Abstract
In order to identify potential sites of hepadnavirus X protein action, we have investigated the subcellular distribution and the stability of woodchuck hepatitis virus (WHV) X protein (WHx) in primary hepatocytes isolated from woodchucks with persistent WHV infection. In vivo cell labeling and cell fractionation studies showed that the majority of WHx is a soluble cytoplasmic protein while a minor part of newly synthesized WHx is associated with a nuclear framework fraction (20%) and with cytoskeletal components (5 to 10%). Pulse-chase experiments revealed that cytoplasmic WHx has a short half-life and decays with bimodal kinetics (approximately 20 min and 3 h). The rates of association and turnover of nucleus-associated WHx suggest that compartmentalization may be responsible for the bimodal turnover observed in the cytoplasm.
Human hepatitis B virus is the prototype member of the hepadnavirus family, which includes viruses that infect woodchucks, ground squirrels, Pekin ducks, and other avian species (14). All mammalian hepadnaviruses can cause both acute and persistent infection, and persistent infection is a recognized risk factor in the development of primary hepatocellular carcinoma (4, 29, 33, 46).
The genetic organization of all the members of the hepadnavirus family is highly conserved. Each of the viruses has a small (approximately 3-kb) circular DNA genome which encodes the envelope, the nucleocapsid, and the polymerase genes in a very compact, overlapping arrangement which utilizes all three reading frames of the DNA (14). However, the mammalian hepadnaviruses have a fourth major open reading frame, which was originally designated the X gene because its function was unknown (48). The fact that the three most pathogenic mammalian hepadnaviruses, human hepatitis B virus, woodchuck hepatitis virus (WHV), and ground squirrel hepatitis virus, contained the X open reading frame and that the less pathogenic avian viruses did not led to the speculation that the X gene might contribute to the pathogenicity of the mammalian viruses. In an effort to elucidate the role of the X gene in viral infection and hepatocarcinogenesis, DNA transfection experiments have demonstrated that overexpression of the human hepatitis B virus X protein (HBx) alters the activity of various endogenous transcription factors and causes transactivation of a wide range of viral elements and cellular promoters (34, 48). The evidence that HBx-responsive enhancers and promoters do not share any DNA sequence and that HBx does not bind double-stranded DNA suggested that HBx might exert its transactivating activity indirectly, through protein-protein interactions. In favor of this idea, several transfection studies have shown that HBx can affect cytoplasmic signal transduction pathways by activating both the Ras–Raf–mitogen-activated protein kinase cascade (3, 9, 10, 12, 27) and the protein kinase C pathway (21). Moreover, in vitro binding studies have shown that HBx has the capability to bind both nuclear (8, 26, 30) and cytoplasmic (19, 45) proteins.
In vivo studies using the woodchuck system as an animal model of hepadnavirus infection now have demonstrated that WHV X protein (WHx) is expressed in liver during infection (11, 20) and that a functional X gene is required for infectivity (7, 49), suggesting an essential role for WHx in viral replication. Furthermore, WHx expression during persistent infection leaves open the possibility that it could affect cellular control mechanisms associated with premalignant changes in hepatocytes. In this regard, transgenic-mouse studies of WHx and HBx also support a role for X protein as a carcinogenic cofactor (11, 22, 25, 39), and studies with immortalized cell lines suggest a possible transforming function (18, 38).
In light of the potential actions of hepadnaviral X protein in cytoplasmic and nuclear compartments of hepatocytes, we have now undertaken a study to determine the distribution and the stability of metabolically labeled WHx in the subcellular fractions of naturally infected woodchuck hepatocytes, where all of the viral protein is expressed at natural levels and the viral life cycle is complete.
Identification and stability of WHx in the cytosol of WHV-infected hepatocytes.
Our previous study utilized immunoprecipitation in combination with a Western blot-enhanced chemiluminescence (ECL) detection method to identify steady-state levels of WHx in the cytoplasmic fraction of hepatocytes isolated from woodchucks persistently infected with WHV (11). In an effort to increase the sensitivity of our detection system and to determine the turnover of WHx in naturally infected hepatocytes, cell fractionation procedures followed by immunoprecipitation of metabolically radiolabeled WHx were performed in this study. Primary hepatocytes were isolated by collagenase perfusion from adult woodchucks with persistent WHV infection and from uninfected animals (11, 17, 28). Hepatocytes (>90% viability) were seeded onto 10-cm tissue culture plates (4 × 106 cells/dish) and maintained for 2 to 3 weeks, at 37°C in a 5% CO2 atmosphere, in a previously described L-15 modified medium (1) supplemented with 5% fetal bovine serum. For metabolic radiolabeling, cells were preincubated for 30 min (37°C) in methionine- and cysteine-free RPMI 1640 (Gibco/BRL) supplemented with 5% dialyzed fetal bovine serum (Sigma), and then 300 μCi of 35S-Promix (Amersham) per ml was added. After 2 h, the labeling medium was removed and the cells were chased with nonradioactive medium for various times. After the chase, hepatocytes were washed in ice-cold phosphate-buffered saline, harvested by scraping, and collected by low-speed centrifugation (50 × g, 5 min).
To answer the question of whether WHx is a soluble cytoplasmic protein or whether it is associated with other cytoplasmic constituents, 35S-labeled hepatocytes were resuspended in 10 volumes of hypotonic TKM buffer (10 mM Tris [pH 7.5], 1 mM MgCl2, 5 mM KCl, 2 mM phenylmethylsulfonyl fluoride [PMSF], 1 mg of leupeptin per ml, 1 mg of pepstatin A per ml) and disrupted by Dounce homogenization. Hepatocyte breakage was monitored by phase-contrast microscopy. Nuclei, plasma membrane sheets, and remaining whole cells were first removed by low-speed centrifugation (1,000 × g, 10 min), and then the postnuclear supernatants were fractionated by high-speed centrifugation (100,000 × g, 2 h) into a soluble S100 fraction (cytosolic fraction) and a particulate P100 fraction (microsomal fraction). After the S100 supernatants were removed, the P100 pellets (microsomal fractions) were dissolved in detergent buffer (TKM buffer supplemented with 150 mM NaCl, 1% Nonidet P-40, and 1% sodium dodecyl sulfate [SDS]), passed through a 26-gauge needle, and then used for the immunoprecipitation with WHx-antiserum or normal rabbit serum as previously described (11). Similarly, equivalent amounts of S100 cytosolic proteins were used for the immunoprecipitation. Immunoprecipitates were finally solubilized in SDS-Laemmli sample buffer, resolved on a 13% SDS-polyacrylamide gel electrophoresis (PAGE), and visualized by autoradiography.
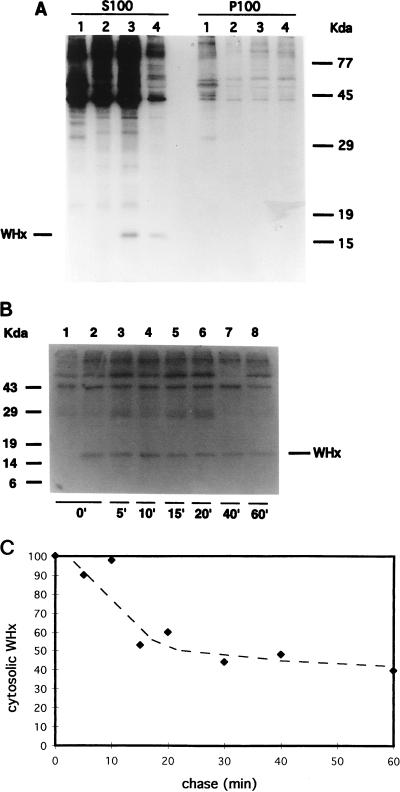
As shown in Fig. 1A, 35S-labeled WHx was specifically immunoprecipitated with the rabbit WHx antiserum from the S100 cytosolic fraction of WHV-positive hepatocytes (S100 lanes 3 and 4), while no protein of similar size (15.5 kDa) was immunoprecipitated from uninfected woodchuck hepatocytes (lane 1). Normal rabbit serum also did not immunoprecipitate a 15.5-kDa protein from the same WHV-infected cell extracts (lane 2). A quantitative evaluation of the amount of labeled WHx present in the S100 fraction after 0 and 30 min of chase (Fig. 1A, S100 lanes 3 and 4, respectively) indicated that cytosolic WHx has a short half-life in chronically infected hepatocytes, since its amount was reduced to 45% after 30 min of chase.
FIG. 1.
Localization and turnover of WHx in the cytosol of hepatocytes chronically infected with WHV (A) Woodchuck hepatocytes were metabolically radiolabeled with 35S-Promix for 2 h and then chased with cold medium. After 0 (lanes 1 to 3) or 30 (lanes 4) min of chase, cell-equivalent amounts (6 × 106 hepatocytes/assay) were fractionated into soluble (S100) and particulate (P100) cytoplasmic fractions, and fractions were immunoprecipitated with WHx antiserum (lanes 1, 3, and 4) or normal rabbit serum (lanes 2). Immunoprecipitates from uninfected (lanes 1) and from WHV-positive hepatocytes (lanes 2, 3, and 4) were then analyzed by SDS-PAGE (12%) and detected by autoradiography (2 weeks’ exposure). WHx was not detected in P100 fractions even after longer exposure of the gel (5 weeks). (B) Cells were labeled for 1 h and chased for the indicated periods (in minutes). Equivalent amounts of total soluble proteins (S100 fractions) per time point were immunoprecipitated with WHx antiserum (lanes 2 to 8) or with normal rabbit serum (lane 1) and fractionated by SDS-PAGE (13%). (C) Kinetics of turnover of cytosolic WHx. The amount of labeled WHx present in the S100 fraction at each time point was quantitated by scanning densitometry and normalized to the amount present at time zero (arbitrary scale).
35S-labeled WHx was not detected in immunoprecipitates from P100 particulate fractions (Fig. 1A), which contained fragments of plasma membrane, intracellular membranes such as the endoplasmic reticulum and the Golgi, and organelles such as lysosomes and mitochondria (32). The distribution of protein disulfide isomerase (43), a specific marker for the endoplasmic reticulum, was analyzed to confirm the purity of the cytosolic and microsomal fractions. Protein disulfide isomerase was exclusively detected by immunoblotting in the P100 microsomal fraction (data not shown). These findings indicate that WHx is not tightly associated with these subcellular compartments.
To determine the kinetics of turnover of cytosolic WHx, cells were metabolically radiolabeled for 1 h and chased up to 60 min (Fig. 1B). The S100 cytosolic fractions, containing equal amounts of total proteins, were immunoprecipitated with WHx antiserum (Fig. 1B, lanes 2 to 8). Densitometric analysis of the fluorographs of immunoprecipitated WHx revealed a bimodal half-life of cytosolic WHx in the first hour of chase. In fact, approximately 50% of labeled WHx was no longer detected at 15 to 20 min of chase. In contrast, the remaining WHx turned over more slowly, since 40% of metabolically radiolabeled WHx was still detected after 1 h of chase (Fig. 1C). Longer chase experiments, extending up to 6 h, also confirmed the presence of the more stable WHx component (see below).
Subcellular distribution of labeled WHx.
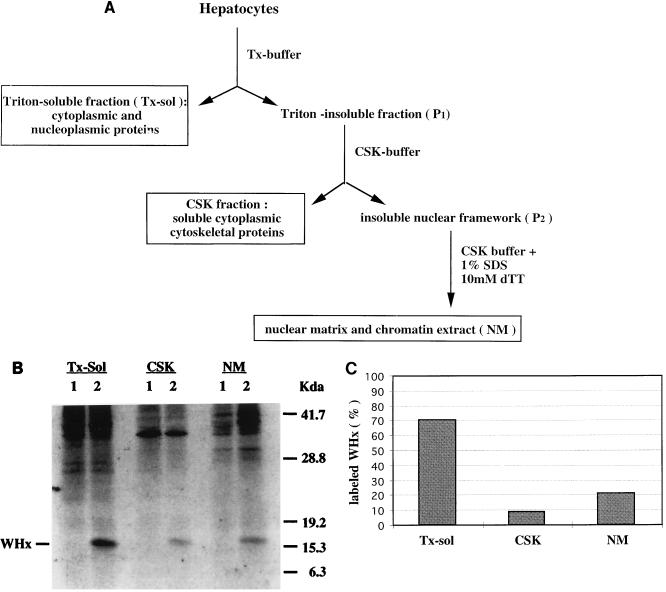
A bimodal degradation of newly synthesized proteins suggested that compartmentalization and/ or posttranslational modifications of WHx might have occurred. To investigate whether a different compartmentalization of WHx could account for the bimodal turnover observed in the cytoplasm, we next used a differential detergent extraction procedure to fractionate hepatocytes chronically infected with WHV (36, 42). Cells were labeled for 1 h with 35S-Promix (300 μCi/ml), harvested as described above, and then resuspended in TX buffer (10 mM Tris [pH 7.5], 250 mM sucrose, 100 mM NaCl, 3 mM MgCl2, 2 mM CaCl2, 0.5% Triton X-100, 2 mM PMSF, 1 mg of leupeptin per ml, 1 mg of pepstatin A per ml). After an 8-min incubation on ice, the cells were centrifuged (600 × g, 3 min) to pellet the Triton-insoluble fraction. As highlighted in Fig. 2A, this procedure first separated soluble cytoplasmic and nucleoplasmic proteins (the TX-sol fraction) from Triton-insoluble cellular components (P1). Next, the P1 pellet was resuspended in CSK buffer (10 mM Tris [pH 7.4], 10 mM NaCl, 1.5 mM MgCl2, 1% Tween 40, 0.5% Na deoxycholate, 2 mM PMSF, 1 mg of leupeptin per ml, 1 mg of pepstatin per ml) to solubilize the cytoskeletal components. The solution was passed through a 26-gauge needle 20 times, and then the CSK fraction was separated by centrifugation (1,000 × g for 5 min) from the insoluble nuclear framework fraction (P2). Finally, the proteins in the nuclear framework pellet, which is mainly composed of nuclear matrix and chromatin (5, 42), were resuspended in CSK buffer supplemented with 1% SDS and passed through a 26-gauge needle to be solubilized (NM fraction).
FIG. 2.
Subcellular distribution of labeled WHx protein in the TX-sol, CSK, and NM fractions. (A) Outline of the differential detergent extraction procedure used to investigate the subcellular distribution of WHx protein in primary hepatocytes isolated from woodchucks persistently infected with WHV. (B) Hepatocytes (6 × 106) from a WHV-positive woodchuck were metabolically radiolabeled with 35S-Promix for 1 h, harvested, and fractionated by differential detergent extraction. Fifty percent of each fraction was then immunoprecipitated with normal rabbit serum (lanes 1) or with WHx antiserum (lanes 2), and the immunoprecipitates were resolved on a SDS-PAGE (12%). (C) The amount of labeled WHx recovered from each fraction was quantitated by densitometry scanning of fluorographs. Each column represents the relative amount of labeled WHx detected in each fraction. These values have been reproduced in three independent experiments.
To determine the subcellular distribution of labeled WHx in the TX-sol, CSK, and NM fractions, we conducted immunoprecipitation-PAGE with either WHx antiserum (Fig. 2B, lanes 2) or rabbit normal serum (Fig. 2B, lanes 1). Quantitative analysis of the amount of labeled WHx recovered from each fraction revealed 70 to 75% of labeled WHx in the TX-sol fraction, 5 to 10% in the CSK fraction, and, to our surprise, approximately 20% in the NM fraction (Fig. 2C).
To check the efficiency of this cell fractionation procedure, the NM fraction was assayed for the presence of contaminant cytoplasmic proteins. Figure 3A shows that ligandin, a cytosolic protein involved in bilirubin intracellular transport (50), was completely extracted into the TX-sol fraction, as determined by SDS-PAGE and ECL-Western blotting with specific antiligandin antibodies (gift from I. Listowsky, Albert Einstein College of Medicine, Bronx, N.Y.). Furthermore, aliquots from each cell fraction were analyzed by SDS-PAGE and Coomassie blue staining. As shown in Fig. 3B, the histone proteins were retained entirely in the NM fraction. Protein concentration analyses and Coomassie blue staining (Fig. 3B) also demonstrated that the CSK and the NM fractions contained much smaller amounts of total proteins (about 15 and 10%, respectively) than the TX-sol fraction (approximately 75 to 78%). Therefore, the Triton-insoluble fractions were considerably enriched for WHx.
FIG. 3.
Distribution of cellular marker proteins in the TX-sol, CSK, and NM fractions. (A) Aliquots representing 0.5% (vol/vol) of the TX-sol and 2% (vol/vol) of the NM fractions were analyzed by SDS-PAGE (13%) and subjected to ECL-Western blot analysis with an antiligandin rabbit antiserum. (B) Total proteins from the TX-sol (0.5%), CSK (1%), and NM (1%) fractions were also subjected to SDS-PAGE (13%) and Coomassie blue staining.
Our results are consistent with those of Schek et al. (36), who previously described a similar subcellular distribution and bimodal half-life of HBx in HepG2 cells when vaccinia virus vectors were used to induce transient overexpression of HBx protein. A similar partition of WHx between soluble and cytoskeletal fractions has also been observed recently in hepatocytes isolated from acutely WHV-infected woodchucks, although nucleus-associated WHx was not detected under the conditions used in that study (20). However, another report by Doria et al. (12) provided evidence, by confocal laser microscopy, for the presence of a small fraction of HBx within the nuclei of transfected cells. It is possible that some discrepancies in terms of X-protein distribution and solubility might be due to differences in the techniques used as well as differences in the expression levels of X protein obtained after infection with recombinant systems (12, 36, 44) versus the low steady-state levels of WHx actually present in naturally infected hepatocytes.
Half-life of TX-sol WHx and kinetics of association of labeled WHx with the nuclear framework.
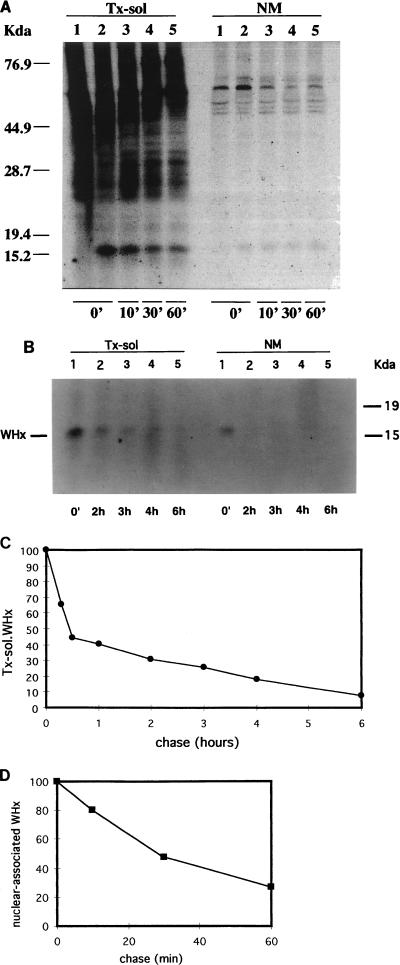
To examine the rate of association of WHx with the NM fraction and to determine the half-lives of WHx in the TX-sol and Triton-insoluble fractions, WHV-positive hepatocytes were pulse-labeled for a short time (10 min) with 600 μCi of 35S-Promix per ml at 37°C. Two 10-cm plates of confluent woodchuck hepatocytes were used per time point. After the chase, cells were washed in ice-cold phosphate-buffered saline and fractionated by using the differential detergent extraction procedure outlined in Fig. 2A. TX-sol fractions containing the same amount of total proteins per chase point were immunoprecipitated with WHx antiserum, and immunoprecipitates were analyzed by SDS-PAGE. Quantitative analyses of the autoradiographs shown in Fig. 4A (chase up to 1 h) and Fig. 4B (chase up to 6 h) demonstrated that the turnover of TX-sol WHx was bimodal. In fact, within 20 min of chase, 50% of TX-sol WHx turned over while the remaining 50% decayed with a half-life of 3 to 4 h (Fig. 4C).
FIG. 4.
Kinetics of the turnover of WHx protein in TX-sol and NM fractions. (A) WHV-infected hepatocytes were pulse-labeled with 35S-Promix for 10 min and chased with cold medium for various times as indicated (in minutes). After 0 min and each chase time, 6 × 106 cells per time point were fractionated as described in the text and in Fig. 2. Fractions were immunoprecipitated with WHx-antiserum (lanes 2 to 5) or with normal rabbit serum (lanes 1), subjected to SDS-PAGE (12%), and visualized by fluorography. (B) Metabolically radiolabeled WHV-positive hepatocytes were chased for longer periods, and immunoprecipitates from TX-sol fractions were analyzed by SDS-PAGE and autoradiography. (C) The amount of WHx present in the TX-sol fraction at each time point was quantitated by scanning densitometry and normalized (on an arbitrary scale) to the amount present at time zero. (D) The amount of labeled WHx associated with the NM fraction in the first hour of chase was normalized to the amount of labeled WHx present at time zero.
Densitometric scanning of the NM data showed that newly synthesized WHx rapidly associated with the NM fraction (Fig. 4A, NM lane 2), since it reached a maximum immediately after the pulse (0 min of chase). Densitometry was difficult to analyze because of the weakness of the signal after only 10 min of pulse, and the amount of labeled WHx in the cytoskeletal fractions was below the limit of detection (data not shown). However, results of several experiments (one of which is shown in Fig. 4A) showed that WHx did not accumulate within the NM fraction, where it appeared to have a short half-life (approximately 30 min). Therefore, our data support the hypothesis that subcellular distribution of WHx may be responsible, at least in part, for the bimodal kinetics of WHx observed in the cytoplasm.
The cytoplasmic cytoskeleton is contiguous with the nuclear matrix (5), and several studies have shown that these structures may support the transport of viral components to the nucleus (31, 35, 42). The presence of small amounts of WHx associated with the CSK fraction would suggest this possibility. However, it is also possible that WHx could enter the nucleus by diffusion, since the X protein is smaller than the exclusion limit of the nuclear pore. Nuclear WHx could then be retained in the nucleus by binding to a nondiffusible nuclear component, like the nuclear matrix. Our finding suggests that a fraction of newly synthesized WHx actually associates with components of the nuclear framework and that the transit from the soluble fraction to the insoluble framework fraction is rapid. This would also explain why we did not detect WHx in nucleoplasmic extracts when standard procedures to detect unlabeled WHx were used (11).
Overall, our subcellular fractionation data indicate that the great majority of WHx is a soluble cytoplasmic protein and that only a minor fraction of WHx is associated with nuclear components in vivo. An accumulating body of evidence (3, 9, 10, 12, 21, 24, 27) suggests that the cytosol is an important site for X-protein transactivating function. Thus, the presence of WHx in the cytosol of chronically infected hepatocytes might lead to a moderate but constitutive activation of several components of the cytoplasmic signaling cascades.
A short half-life is a typical feature of many viral and cellular proteins that have transcriptional regulatory functions, such as the Tax protein of human T-cell leukemia virus type 1 (40), the E1A protein of adenovirus (41), and the c-Myc protein (47). Furthermore, the presence of newly synthesized WHx associated with the NM fraction gives rise to the idea of a possible functional interaction between nuclear transcription factors and structural components of the nuclear matrix. It is known that the nuclear matrix plays an important role in regulating gene expression, and it has been shown that several transcription factors, like the ubiquitous transcription factor YY1 (15), ATF1 (16), and Oct-1 (23), as well as the retinoblastoma gene product Rb (2), the glucocorticoid receptor (13), the simian virus 40 large T antigen (37), and the E1A herpesvirus protein (6), are partially sequestered in the nuclear matrix. Studies targeted towards investigating such interactions may shed further light on the natural mechanism of action of WHx in hepatocytes.
Acknowledgments
We thank L. Johnson and B. Tennant for providing woodchucks from the experimental woodchuck colony at Cornell University, Ithaca, N.Y., William Mason for providing WHx antiserum; M. L. Schilsky for the PDI antiserum; and I. Listowsky for the ligandin antiserum. Many thanks to S. Gupta and members of his laboratory for their assistance during woodchuck liver perfusions and to H. Will for helpful discussions.
This work was supported by Public Health Service grants CA37232 and DK 46952, Center grants P30CA13330 and P30DK41296, and a grant from the Council for Tobacco Research. C.E.R. is the recipient of an Irma T. Hirschl-Weiler career scientific award. M.D. was supported in part by a stipend from the Deutsche Krebshilfe. J.P. was supported by the Deutsche Forschungsgemeinschaft (Pe/608 2-1), Bonn, Germany.
REFERENCES
- 1.Aldrich C E, Coates L, Wu T T, Newbold J, Tennant B C, Summers J, Seeger C, Mason W S. In vitro infection of woodchuck hepatocytes with woodchuck hepatitis virus and ground squirrel hepatitis virus. Virology. 1989;172:247–252. doi: 10.1016/0042-6822(89)90126-8. [DOI] [PubMed] [Google Scholar]
- 2.Amellem O, Stokke T, Sandvik J A, Pettersen E O. The retinoblastoma gene product is reversibly dephosphorylated and bound in the nucleus in S and G2 phases during hypoxic stress. Exp Cell Res. 1996;227:106–115. doi: 10.1006/excr.1996.0255. [DOI] [PubMed] [Google Scholar]
- 3.Benn J, Su F, Doria M, Schneider R J. Hepatitis B virus HBx protein induces transcription factor AP-1 by activation of extracellular signal-regulated and c-Jun N-terminal mitogen-activated protein kinases. J Virol. 1996;70:4978–4985. doi: 10.1128/jvi.70.8.4978-4985.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buendia M A. Hepatitis B viruses and hepatocellular carcinoma. Adv Cancer Res. 1992;59:167–226. doi: 10.1016/s0065-230x(08)60306-1. [DOI] [PubMed] [Google Scholar]
- 5.Capco D G, Wan K M, Penman S. The nuclear matrix: three-dimensional architecture and protein composition. Cell. 1982;29:847–858. doi: 10.1016/0092-8674(82)90446-9. [DOI] [PubMed] [Google Scholar]
- 6.Chatterjee P K, Flint S J. Partition of E1A proteins between soluble and structural fractions of adenovirus-infected and -transformed cells. J Virol. 1986;60:1018–1026. doi: 10.1128/jvi.60.3.1018-1026.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen H-S, Kaneko S, Girones R, Anderson R W, Hornbuckle W E, Tennant B C, Cote P J, Gerin J L, Purcell R H, Miller R H. The woodchuck hepatitis virus X gene is important for establishment of virus infection in woodchucks. J Virol. 1993;67:1218–1226. doi: 10.1128/jvi.67.3.1218-1226.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheong J-H, Yi M-K, Lin Y, Murakami S. Human RPB5, a subunit shared by eukaryotic nuclear polymerases, binds human hepatitis B virus X protein and may play a role in X transactivation. EMBO J. 1995;14:143–150. doi: 10.1002/j.1460-2075.1995.tb06984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chirillo P, Falco M, Puri P L, Artini M, Balsano C, Levrero M, Natoli G. Hepatitis B virus pX activates NF-κB-dependent transcription through a Raf-independent pathway. J Virol. 1996;70:641–646. doi: 10.1128/jvi.70.1.641-646.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cross J C, Wen P, Rutter W J. Transactivation by hepatitis B virus X protein is promiscuous and dependent on mitogen-activated cellular serine/threonine kinases. Proc Natl Acad Sci USA. 1993;90:8078–8082. doi: 10.1073/pnas.90.17.8078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dandri M, Schirmacher P, Rogler C E. Woodchuck hepatitis virus X protein is present in chronically infected woodchuck liver and woodchuck hepatocellular carcinomas which are permissive for viral replication. J Virol. 1996;70:5246–5254. doi: 10.1128/jvi.70.8.5246-5254.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doria M, Klein N, Lucito R, Schneider R J. Hepatitis B virus HBx protein is a dual specificity cytoplasmic activator of Ras and nuclear activator of transcription factors. EMBO J. 1995;14:4747–4757. doi: 10.1002/j.1460-2075.1995.tb00156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eggert M, Michel J, Schneider S, Bornfleth H, Baniahmad A, Fackelmayer F O, Schmidt S, Renkawitz R. The glucocorticoid receptor is associated with the RNA-binding nuclear matrix protein hnRNP U. J Biol Chem. 1997;272:28471–28478. doi: 10.1074/jbc.272.45.28471. [DOI] [PubMed] [Google Scholar]
- 14.Ganem D. Hepadnaviridae and their replication. In: Fields B N, Knipe D N, Howley P M, editors. Virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 2703–2739. [Google Scholar]
- 15.Guo B, Odgren P R, Van Wijnen A J, Last T J, Nickerson J, Penman S, Lian J B, Stein J L, Stein G S. The nuclear matrix protein NMP-1 is the transcription factor YY1. Proc Natl Acad Sci USA. 1995;92:10526–10530. doi: 10.1073/pnas.92.23.10526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo B, Stein J L, Van Wijnen A J, Stein G S. ATF1 and CREB transactivate a cell cycle regulated histone H4 gene at a distal nuclear matrix associated promoter element. Biochemistry. 1997;36:14447–14455. doi: 10.1021/bi971781s. [DOI] [PubMed] [Google Scholar]
- 17.Gupta S, Vemuru R P, Lee C D, Yerneni P, Aragona E, Burk R D. Hepatocytes exhibit superior transgene expression after transplantation into liver and spleen compared with peritoneal cavity or dorsal fat pad: implications for hepatic gene therapy. Hum Gene Ther. 1994;5:959–967. doi: 10.1089/hum.1994.5.8-959. [DOI] [PubMed] [Google Scholar]
- 18.Hohne M, Schaefer S, Seifer M, Feitelson M A, Paul D, Gerlich W H. Malignant transformation of immortalized transgenic hepatocytes after transfection with hepatitis B virus DNA. EMBO J. 1990;9:1137–1145. doi: 10.1002/j.1460-2075.1990.tb08220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang J, Kwong J, Sun E C-Y, Liang T J. Proteasome complex as a potential cellular target of hepatitis B virus X protein. J Virol. 1996;70:5582–5591. doi: 10.1128/jvi.70.8.5582-5591.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jacob J R, Ascenzi M A, Roneker C A, Toshkov I A, Cote P J, Gerin J L, Tennant B C. Hepatic expression of the woodchuck hepatitis virus X-antigen during acute and chronic infection and detection of a woodchuck hepatitis virus X-antigen antibody response. Hepatology. 1997;26:1607–1615. doi: 10.1002/hep.510260632. [DOI] [PubMed] [Google Scholar]
- 21.Kekule A S, Lauer U, Weiss L, Luber B, Hofschneider P H. Hepatitis B virus transactivator HBx uses a tumor promoter signalling pathway. Nature (London) 1993;361:742–745. doi: 10.1038/361742a0. [DOI] [PubMed] [Google Scholar]
- 22.Kim C M, Koike K, Saito I, Miyamura T, Jay G. HBx gene of hepatitis B virus induces liver cancer in transgenic mice. Nature (London) 1991;353:317–320. doi: 10.1038/351317a0. [DOI] [PubMed] [Google Scholar]
- 23.Kim M K, Lesoon-Wood L A, Weintraub B D, Chung J H. A soluble transcription factor, Oct-1, is also found in the insoluble nuclear matrix and possesses silencing activity in its alanine-rich domain. Mol Cell Biol. 1996;16:4366–4377. doi: 10.1128/mcb.16.8.4366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klein N P, Schneider R J. Activation of Src family kinases by hepatitis B virus HBx protein and coupled signaling to Ras. Mol Cell Biol. 1997;17:6427–6436. doi: 10.1128/mcb.17.11.6427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koike K, Moriya K, Iino S, Yotsuyanagi H, Endo Y, Miyamura T, Kurokawa K. High-level expression of hepatitis B virus HBx gene and hepatocellular carcinogenesis in transgenic mice. Hepatology. 1994;19:810–819. [PubMed] [Google Scholar]
- 26.Lee T-H, Elledge S J, Butel J S. Hepatitis B virus X protein interacts with a probable cellular DNA repair protein. J Virol. 1995;69:1107–1114. doi: 10.1128/jvi.69.2.1107-1114.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Natoli G, Avantaggiati M L, Chirillo P, Puri P L, Ianni A, Balsano C, Levrero M. Ras- and Raf-dependent activation of c-Jun transcriptional activity by the hepatitis B virus transactivator pX. Oncogene. 1994;9:2837–2843. [PubMed] [Google Scholar]
- 28.Petersen J, Dandri M, Gupta S, Rogler C E. Liver repopulation with xenogenic hepatocytes in B and T cell-deficient mice leads to chronic hepadnavirus infection and clonal growth of hepatocellular carcinoma. Proc Natl Acad Sci USA. 1998;95:310–315. doi: 10.1073/pnas.95.1.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Popper H, Roth L, Purcell R H, Tennant B D, Gerin J L. Hepatocarcinogenicity of the woodchuck hepatitis virus. Proc Natl Acad Sci USA. 1987;84:866–870. doi: 10.1073/pnas.84.3.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qadri I, Maguire H F, Siddiqui A. Hepatitis B virus transactivator protein X interacts with the TATA-binding protein. Proc Natl Acad Sci USA. 1995;92:1003–1007. doi: 10.1073/pnas.92.4.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Quinlan M P, Knipe D M. Nuclear localization of herpesvirus proteins: potential role for the cellular framework. Mol Cell Biol. 1983;3:315–324. doi: 10.1128/mcb.3.3.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Radke K, Carter V C, Moss P, Dehazya P, Schliwa M, Martin G S. Membrane association of a 36,000-dalton substrate for tyrosine phosphorylation in chicken embryo fibroblasts transformed by avian sarcoma viruses. J Cell Biol. 1983;97:1601–1611. doi: 10.1083/jcb.97.5.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rogler C E. Cellular and molecular mechanisms of hepatocarcinogenesis associated with hepadnavirus infection. Curr Top Microbiol Immunol. 1991;168:104–140. doi: 10.1007/978-3-642-76015-0_6. [DOI] [PubMed] [Google Scholar]
- 34.Rossner M T. Review: hepatitis B virus X-gene product: a promiscuous transcriptional activator. J Med Virol. 1992;36:101–117. doi: 10.1002/jmv.1890360207. [DOI] [PubMed] [Google Scholar]
- 35.Sanderson C M, Avalos R, Kundu A, Nayak D P. Interaction of Sendai viral F, HN, and M protein cytoskeletal and lipid components in Sendai virus-infected BHK cells. Virology. 1995;209:701–707. doi: 10.1006/viro.1995.1308. [DOI] [PubMed] [Google Scholar]
- 36.Schek N, Bartenschlager R, Kuhn C, Schaller H. Phosphorylation and rapid turnover of hepatitis B virus X protein expressed in HepG2 cells from a recombinant vaccinia virus. Oncogene. 1991;6:1735–1744. [PubMed] [Google Scholar]
- 37.Schirmbeck R, Deppert W. Specific interaction of simian virus 40 large T antigen with cellular chromatin and nuclear matrix during the course of infection. J Virol. 1987;61:3561–3569. doi: 10.1128/jvi.61.11.3561-3569.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shirakata Y, Kawada M, Fujiki Y, Sano H, Oda M, Kobayashi M, Koike K. The X gene of hepatitis B virus induced growth stimulation and tumorigenic transformation of mouse NIH3T3 cells. Jpn J Cancer Res. 1989;80:617–621. doi: 10.1111/j.1349-7006.1989.tb01686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Slagle B L, Lee T H, Medina D, Finegold M J, Butel J S. Increased sensitivity to the hepatocarcinogen diethylnitrosamine in transgenic mice carrying the hepatitis B virus X gene. Mol Carcinog. 1996;15:261–269. doi: 10.1002/(SICI)1098-2744(199604)15:4<261::AID-MC3>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 40.Slamon D J, Press M F, Souza L M, Murdock D C, Cline M J, Golde D W, Gasson J C, Chen I S. Studies of the putative transforming protein of the type I human T-cell leukemia virus. Science. 1985;228:1427–1430. doi: 10.1126/science.2990027. [DOI] [PubMed] [Google Scholar]
- 41.Spindler K R, Berk A J. Rapid intracellular turnover of adenovirus 5 early region 1A proteins. J Virol. 1984;52:706–710. doi: 10.1128/jvi.52.2.706-710.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stamatos N M, Chakrabarti S, Moss B, Hare J D. Expression of polyomavirus virion proteins by a vaccinia virus vector: association of VP1 and VP2 with the nuclear framework. J Virol. 1987;61:516–525. doi: 10.1128/jvi.61.2.516-525.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Terada K, Manchikalapudi P, Noiva R, Jauregui H O, Stockert R J, Schilsky M L. Secretion, surface localization, turnover, and steady state expression of protein disulfide isomerase in rat hepatocytes. J Biol Chem. 1995;270:20410–20416. doi: 10.1074/jbc.270.35.20410. [DOI] [PubMed] [Google Scholar]
- 44.Urban S, Hildt E, Eckerskorn C, Sirma H, Kekule’ A, Hofschneider P H. Isolation and molecular characterization of hepatitis B virus X-protein from a baculovirus expression system. Hepatology. 1997;26:1045–1053. doi: 10.1002/hep.510260437. [DOI] [PubMed] [Google Scholar]
- 45.Wang X W, Forrester K, Yeh H, Feitelson M A, Gu J-R, Harris C C. Hepatitis B virus X protein inhibits p53 sequence-specific DNA binding, transcriptional activity, and association with transcription factor ERCC3. Proc Natl Acad Sci USA. 1994;91:2230–2234. doi: 10.1073/pnas.91.6.2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang D, Alt E, Rogler C E. Coordinate expression of N-myc2 and insulin-like growth factor II in precancerous altered hepatic foci in woodchuck hepatitis virus carriers. Cancer Res. 1993;53:2020–2027. [PubMed] [Google Scholar]
- 47.Yeilding N M, Rehman M T, Lee W M F. Identification of sequences in c-myc mRNA that regulate its steady-state levels. Mol Cell Biol. 1996;16:3511–3522. doi: 10.1128/mcb.16.7.3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yen T S B. Hepadnaviral X protein: review of recent progress. J Biomed Sci. 1996;3:20–30. doi: 10.1007/BF02253575. [DOI] [PubMed] [Google Scholar]
- 49.Zoulim F, Saputelli J, Seeger C. Woodchuck hepatitis virus X protein is required for viral infection in vivo. J Virol. 1994;68:2026–2030. doi: 10.1128/jvi.68.3.2026-2030.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zucker S D, Goessling W, Ransil B J, Gollan J L. Influence of glutathione S-transferase B (ligandin) on the intermembrane transfer of bilirubin. Implications for the intracellular transport of nonsubstrate ligands in hepatocytes. J Clin Investig. 1995;96:1927–1935. doi: 10.1172/JCI118238. [DOI] [PMC free article] [PubMed] [Google Scholar]