the localization of this receptor in human airway epithelial cultures. Retrovirus-mediated overexpression of hCAR cDNA in CHO cells (normally resistant to AdV-mediated gene transfer) leads to increased specific AdV attachment and increased specific AdV-mediated gene transfer to levels above that observed for HeLa cells (Fig. 5A). Immunofluorescence detection of hCAR was performed with MAb RmcB on HeLa, CHO, and CAR-CHO cells. RmcB detected hCAR on HeLa and CAR-CHO cells but not on control CHO cells (Fig. 5B, panels I, III, and II, respectively). In addition, coincubation of the cells with RmcB and CyAdV showed a similar distribution for the receptor and ligand, respectively, indicating that increased hCAR expression led to increased AdV attachment. These results show that hCAR mediates AdV attachment and AdV-mediated gene transfer in these cell lines and that RmcB can detect the presence of hCAR.
FIG. 5.
Expression of hCAR mediates AdV attachment and gene expression. (A) Specific AdV attachment (i) and gene expression (ii) were measured for the HeLa, CHO, and CAR-CHO cell lines by incubating cells in the absence (solid bars) or presence (open bars) of purified fiber-knob protein (10 μg/ml) for 1 h at 4°C before adding 35S-AdV (1010 particles/ml for 2 h at 4°C). Values shown represent the mean ± SE (n = 6 and 3 for solid and open bars, respectively). (B) Representative immunofluorescent detection of hCAR with HeLa (i), CHO (ii), and CAR-CHO (iii) cell lines exposed at 4°C to anti-hCAR MAb with fluorescein isothiocyanate-conjugated goat anti-mouse IgG (green) and CyAdV (red). Cell nuclei are counterstained with DAPI (blue). Localization of hCAR and CyAdV was restricted to HeLa and CAR-CHO cells; there was no localization of hCAR and little CyAdV associated with CHO cells. Magnification, ×250.
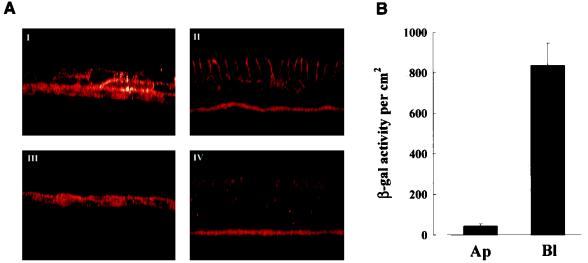
To determine whether hCAR is expressed in human airway epithelia, human PD and WD cultures were permeabilized and probed for hCAR expression with RmcB. In permeabilized human PD cultures, hCAR immunoreactivity was detected on the surfaces of all of the epithelial cells (Fig. 6A, panel I) whereas in permeabilized WD cultures hCAR was restricted to the basolateral membranes of the columnar cells (panel II). Control cultures probed with an irrelevant MAb showed little immunoreactivity above autofluorescence (panels III and IV). These results parallel the earlier findings of the AdV attachment and expression profiles for these culture types.
FIG. 6.
Distribution of endogenous hCAR in human PD and WD cultures. (A) Human PD (i and iii) and WD (ii and iv) cultures after permeabilization were exposed to either RmcB MAb (i and ii) or an irrelevant isotyped MAb (iii and iv), and binding was detected with goat anti-mouse IgG-Texas Red. For PD cultures, hCAR was detected on all surfaces of epithelial cells, whereas WD cultures show a basolateral distribution of hCAR. Magnification, ×100. (B) Exposure of AdV to apical versus basolateral surfaces of human WD cultures. AdV (1010 particles/ml) was applied to the respective surfaces for 6 h at 37°C, and gene expression was measured 48 h later. The values shown represent mean ± SE (n = 3).
The basolateral distribution of both hCAR and αvβ3/5 integrins (see below and reference 14) in WD cells suggests that these cultures may be more susceptible to AdV gene transfer if access to the basolateral membrane was feasible. To test this notion, AdV was applied to either the basolateral or apical surface of human WD cultures. These studies showed that the gene transfer efficiency is enhanced when the basolateral surface rather than the apical surface is exposed to AdV (Fig. 6B).
(ii) Rat cultures.
Preliminary experiments suggested that PD airway epithelial cells derived from the rat tracheal epithelium exhibited less nonspecific AdV attachment than human PD cells (26). We therefore compared this well-characterized system (Fig. 7A) with our human studies. In agreement with the human model and in vivo studies, WD cultures were resistant and PD cultures were susceptible to AdV-mediated gene transfer (Fig. 7B). In contrast to human airway epithelia, the attachment of AdV to PD and WD cultures was similar (Fig. 7D). Because of the lower total attachment, specific fiber-knob inhibitable attachment could be detected and accounted for approximately 50% of the total attachment (rat PD, 648 ± 125 and 320 ± 73 cpm per cm2 in the absence and presence, respectively, of fiber-knob protein [n = 9 for each]; rat WD, 449 ± 49 and 224 ± 60 cpm per cm2 in the absence and presence, respectively, of fiber-knob protein [n = 9 for each]). Thus, the rat model allows a comparison of AdV internalization when specific and nonspecific AdV attachment was similar on both culture types. This comparison shows that the amount of AdV internalized into WD cells is far smaller than that internalized in PD cells, which correlates with the gene transfer efficiencies (Fig. 7C). These data indicate that the rate-limiting step for efficient gene transfer to the rat WD cultures is the internalization of AdV.
FIG. 7.
Interaction of AdV with rat airway epithelial cell cultures. (A) Representative histological cross-sections of rat airway cells after 5 days in culture showing PD epithelial cells and after >19 days in culture showing WD pseudostratified mucociliary epithelial cells. Hematoxylin and eosin counterstain; magnification, ×220. (B to D) Comparative analyses of lacZ gene expression in PD and WD cultures 48 h after exposure to AdVlacZ (6 h at 37°C) (B), internalization of AdV into PD and WD cultures after exposure to 35S-AdVlacZ (6 h at 37°C) (C), and attachment of AdV to PD and WD cultures after exposure to 35S-AdVlacZ (6 h at 4°C) (D). Only the apical surfaces of cultures were exposed to AdV (1010 particles/ml). β-Gal activity and counts per minute (CPM) were measured per square centimeter of epithelial surface area. Values shown are mean ± SE (n > 8).
Mechanisms of internalization. (i) αvβ3/5 integrin localization and interactions with AdV.
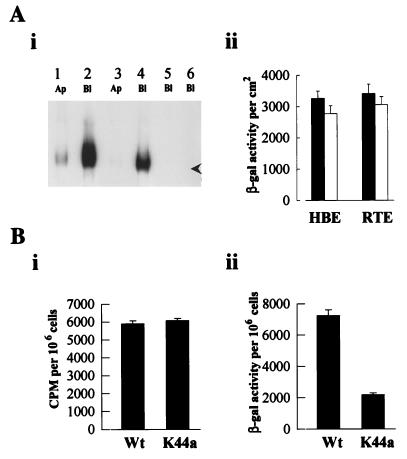
AdV interactions with fiber-knob receptors and/or αvβ3/5 integrins are thought to initiate endosomogenesis via a mechanism involving receptor clustering. To test the hypothesis that reduced gene transfer in WD cultures compared to PD cultures reflects the absence of αvβ3/5 integrin “initiation” of coated-pit endosomogenesis, we measured the distribution of αvβ3/5 integrins in the apical and basolateral regions of PD and WD cultures. For human cultures (Fig. 8A, panel i) and rat cultures (data not shown), the αvβ3/5 integrins are distributed predominantly in the basolateral membranes of both PD and WD cultures. The αvβ3/5 integrins are absent from the WD apical membranes of both species and were expressed at low levels in the apical membranes of the PD cultures, a finding in agreement with that of Goldman and Wilson (14).
FIG. 8.
αvβ3/5 integrin localization in human airway cultures and entry of AdV mediated by dynamin-associated uptake pathways. (A) Panel i shows immunoprecipitates of biotinylated human αvβ3/5 integrins with a rabbit anti-human αvβ3/5 integrin polyclonal antibody. Apical (Ap) or basolateral (Bl) membranes of either PD (lanes 1, 2, and 5) or WD (lanes 3, 4, and 6) cultures were biotinylated and, after immunoprecipitation, probed with streptavidin conjugated to horseradish peroxidase for detection. Lanes 5 and 6 show immunoprecipitation with a rabbit IgG control. The arrowhead shows approximately 120 kDa. The experiment shown is representative of four experiments. Panel ii shows lack of inhibition of gene transfer with αvβ3/5 integrin-interacting peptides. Neither cRGD peptide (0.4 mg/ml) with human cultures (HBE) nor RGD peptide (4 mg/ml) with rat cultures (RTE) significantly altered the level of transgene expression in the respective cultures after exposure to AdVlacZ (1010 particles/ml). Values shown represent mean ± SE (n = 4 and 3 for cultures in the absence [closed bars] and presence [open bars] of RGD peptides, respectively). (B) Comparison of AdV attachment (i) and AdV-mediated gene transfer (ii) to HeLa cells overexpressing either Wt or mutant (K44a) dynamin. Cells were exposed to AdVlacZ (1010 particles/ml) for 2 h at 4°C, and either attachment was measured immediately or expression was measured 24 h later. Values shown represent mean ± SE (n = 6 for each).
Whereas these data on integrin distribution correlate with the high and low levels of gene transfer to the respective culture types, attempts to demonstrate the functional importance of integrins in AdV-mediated gene transfer were unsuccessful. In our models of airway epithelial cells, neither cyclical RGD (cRGD) peptide (0.4 mg/ml) nor RGD peptide (4 mg/ml) significantly altered the level of transgene expression in human or rat PD cultures, respectively (Fig. 8A, panel ii). AdV attachment to human or rat cultures was also not altered by cRGD or RGD peptides, respectively (results not shown). These data suggest that mediation of endosomogenesis and endosomolysis leading to internalization of AdV into PD cultures may occur by mechanisms other than αvβ3/5 integrin interactions, suggesting that the absence of αvβ3/5 integrins from the apical membrane of WD cells may not entirely account for the resistance of these cells to gene transfer.
(ii) Dynamin-mutant cells deficient in receptor-mediated endocytosis.
The data from rat WD cultures emphasize the importance of entry across the apical membrane as a limiting variable for gene transfer. Whereas there are multiple modes of entry across the cellular membrane (see below), morphological studies have identified coated-pit vesicles as an important path for AdV entry (11, 30). We initiated studies to functionally test the importance of this path for AdV-mediated gene transfer with HeLa cells and HeLa cell mutants that have reduced coated-pit-mediated endocytosis. Dynamin is responsible for “pinching off” endocytotic invaginations formed during receptor-mediated endocytosis (7). While overexpression of wild-type (Wt) dynamin in HeLa cells does not affect endocytotic processes (7), overexpression of the K44a dynamin mutant selectively and reproducibly reduces receptor-mediated endocytosis. Overexpression of either Wt or K44a dynamin did not affect AdV attachment to the cells (Fig. 8B, panel i), but β-gal expression was significantly reduced in K44a dynamin-expressing cells compared to Wt dynamin-expressing cells (panel ii). These findings show that the predominant route of entry of AdV into HeLa cells occurs via receptor-mediated endocytosis and that cells expressing identical amounts of AdV attachment/internalization receptors can display a reduced gene transfer efficiency reflecting a reduced plasma membrane uptake pathway process.
Relationship between attachment and expression in PD and WD cultures.
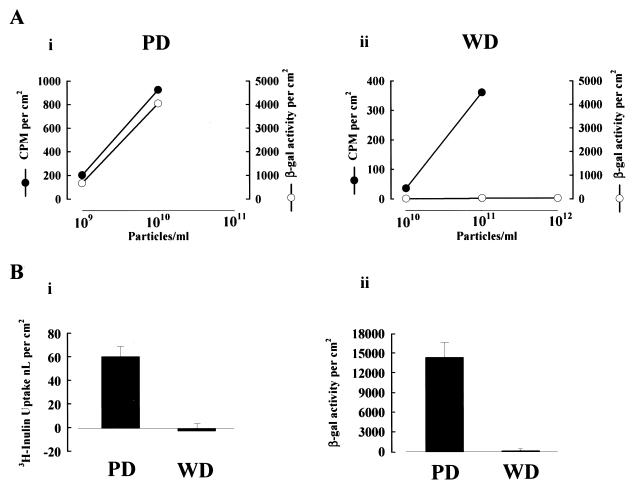
Increased efficiency of AdV-mediated gene transfer to cells in vitro has been achieved by the manipulation of the AdV capsid coat to increase cellular attachment (10, 33). To determine if increased attachment of AdV to human PD and WD cultures leads to enhanced gene transfer efficiency, increasing concentrations of AdV were applied to both culture types. As shown in Fig. 9A, panel i, exposure of PD cultures to a 10-fold increase in AdV concentration resulted in significant enhancement of both the amount of AdV attached to the cultures and transgene expression. These data suggest that in this culture type, similar in morphology to many cell types grown in vitro, the absolute amount of AdV attachment directly correlates with the subsequent level of gene transfer. For WD cultures (panel ii) exposed to AdV concentrations similar to or up to 1,000-fold higher than those to which PD cultures were exposed, the level of gene transfer was not enhanced even though the absolute amount of AdV attachment was increased to levels associated with significant gene transfer in the PD cultures. These data clearly show that increased AdV attachment to WD cultures failed to overcome the inefficient gene transfer.
FIG. 9.
Increased AdV attachment leads to increased gene transfer in cells with active nonspecific uptake pathways. (A) Effect on AdV attachment and gene transfer in human PD (i) and WD (ii) cultures with exposure to different numbers of AdV particles. Values shown represent mean ± SE (n > 3). (B) Panel i shows measurement of nonspecific fluid-phase uptake pathways in human PD and WD cells with [3H]inulin exposed to the luminal surface of the cultures at 37°C for 6 h. Values shown represent mean ± SE (n > 5). Panel ii shows gene expression in parallel cultures exposed to AdVlacZ (1010 particles/ml) for 6 h at 37°C, with enzyme activity measured 24 h later. Values shown represent mean ± SE (n > 8).
The direct relationship between the quantity of attached vector and expression in PD cells suggests that once attachment has occurred, entry can be achieved by constitutive nonspecific pathways, e.g., pinocytosis and phagocytosis. In contrast, although vector can nonspecifically attach to WD cells, the absence of expression suggests that few or no constitutive or nonspecific entry pathways exist in the apical surface of this cell type. To test the capacity for constitutive (unstimulated) uptake of luminal solutions by airway epithelia in different states of differentiation, we measured the uptake of a fluid-phase marker and correlated this parameter with gene transfer efficiency. Human cells grown on tissue culture plastic (i.e., PD cells) internalized measurable amounts of [3H]inulin, whereas WD cells did not (Fig. 9B, panel i). These differences were paralleled by the differences in gene transfer (panel ii). These findings suggest that a relative reduction in the constitutive activity of the aggregate apical membrane entry pathways in WD cells contributes to the resistance of these cells to gene transfer.
DISCUSSION
The WD ciliated airway epithelium is the target tissue for AdV-mediated gene transfer approaches for the treatment of the pulmonary manifestations of CF. However, AdV are inefficient gene transfer vectors for the respiratory epithelium in vivo (18, 25, 35, 36) and high AdV doses delivered to the lung are inflammatory (5, 6, 27), limiting the usefulness of the currently available vectors. Therefore, efforts are being directed at increasing the efficiency of AdV-mediated gene transfer in the hope that lower, less inflammatory doses can be administered to provide effective gene transfer to the airway epithelium.
A strategy to identify the mechanisms that account for the low in vivo gene transfer efficiency of AdV has emanated from studies that demonstrate that WD epithelial cells of human and rodent airways are resistant to AdV gene transfer whereas injured or PD airway epithelial cells are efficiently transduced (8, 25, 36). Since quantitative studies of the interactions of AdV with the airway epithelium in vivo are difficult and prone to considerable variation, we have used cell culture models that reproduce (i) the WD (ciliated) and PD cellular phenotypes and (ii) the relative resistance of WD cells and permissiveness of PD cells to AdV-mediated gene transfer as observed in vivo (Fig. 1 and 7).
Our analyses of AdV interactions with human airway epithelial cells show that decreased gene transfer efficiency in WD cultures compared to PD cultures is due to limited entry of AdV across the apical membrane of WD cultures, which may reflect as many as three independent steps: (i) reduced specific AdV attachment to the apical surface of WD cells; (ii) the absence of αvβ3/5 integrins at the apical surface of WD cells; and (iii) a reduced rate of AdV internalization across the apical membrane of WD cells.
Using radiolabeled virus, we demonstrate that the specific attachment of AdV to human WD cultures is reduced compared to attachment to PD cultures (Fig. 1, 3, and 4) and that differences in specific attachment mirror differences in AdV-mediated gene transfer efficiency (Fig. 1 and 3). In support of these functional attachment data, immunofluorescence studies with the recently identified specific AdV fiber-knob attachment receptor (hCAR [1, 29]) demonstrated that hCAR is not expressed at the luminal surface of human WD cultures but is expressed on all surfaces of PD cells. The distribution of hCAR and the attachment data correlate with the relative degree of AdV-mediated transgene expression in the respective cultures (Fig. 6). These findings are consistent with those from a study recently reported by Zabner et al. (35) with a similar model of human WD airway epithelial cells, which concluded that a determining factor for inefficient gene transfer is the lack of high-affinity fiber receptors on the apical surface of WD cells.
While the results of the present study are consistent with those of Zabner et al. (35), additional steps may also determine the efficiency of AdV-mediated gene transfer. It has been reported that fiber-knob receptors and/or αvβ3/5 integrins mediate endosomogenesis by a mechanism involving receptor-mediated clustering of clathrin-rich membrane regions (11, 30). The apical membrane of WD epithelia may have a generally low capacity for performing endosomogenesis because of a low expression of fiber-knob/αvβ3/5 integrin receptors (Fig. 6 and 8A) (14, 35). However, the αvβ3/5 integrins do not appear to be necessary for efficient gene transfer to PD cultures since RGD peptides failed to reduce gene expression in these culture types (Fig. 8A), a finding that is supported by studies with RGD-mutated AdV (12) and efficient gene transfer to airway epithelial cells derived from a β5 integrin knockout mouse model (17). These observations cast doubt on the absolute requirement of these integrins for gene transfer in WD cells.
With respect to the importance of apical membrane internalization capacity, we observed that internalization of AdV into human WD cultures is lower than into human PD cultures but that differences in attachment efficiency precluded an analysis of the direct effects of entry rates on this parameter. However, an indication that absolute rates of entry (endocytosis) are important for gene transfer efficiency came from studies with rat airway cultures. In contrast to human cultures, AdV attachment is similar in rat PD and WD cells and does not reflect the differences in gene transfer efficiency (Fig. 7). However, the quantity of AdV internalized into WD rat cultures is greatly reduced compared to the quantity internalized into the PD cultures. The rates of internalization of AdV directly correlate with the relative gene transfer efficiencies observed with the two cellular phenotypes (Fig. 7), indicating that the internalization process for the entry of AdV into WD cells is rate limiting.
PD airway epithelial cells are susceptible to AdV-mediated gene transfer via specific hCAR-mediated pathways. However, PD cells also appear transducible via nonspecific attachment and nonspecific uptake processes. Both human and rat PD cultures, but not WD cells, appear capable of internalizing nonspecifically attached AdV via nonspecific mechanisms such as pinocytosis (Fig. 9B). This observation is important to the design of targeted vectors that attempt to increase gene transfer efficiency based on the assumption that attachment alone is the rate-limiting step to efficient gene transfer (10, 33). Retargeted vectors attached via nonspecific interactions or to noninternalizing receptors will probably depend on nonspecific uptake pathways to enter cells; while this approach is useful for PD cells in vitro, increasing attachment to WD cultures which do not exhibit these cellular entry pathways does not increase gene transfer efficiency (Fig. 9A). In addition, nonspecific entry pathways that do not use dynamin-associated coated pits may not efficiently allow the productive entry of AdV into cells (Fig. 8B).
In summary, human WD cultures are resistant to AdV-mediated gene transfer because of decreased specific attachment sites and reduced nonspecific entry paths that internalize a fraction of a large vector load typical of Ad CF gene therapy protocols. To circumvent the inefficiency of AdV-mediated gene transfer to the respiratory epithelium, AdV will require retargeting to receptor types that both undergo endocytosis via coated-pit mechanisms and are present in sufficient numbers on the airway epithelial luminal surface.
ACKNOWLEDGMENTS
We gratefully thank R. J. Samulski for helpful discussions during the preparation of the manuscript and Steven Albelda, Jeffrey Bergelson, Robert Gerard, John Olsen, Sandra Schmid, and James Yankaskas for generous gifts of reagents and human tissue.
This work was supported by NIH grant SCOR CF HL42384 and Cystic Fibrosis Foundation (CFF) grant S880. R.J.P. was in receipt of a CFF Research Fellowship.
REFERENCES
- 1.Bergelson J M, Cunningham J A, Droguett G, Kurt-Jones E A, Krivithas A, Hong J S, Horowitz M S, Crowell R L, Finberg R W. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- 2.Boucher R C. Current status of CF gene therapy. Trends Genet. 1996;12:81–84. doi: 10.1016/0168-9525(96)81410-7. [DOI] [PubMed] [Google Scholar]
- 3.Brighton C T, Albelda S M. Identification of cell substratum adhesion receptors of integrins on cultured rat bone cells. J Orthop Res. 1992;10:766–773. doi: 10.1002/jor.1100100604. [DOI] [PubMed] [Google Scholar]
- 4.Comstock K E, Watson N F, Olsen J C. Design of retroviral expression vectors. In: Tuan R, editor. Recombinant gene expression protocols. Totowa, N.J: Humana Press, Inc.; 1996. p. 207. [DOI] [PubMed] [Google Scholar]
- 5.Crystal R G, Jaffe A, Brody S, Mastrangeli A, McElvaney N G, Rosenfeld M E, Chu C, Danel C, Hay J, Eissa T. A phase I study, in cystic fibrosis patients, of the safety, toxicity, and biological efficacy of a single administration of a replication-deficient, recombinant adenovirus carrying the cDNA of the normal cystic fibrosis transmembrane conductance regulator gene in the lung. Hum Gene Ther. 1995;6:643–666. doi: 10.1089/hum.1995.6.5-643. [DOI] [PubMed] [Google Scholar]
- 6.Crystal R G, McElvaney N G, Rosenfeld M A, Chu C, Mastrangeli A, Hay J G, Brody S L, Jaffe H A, Eissa N T, Danel C. Administration of an adenovirus containing the human CFTR cDNA to the respiratory tract of individuals with cystic fibrosis. Nat Genet. 1994;8:42–51. doi: 10.1038/ng0994-42. [DOI] [PubMed] [Google Scholar]
- 7.Damke H, Baba T, Warnock D E, Schmid S L. Induction of mutant dynamin specifically blocks endocytic coated vesicle formation. J Cell Biol. 1994;127:915–934. doi: 10.1083/jcb.127.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dupuit F, Zahm J, Pierrot D, Brezillion S, Bonnet N, Imler J, Pavirani A, Puchelle E. Regenerating cells in the human airway surface epithelium represent preferential targets for recombinant adenovirus. Hum Gene Ther. 1995;6:1185–1193. doi: 10.1089/hum.1995.6.9-1185. [DOI] [PubMed] [Google Scholar]
- 9.Engelhardt J F, Yang Y, Stratford-Perricaudet L D, Allen E D, Kozarsky K, Perricaudet M, Yankaskas J R, Wilson J M. Direct gene transfer of human CFTR into human bronchial epithelia of xenografts with E1-deleted adenoviruses. Nat Genet. 1993;4:27–34. doi: 10.1038/ng0593-27. [DOI] [PubMed] [Google Scholar]
- 10.Fasbender A, Zabner J, Chillon M, Moninger T O, Puga A P, Davidson B L, Welsh M J. Complexes of adenovirus with polycationic polymers and cationic lipids increase the efficiency of gene transfer in vitro and in vivo. J Biol Chem. 1997;272:6479–6489. doi: 10.1074/jbc.272.10.6479. [DOI] [PubMed] [Google Scholar]
- 11.Fitzgerald D J P, Padamanabhan R, Pastan I, Willingham M C. Adenovirus-induced release of epidermal growth factor and Pseudomonas toxin into the cytosol of KB cells during receptor-mediated endocytosis. Cell. 1983;32:607–617. doi: 10.1016/0092-8674(83)90480-4. [DOI] [PubMed] [Google Scholar]
- 12.Freimuth P. A human cell line selected for resistance to adenovirus infection has reduced levels of the virus receptor. J Virol. 1996;70:4081–4085. doi: 10.1128/jvi.70.6.4081-4085.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldman M J, Su Q, Wilson J M. Gradient of RGD-dependent entry of adenoviral vector in nasal and intrapulmonary epithelia: implications for gene therapy of cystic fibrosis. Gene Ther. 1996;3:811–818. [PubMed] [Google Scholar]
- 14.Goldman M J, Wilson J M. Expression of avb5 integrin is necessary for efficient adenovirus-mediated gene transfer in the human airway. J Virol. 1995;69:5951–5958. doi: 10.1128/jvi.69.10.5951-5958.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gray T E, Guzman K, Davis C W, Abdullah L H, Nettesheim P. Mucociliary differentiation of serially passaged normal human tracheobronchial epithelial cells. Am J Respir Cell Mol Biol. 1996;14:104–112. doi: 10.1165/ajrcmb.14.1.8534481. [DOI] [PubMed] [Google Scholar]
- 16.Greber U F, Willets M, Webster P, Helenius A. Stepwise dismantling of adenovirus type 2 during entry into cells. Cell. 1993;75:477–486. doi: 10.1016/0092-8674(93)90382-z. [DOI] [PubMed] [Google Scholar]
- 17.Griffiths M, Huang X, Wu J F, Driscoll R, Sheppard D. Inactivation of the b5 integrin subunit gene does not prevent expression of adenovirus genes in mouse airway epithelium. Respir Crit Care Med. 1997;155:A459. . (Abstract.) [Google Scholar]
- 18.Grubb B R, Pickles R J, Ye H, Yankaskas J R, Vick R N, Engelhardt J F, Wilson J M, Johnson L G, Boucher R C. Inefficient gene transfer by adenovirus vector to cystic fibrosis airway epithelia of mice and humans. Nature. 1994;371:802–806. doi: 10.1038/371802a0. [DOI] [PubMed] [Google Scholar]
- 19.Henry L J, Xia D, Wilke M E, Deisenhofer J, Gerard R D. Characterization of the knob domain of the adenovirus type 5 fiber protein expressed in Escherichia coli. J Virol. 1994;68:5239–5246. doi: 10.1128/jvi.68.8.5239-5246.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsu K L, Lonberg-Holm K, Alstein B, Crowell R L. A monoclonal antibody specific for the cellular receptor for the group B coxsackieviruses. J Virol. 1988;62:1647–1652. doi: 10.1128/jvi.62.5.1647-1652.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaartinen L, Nettesheim P, Adler K B, Randell S H. Rat tracheal epithelial cell differentiation in vitro. In Vitro Cell Dev Biol. 1993;29:481–492. doi: 10.1007/BF02639383. [DOI] [PubMed] [Google Scholar]
- 22.Lechner J F, LaVeck M A. A serum-free method for culturing normal human bronchial epithelial cells at clonal density. J Tissue Cult Methods. 1985;9:43–48. [Google Scholar]
- 23.Leopold P L, Ferris B, Grinberg I, Worgall S, Hackett N R, Crystal R G. Fluorescent virions: dynamic tracking of the pathway of adenoviral gene transfer vectors in living cells. Hum Gene Ther. 1998;9:367–378. doi: 10.1089/hum.1998.9.3-367. [DOI] [PubMed] [Google Scholar]
- 24.Matsui H, Johnson L G, Randell S H, Boucher R C. Loss of binding and entry of liposomal-DNA complexes decreases transfection efficiency in differentiated airway epithelial cells. J Biol Chem. 1997;272:1117–1126. doi: 10.1074/jbc.272.2.1117. [DOI] [PubMed] [Google Scholar]
- 24a.Olsen, J. Personal communication.
- 25.Pickles R J, Barker P M, Ye H, Boucher R C. Efficient adenovirus-mediated gene transfer to basal but not columnar cells of cartilaginous airway epithelia. Hum Gene Ther. 1996;7:921–931. doi: 10.1089/hum.1996.7.8-921. [DOI] [PubMed] [Google Scholar]
- 26.Pickles R J, McCarty D, Randell S H, Boucher R C. Internalisation, not attachment is the rate limiting step responsible for the inefficiency of adenoviral-mediated gene transfer to well differentiated respiratory epithelium. Pediatr Pulmonol. 1997;14:A193. . (Abstract.) [Google Scholar]
- 27.Piedimonte G, Pickles R J, Lehmann J R, McCarty D, Costa D L, Boucher R C. Replication-deficient adenoviral vector for gene transfer potentiates airway neurogenic inflammation. Am J Respir Cell Mol Biol. 1997;16:250–258. doi: 10.1165/ajrcmb.16.3.9070609. [DOI] [PubMed] [Google Scholar]
- 28.Robinson G, Gray T. Electron microscopy 2: practical procedures. In: Bancroft J D, Stevens A, editors. Theory and practice of histological techniques. New York, N.Y: Churchill Livingstone, Inc.; 1996. pp. 585–627. [Google Scholar]
- 29.Tomko R P, Xu R, Philipson L. HCAR and MCAR: the human and mouse cellular receptors for subgroup C adenoviruses and group B coxsackieviruses. Proc Natl Acad Sci USA. 1997;94:3352–3356. doi: 10.1073/pnas.94.7.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Varga M J, Weibull C, Everitt E. Infectious entry pathway of adenovirus type 2. J Virol. 1991;65:6061–6070. doi: 10.1128/jvi.65.11.6061-6070.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.von Bonsdorff C, Fuller S D, Simons K. Apical and basolateral endocytosis in Madin-Darby canine kidney (MDCK) cells grown on nitrocellulose filters. EMBO J. 1985;4:2781–2792. doi: 10.1002/j.1460-2075.1985.tb04004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wickham T J, Mathias P, Cheresh D A, Nemerow G R. Integrins avb3 and avb5 promote adenovirus internalisation but not virus attachment. Cell. 1993;73:309–319. doi: 10.1016/0092-8674(93)90231-e. [DOI] [PubMed] [Google Scholar]
- 33.Wickham T J, Roelvink P W, Brough D E, Kovesdi I. Adenovirus targeted to heparan-containing receptors increases its gene delivery efficiency to multiple cell-types. Nat Biotechnol. 1996;14:1570–1573. doi: 10.1038/nbt1196-1570. [DOI] [PubMed] [Google Scholar]
- 34.Wu R, Yankaskas J, Cheng E, Knowles M R, Boucher R C. Growth and differentiation of human nasal epithelial cells in culture. Serum-free, hormone-supplemented medium and proteoglycan synthesis. Am Rev Respir Dis. 1985;132:311–320. doi: 10.1164/arrd.1985.132.2.311. [DOI] [PubMed] [Google Scholar]
- 35.Zabner J, Freimuth P, Puga A, Fabrega A, Welsh M J. Lack of high affinity fiber receptor activity explains the resistance of ciliated airway epithelia to adenovirus infection. J Clin Invest. 1997;100:1144–1149. doi: 10.1172/JCI119625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zabner J, Zeiher B G, Friedman E, Welsh M J. Adenovirus-mediated gene transfer to ciliated airway epithelia requires prolonged incubation times. J Virol. 1996;70:6994–7003. doi: 10.1128/jvi.70.10.6994-7003.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]