Abstract
Adeno-associated virus (AAV) vectors appear promising for use in gene therapy in cystic fibrosis (CF) patients, yet many features of AAV-mediated gene transfer to airway epithelial cells are not well understood. We compared the transduction efficiencies of AAV vectors and adenovirus (Ad) vectors in immortalized cell lines from CF patients and in nasal epithelial primary cultures from normal humans and CF patients. Similar dose-dependent relationships between the vector multiplicities of infection and the efficiencies of lacZ gene transfer were observed. However, levels of transduction for both Ad and recombinant AAV (rAAV) were significantly lower in the airway epithelial cell than in the control cell lines HeLa and HEK 293. Transduction efficiencies differed among cultured epithelial cell types, with poorly differentiated cells transducing more efficiently than well-differentiated cells. A time-dependent increase in gene expression was observed after infection for both vectors. For Ad, but not for AAV, this increase was dependent on prolonged incubation of cells with the vector. Furthermore, for rAAV (but not for rAd), the delay in maximal transduction could be abrogated by wild-type Ad helper infection. Thus, although helper virus is not required for maximal transduction, it increases the kinetics by which this is achieved. Expression of Ad E4 open reading frame 6 or addition of either hydroxyurea or camptothecin resulted in increased AAV transduction, as previously demonstrated for nonairway cells (albeit to lower final levels), suggesting that second-strand synthesis may not be the sole cause of inefficient transduction. Finally, the efficiency of AAV-mediated ex vivo gene transfer to lung cells was similar to that previously described for Ad vectors in that transduction was limited to regions of epithelial injury and preferentially targeted basal-like cells. These studies address the primary factors influencing rAAV infection of human airway cells and should impact successful gene delivery in CF patients.
Two DNA viruses, adeno-associated virus (AAV) and adenovirus (Ad), are currently being evaluated as vectors for human gene therapy of cystic fibrosis (CF) (9, 14, 15, 27–29, 41). Outcomes of several human and preclinical studies of gene therapy in CF patients, using Ad vectors, revealed that E1-deleted Ad vectors need to resolve several obstacles in terms of efficiency and safety (3, 17, 24, 40). At present, Ad vectors are known to cause inflammation (3, 33, 39), to induce immune responses (11, 36, 37, 39), and to be susceptible to recombination with endogenous virus (8, 10, 27). Fewer studies have been carried out with AAV, but primary differences between AAV and Ad vectors with regard to immune response and safety have been noted (4). In addition, since wild-type virus is not known to have the potential to cause disease in humans, recombination with endogenous virus seems less problematic (20). Long-term expression of the CF transmembrane conductance regulator (CFTR) gene by AAV vectors has been described for up to 180 days after vector administration to airway epithelia of New Zealand White rabbits (15) and rhesus monkeys (4). Gene expression by first-generation Ad vectors in airway epithelia has been transient in vivo. Such comparisons support the further characterization of AAV vectors for use in gene therapy in CF patients (2, 25).
Since a common observation with the use of Ad vectors in primary airway cells has been inefficient transduction, we initiated a study to measure the requirements for AAV-mediated gene transfer. Recently it has been shown that an immediate-early event—namely, second-strand DNA synthesis—can be rate limiting in the absence of Ad coinfection (12, 13). These studies suggest that the limiting step in our ability to score gene transduction in some cell types is not internalization of the virus but rather the synthesis of a transcriptionally active double-stranded version of the AAV genome. This genomic conversion and the subsequent expression of the recombinant reporter or therapeutic gene are greatly facilitated by expression of the Ad E4 open reading frame 6 (ORF6) protein (12, 13). Interestingly, the phenomenon elicited by the Ad E4 ORF6 protein can be reproduced to different degrees in recombinant AAV (rAAV)-infected cells by exposure of the cells to heat shock or genotoxic reagents (12). It is assumed that Ad E4 ORF6 and genotoxic and physical stresses are acting through a common mechanism and that their effects are linked to the induction of the host cell DNA repair machinery rather than the cell cycle (12, 13). However, the precise mechanism for the increase in AAV transduction has not been defined. The impact of these findings on the use of AAV vectors for gene therapy in CF patients is unclear. However, it is apparent that rAAV vector transduction in some cultured cells can be improved dramatically by physical and chemical manipulations (1, 12, 13, 30), which suggests that similar reagents could be coupled with current AAV vector strategies to enhance the delivery of genes to the airway epithelia of human CF patients. Thus, one goal of this study was to determine the importance of second-strand synthesis to AAV-mediated gene delivery to the airway epithelium. Factors influencing the transduction efficiency of AAV vectors relevant to the airway epithelium of CF patients were compared to those of first-generation Ad vectors. AAV and Ad vectors were used to deliver the lacZ reporter gene to primary cultures of airway epithelial cells as well as a spectrum of poorly differentiated (CF/T43 cells) and well-differentiated (CFT1 cells) airway epithelial cell lines. We observed low transduction efficiencies in these cells when using these vectors, suggesting the existence of a potential barrier to effective gene delivery in vivo.
Finally, we tested the efficiency of rAAV vectors for in vivo gene transfer in human airways, using freshly excised human tracheal specimens from normal humans and CF patients as a model for the well-differentiated columnar epithelia and injured epithelia characteristic of these individuals. Our results support previous Ad vector studies demonstrating that basal-like cell types, not columnar cell types, are preferentially transduced by AAV vectors and that areas of transduction are limited to regions of epithelial injury.
MATERIALS AND METHODS
Viruses and recombinant viral vectors.
rAAV vectors were prepared in human HEK 293 cells as previously described (26, 31, 34). Vectors were purified by double CsCl gradient ultracentrifugation, dialyzed into 10 mM Tris-HCl–150 mM NaCl–10% glycerol, and stored at −20°C until used for infection of airway epithelial cells. Vector titers, depicted as transducing units (TU) per milliliter, were determined on Ad-infected HeLa cells by staining for β-galactosidase activity (32). This procedure typically produced vector lots with titers of 108 to 109 TU/ml. The ratio of TU to the genome-based particle number was approximately 1:100. The AAV vector lots were not heat inactivated, since this AAV vector preparation method produces AAV free of wild-type Ad.
The Ad vector AdCMVβgal was used in this study. This vector encodes a cytoplasmic β-galactosidase gene driven by the cytomegalovirus promoter-enhancer (9, 37). Ad vectors were propagated in HEK 293 cells, purified by CsCl gradient ultracentrifugation, and stored at −20°C until use. Vectors titers were determined on HeLa cells by staining for β-galactosidase activity. These titers ranged from 2 × 1011 to 2 × 1012 TU/ml. The ratio of TU to the number of viral particles (as determined by measuring the optical density at 260 nm) was approximately 1:20.
Wild-type Ad dl309 (23) was used to enhance AAV transduction. The Ad mutants dl366* (19) (designated Ad E4−) and E4dl ORF1–4 (21) (designated Ad E4− ORF6+ 6/7+) have been described previously. These mutants were used in the studies of the helper function in AAV-mediated gene transfer in human airway cells.
Ad was inactivated with long-wave UV irradiation in the presence of the DNA intercalator psolaren (5). In brief, samples of Ad were added to a freshly prepared 8-methoxypsoralen solution (0.33 μg/μl; Sigma) and, while on ice, exposed to a 366-nm UV light source (model UVL-56; UVP Inc., Upland, Calif.) for 30 min. Following UV irradiation, the virus was purified by gel filtration (G-50 Sephadex; Boehringer Mannheim Corp., Indianapolis, Ind.) into phosphate-buffered saline (PBS) containing 3% (vol/vol) glycerol.
Cell culture.
Human nasal epithelial cells were isolated from nasal polyps of CF patients and from turbinates of normal subjects and cultured as described in detail elsewhere (35). All procedures were approved by the University of North Carolina committee for the rights of human subjects. The cells were fed on alternate days with serum-free, hormone-supplemented F-12 medium (supplements included insulin [5 μg/ml], endothelial cell growth supplement [3.7 μg/ml], epidermal growth factor [25 ng/ml], triiodothyroxine [3 × 10−8 M], hydrocortisone [10−6 M], transferrin [5 μg/ml], and cholera toxin [10 ng/ml], plus penicillin and streptomycin) (35).
Two immortalized airway epithelial cell lines from CF patients were also used in this study. CF/T43 cells were developed from the nasal epithelium of a homozygous ΔF508 CF patient and transformed with the simian virus 40 (SV40) T antigen (22). CFT1 cells were derived from the tracheal epithelium of a homozygous ΔF508 CF patient and transformed with the E6 and E7 genes of human papillomavirus (38). All experiments using CF/T43 cells or CFT1 cells were performed on a single clone, between passages 20 and 30. The cells were fed on alternate days with serum-free, hormone-supplemented F-12 medium.
Transduction of human airway epithelial cells.
Primary human nasal epithelial cells or immortalized airway epithelial cell lines from CF patients were plated at a density of 3 × 104 cells per well in 24-well dishes (Costar, Cambridge, Mass.) and allowed to attach for 16 h, and then nonadherent cells were removed by gentle washing with PBS. Two days after plating, the total number of cells per dish was approximately 1 × 104 to 3 × 104. AAV vector (AAVβgal) or Ad vector (AdCMVβgal) was added to the dishes at various multiplicities of infection (MOIs) in 0.3 ml of medium. After incubation for various periods of time at 37°C in air plus 5% CO2, the cells were washed with PBS and then fed with fresh, hormone-supplemented F-12 medium. Control groups were exposed to 0.3 ml of vehicle (F-12 medium) alone for comparable time periods and washed as described above.
Two days after infection, the transduction efficiency was calculated by determining the percentage of β-galactosidase-expressing cells by histochemical staining (32). To assess the time course of transgene expression, staining was performed at 1, 2, 3, 5, 7, and 10 days after vector exposure. All experiments were performed at least in triplicate, and a minimum of 500 cells/well were counted to determine the percentage of transduced cells.
Effect of Ad on AAV transduction.
Two days after being plated, airway cells were incubated with AAV or Ad vector and then exposed to Ad dl309 for 1 h at 37°C. Forty-eight hours later, the transduction efficiency was determined as described above.
To elucidate the role of Ad in AAVβgal transduction of airway cells from CF patients, these cells were exposed to AAV vector at different MOIs for 1 h in the presence of either Ad dl309, UV- and psoralen-inactivated Ad dl309, Ad dl366* (Ad E4−), or E4dl ORF1–4 (Ad E4− ORF6+ 6/7+) (100 particles/cell). The cells were then washed with PBS and fed with fresh, hormone-supplemented F-12 medium. Two days later, transduction efficiencies were determined by staining for β-galactosidase activity as described above.
Transduction of human tracheal explants with AAV.
Freshly excised human tracheal tissue (n = 3 [CF patients] or 4 [normal subjects]) was obtained from lung transplant CF patients and normal donors. Tracheal tissue was cut into 0.25-cm2 samples and exposed to 20 μl of a solution containing 108 TU (1010 genome-based particles) of AAVβgal vector/ml (MOI, ca. 1) for 30 min in the presence or absence of Ad dl309 (100 particles/cell). Tracheal specimens were then rinsed with PBS, incubated for 48 h at 37°C, fixed with 0.5% glutaraldehyde in PBS, and stained for β-galactosidase activity. The tissue was postfixed with OmniFixII (Aaron Medical Industries, St. Petersburg, Fla.), embedded in paraffin, sectioned, and counterstained with hematoxylin plus eosin.
RESULTS
AAV transduction in airway cells of CF patients and normal subjects.
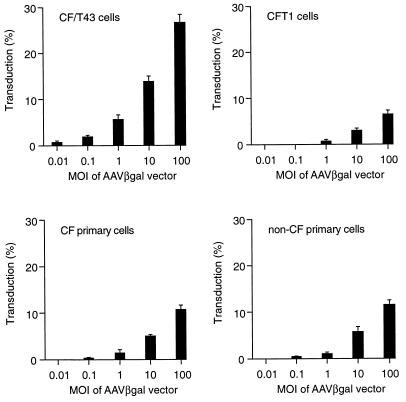
We first tested the transduction efficiency of rAAV by infecting immortalized airway-specific cell lines and scoring for vector transduction by measuring lacZ expression. The transduction efficiency of the AAV vector increased in a dose-dependent manner in all cell types (Fig. 1). There was no difference in the transduction efficiency in primary nasal epithelial cells of CF patients versus those of normal subjects throughout the range of vector doses used (Fig. 1). The transduction efficiencies in the CF/T43 and CFT1 cell lines, however, differed dramatically. At the same MOI of AAV vector, the transduction efficiency in CF/T43 cells was approximately 4- to 10-fold higher than that in the CFT1 cell line (Fig. 1).
FIG. 1.
Efficiency of AAVβgal-mediated gene transfer to human airway epithelial cells. Values are presented as means ± standard errors of the means (n = 5). Cells were infected for 1 h at 37°C, and the transduction efficiencies were determined by histochemical staining for β-galactosidase activity 2 days after infection, as described in Materials and Methods.
Ad and AAV vectors transduce airway epithelial cells with similar efficiencies.
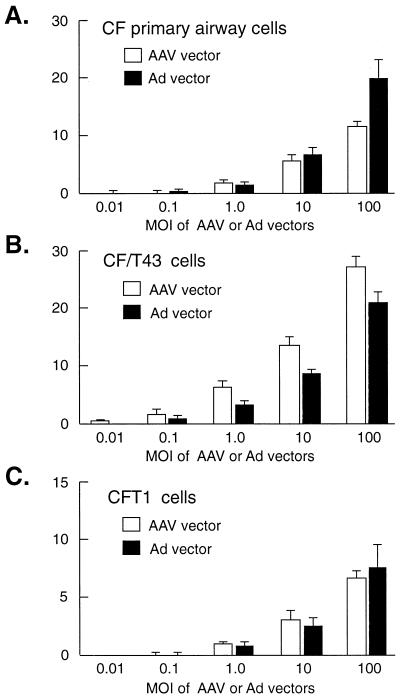
We next compared the transduction efficiencies of AAV, lacZ, and Ad lacZ vectors in various airway-specific cell lines. The dose-effect relationships of vector MOI and transduction efficiency of airway cells from CF patients were remarkably similar for the AAV and Ad vectors (Fig. 2). The Ad vector was slightly more efficient in transduction of primary cells than was the AAV vector at the highest MOI (Fig. 2A). However, the efficiency of Ad vector transduction of the CF/T43 cell line was somewhat less than that of the AAV vector (Fig. 2B). In CFT1 cells there was no difference in the transduction efficiencies of the AAV and Ad vectors (Fig. 2C). These results suggest that transduction efficiencies can vary, depending on the specific cell line and vector being tested.
FIG. 2.
The transduction efficiencies of AAV and Ad vectors in primary cultures of human airway epithelial cells (A), CF/T43 cells (B), and CFT1 cells (C). Values are presented as means ± standard errors of the means (n = 3). Experimental conditions were the same as described in the legend to Fig. 1 and are detailed in Materials and Methods.
Enhancement of rAAV but not rAd transduction by subsequent wild-type Ad infection.
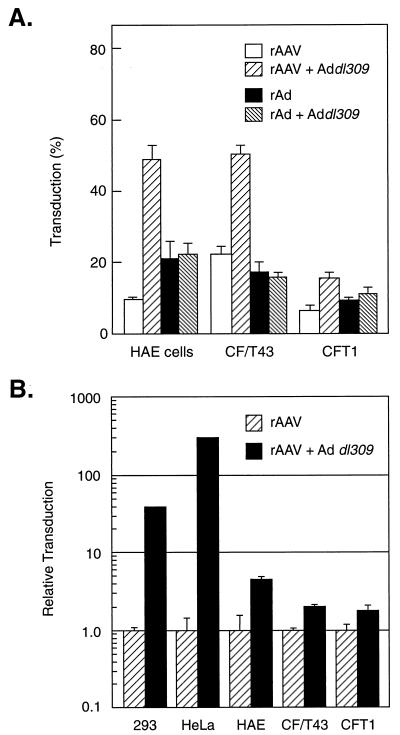
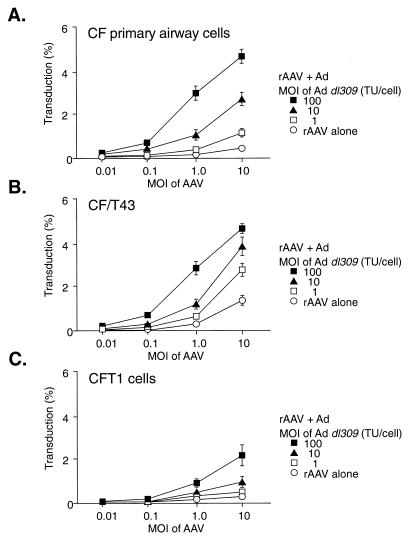
Previous work has demonstrated a rate-limiting step in rAAV-mediated transduction that can be alleviated by helper Ad coinfection (12, 13). To test this premise in airway epithelial cells, coinfections with rAAV and wild-type Ad were performed. AAV-mediated gene transfer to airway epithelial cells was enhanced in the presence of Ad dl309, whereas Ad-mediated gene transfer was unaffected by Ad dl309 (Fig. 3). In the presence of Ad, the AAV vector was considerably more efficient at transducing airway cells than was the Ad vector (Fig. 3A). We next compared the enhancement seen in airway cells after Ad coinfection to the reported observations in HeLa and 293 cells (12, 13). At 100 IU of Ad dl309 per cell, AAV transduction increased only two- to sevenfold, whereas at 2 IU of Ad dl309 per cell, AAV transduction of HEK 293 and HeLa cells increased by several orders of magnitude (Fig. 3B). The effect of Ad on AAV transduction was dose dependent for each CF cell type analyzed, although the magnitude of the effect differed among the cell lines (Fig. 4).
FIG. 3.
Effect of the presence of Ad on transduction of AAVβgal. (A) Airway epithelial cells infected with AAV or Ad vector, alone or with Ad dl309. Values are presented as means ± standard errors of the means (n = 3). Airway cells were infected with AAVβgal vector (100 TU/cell; 104 particles/cell) or AdCMVβgal vector (100 TU/cell; 2 × 103 particles/cell) in the presence or absence of Ad dl309 (100 IU/cell) for 1 h. Two days later, the transduction efficiencies were determined by staining for β-galactosidase activity as described in Materials and Methods. (B) The relative effect of Ad on AAVβgal transduction of HEK 293 cells, HeLa cells, and human airway epithelial cells. Levels of transduction in the presence of Ad are shown relative to the level of transduction in the absence of Ad, which has been assigned a value of 1. Airway cells were infected with the AAVβgal vector (100 TU/cell; 104 particles/cell) or the AdCMVβgal vector (100 TU/cell; 2 × 103 particles/cell) in the presence or absence of Ad dl309 (100 IU/cell) as described for panel A. To allow for increased transduction in the presence of Ad, HEK 293 and HeLa cells were infected with 0.1 particle of AAVβgal vector or 0.5 particle of AdCMVβgal per cell in the presence or absence of Ad dl309 (50 particles/cell).
FIG. 4.
Relationship between Ad dose and enhancement of AAVβgal transduction in primary cultures of human airway epithelial cells (A), CF/T43 cells (B), and CFT1 cells (C). Cells were infected for 1 h at 37°C, and the transduction efficiencies were determined by histochemical staining for β-galactosidase activity 24 h later. Each value is presented as the mean ± the standard error of the mean (n = 3).
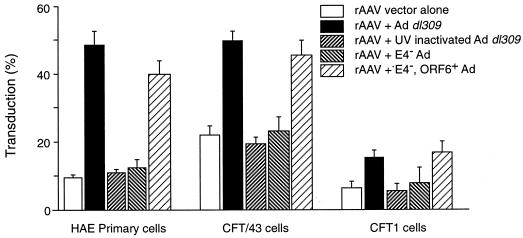
The effect of Ad on AAV transduction was abolished by UV-psoralen inactivation of Ad gene expression (Fig. 5). In addition, an Ad mutant lacking the E4 region (E4− Ad) was unable to enhance AAV transduction (Fig. 5). However, a partially deleted Ad mutant, lacking E4 ORF1–4 but containing ORF6 and ORF6/7 (Ad E4− ORF6+ 6/7+), maintained the ability to increase AAV vector transduction similarly to that of Ad dl309 (Fig. 5), supporting previous published observations for the role of Ad gene expression in rAAV transduction (12, 13).
FIG. 5.
Transduction of airway epithelial cells with rAAV in the presence of different Ad mutants. Values are presented as means ± standard errors of the means (n = 3). Vector AAVβgal (104 particles/cell) was administered to cells for 1 h in the presence or absence of Ad dl309, UV- and psoralen-inactivated Ad dl309, E4− Ad, or E4− Ad ORF6+ 6/7+ (each at 1,000 particles/cells). At 24 h after infection, the cells were stained for β-galactosidase activity as described in Materials and Methods.
Prolonged incubation increases transduction of cultured human airway epithelial cells.
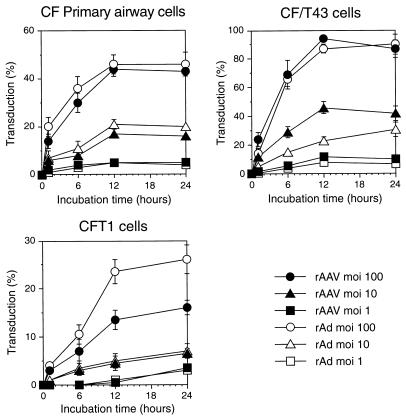
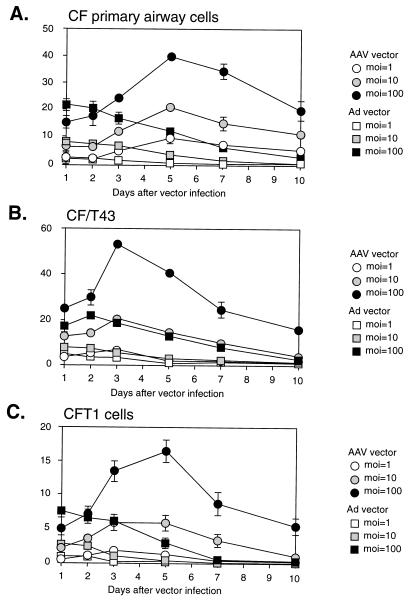
We tested another variable, involving extended vector exposure, for its effect on vector transduction. Both the AAV and Ad vectors exhibited a relationship between the length of exposure of airway epithelial cells and the efficiency of gene transfer (Fig. 6). All of the cell types showed similar requirements of prolonged exposure to achieve maximal transduction. No additional effect was detected when incubation times were extended longer than 24 h (data not shown). However, since transduction was determined 48 h after each of the different exposure periods (1, 6, 12, or 24 h), the cells from each experimental group were ultimately maintained in culture for different periods of time (2 to 3 days). Thus, we sought to determine if the duration of exposure or the duration of time in culture was responsible for the increase in transduction. Cells were infected for 1 h, washed free of unbound vector, and then monitored for different lengths of time in culture prior to staining for LacZ to access the amount of gene transfer. For AAV vectors, the percentage of LacZ-expressing cells increased with increased time in culture (Fig. 7). The highest levels of transduction were seen 3 to 5 days after infection of primary airway cells and CFT1 cells (Fig. 7A and C) and 3 days after infection of CF/T43 cells (Fig. 7B). These results are similar to those of the previous experiment, in which the highest level of transduction occurred following a 24-h incubation period and 48 h of growth in culture, i.e., 3 days after initial vector administration.
FIG. 6.
Effect of prolonged vector incubation time on the transduction of primary cultures of human airway epithelial cells (A), CF/T43 cells (B), and CFT1 cells (C) by AAVβgal. Values are presented as means ± standard errors of the means (n = 3). Cells were incubated with the vector for the indicated period of time, washed, cultured for an additional 48 h in the absence of vector, and stained for β-galactosidase activity to determine the efficiency of transduction.
FIG. 7.
Activation of rAAV vector over time in culture. Primary human airway epithelial cells from CF patients (A), CF/T43 cells (B), and CFT1 cells (C) were infected with AAVβgal for 1 h at 37°C. Transduction efficiencies were determined on the indicated days postinfection by staining for β-galactosidase activity as described in Materials and Methods. Values are presented as means ± standard errors of the means (n = 3).
For the Ad vector, maximal transduction was observed at the earliest time points (Fig. 7). The percentage of transduced cells decreased over time in culture and was nearly zero at 10 days postinfection (Fig. 5). Because of these differences in the effect of time on expression from Ad and AAV vectors, there was a significant difference in the efficiencies of transduction of airway cells by AAV and Ad vectors at three or more days following vector administration. The maximal effect of time in culture on transduction was approximately three- to sevenfold. Interestingly, this was similar to the increase in transduction observed during coinfection with Ad.
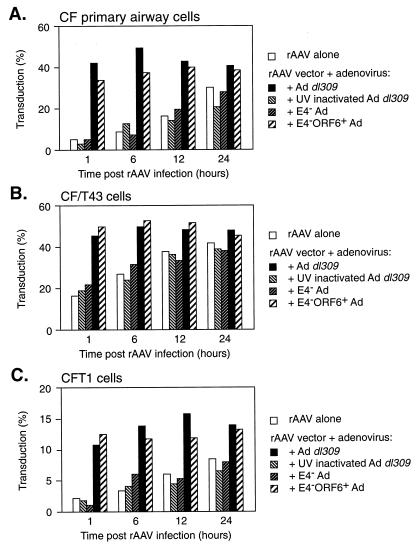
To investigate the relationship between the effect of the presence of Ad and the duration of incubation on AAV transduction, airway epithelial cells were infected with AAV for a short period of time and then infected with Ad at various times following the initial vector infection. It was determined that either time or Ad coinfection could be used to increase AAV transduction, but the effects were not additive (Fig. 8). When exposure to the AAV vector was limited to 1 h, nearly the same level of transduction could be achieved with a 1-h incubation in the presence of Ad as could be achieved with a 24-h incubation in the absence of Ad (Fig. 8). Ad was able to increase AAV transduction at the early time points. However, given time, transduction increased to the same level on its own and Ad no longer had an effect. UV- and psoralen-inactivated Ad or E4− Ad was unable to alleviate the need for a prolonged incubation time, whereas the E4− ORF6+ 6/7+ virus functioned similarly to Ad dl309 (Fig. 8). Since the effects of time and the presence of Ad on AAV transduction of airway epithelial cells were not additive and could substitute for one another, it is assumed that they are acting in a similar manner; i.e., both result in the accumulation of functional genomes.
FIG. 8.
Effect of Ad on AAVβgal transduction of primary airway cells from CF patients (A), CF/T43 cells (B), or CFT1 cells (C) at various times postinfection. Cells were initially infected with AAVβgal for 1 h at 37°C, washed, and then exposed to Ad dl309, UV- and psoralen-inactivated Ad dl309, E4− Ad, or E4− ORF6+ 6/7+ Ad for 1 h at the indicated times post-rAAV infection. All cells were stained for LacZ activity 48 h following rAAV infection as described in the text. Values represent the means of data from triplicate experiments.
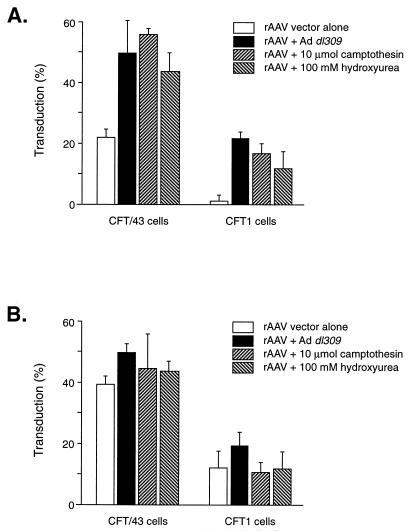
As mentioned above, a number of other agents have also been shown to increase AAV-mediated gene transduction. Both camptothesin and hydroxyurea were able to increase AAV transduction of airway epithelial cells (Fig. 9). However, by 3 days after vector delivery, these agents were no longer able to increase gene transduction (Fig. 9). These results support the above-mentioned interpretation of the conversion of singled-stranded AAV genomes to doubled-stranded templates capable of gene expression over time or enhanced by the addition of genetic (Ad coinfection) or chemical (hydroxyurea) stimuli.
FIG. 9.
Effect of Ad and DNA-damaging agents on rAAV transduction of airway epithelial cell lines. (A) The indicated cell lines were infected with AAVβgal (1,000 particles/cell) for 1 h at 37°C and then treated with the indicated agents and stained for β-galactosidase activity following 48 h in culture, as described in Materials and Methods. (B) The cells were cultured for 48 h after infection, prior to treatment with the indicated agents, and then cultured for an additional 48 h prior to assessment of transduction.
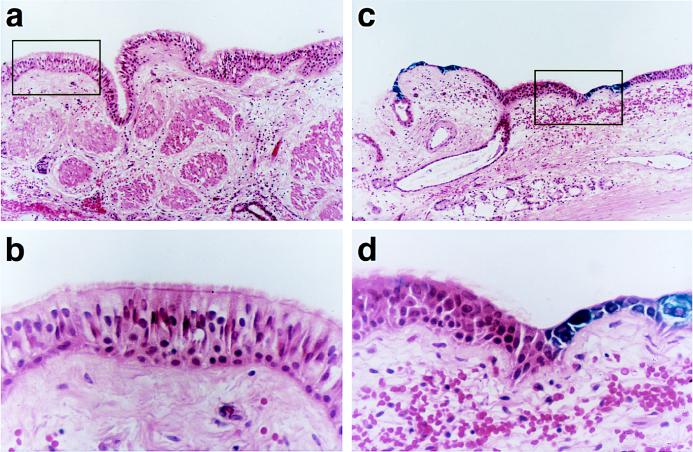
Transduction of human tracheal explants with rAAV is limited to basal-like cells.
In the absence of Ad, three of eight freshly exercised human tracheal specimens infected with rAAV exhibited lacZ gene expression; however, the transduction efficiency, as assessed by determining the percentage of LacZ-expressing cells, was less than 0.1% (data not shown). Whereas basal-like cells were transduced in relatively ample quantities by the AAV vector (Fig. 10C and D), well-differentiated, ciliated columnar cells were not transduced by this vector (Fig. 10A and B). In the presence of Ad, LacZ-positive cells were found in one of three specimens. Because transduction efficiency, as assessed by determination of LacZ-positive cells, was less than 0.1%, there was no obvious enhancement of AAV transduction in the presence of Ad (data not shown). Further, features of cell tropism (columnar cells versus basal cells) of AAV vectors were not affected by coadministration of Ad.
FIG. 10.
Histological sections of freshly excised tissue from CF patients. Human tracheal specimens were cut into 0.25-cm2 samples and infected with AAVβgal for 30 min. At 48 h after exposure, specimens were fixed with 0.5% glutaraldehyde and stained for β-galactosidase activity as described in Materials and Methods. The sections were counterstained with hematoxylin and eosin. Regions of the epithelium containing well-differentiated columnar cells were not transduced by AAVβgal (A and B), whereas areas of the epithelium that were injured and had exposed cuboidal, basal-like cells were moderately well transduced by this vector (C and D). Magnifications: A, C, and D, ×250; B, ×1,000.
DISCUSSION
Several studies reporting successful CFTR gene expression in airway epithelia by the use of AAV vectors have suggested that AAV may be a promising vector for gene therapy of CF airway disease (4, 14, 15). However, compared with the detailed descriptions of gene expression in airway epithelia in animal models, the features of AAV vectors in human airway epithelial cells have not been fully elucidated.
As a part of our initial characterization of AAV vectors in human airway epithelial cells, we tested whether the CF phenotype affected the efficiency of AAV vector-mediated gene transfer. The dose-dependent relationships between the amount of AAV vector applied and the percentage of transduced cells were similar in primary cultures of airway epithelial cells derived from both normal and CF subjects (Fig. 1). These results indicate that the absence of functional cell surface CFTR activity does not effect the efficiency of AAV gene transfer. Previous studies of AAV-mediated gene transfer to human airway epithelial cells have been performed with immortalized cell lines from CF patients, such as IB3 (16). Because IB3 cells have been transformed by a chimeric virus (Ad type 12 [Ad12]/SV40), it was not clear if the efficiency of vector transduction in these cells would hold true for AAV transduction of primary, untransformed human airway epithelial cells from CF patients. Therefore, we performed a comprehensive study comparing the transduction efficiencies of AAV vectors in primary cultures of airway epithelial cells from normal subjects and CF patients and two additional immortalized CF cell lines (Fig. 1). Interestingly, the ability of AAV to transduce these cells varied by severalfold. While the CF/T43 cell line was more efficiently transduced by an AAV vector than were primary airway cells, the CFT1 cell line was considerably less-efficiently transduced than the primary cells. These observations with the CF/T43 cell line are in agreement with the data of Halbert and coworkers (18), who reported that the transduction efficiency of AAV vectors was 10 to 60 times higher in immortalized human cells than in primary cells. However, our results with the CFT1 cell line suggest that the transduction efficiency of airway epithelial cells cannot be predicted simply as a function of immortalization. In addition, it should be noted that these results may be influenced by the clonal isolation of these cell lines and may not be related to the mechanism of immortalization.
Several factors may be pertinent to these observations. It is possible that the differences in transduction efficiency reflect influences of the immortalizing genes (e.g., the SV40 T-antigen gene for cell line CF/T43 and the human papillomavirus E6/E7 gene for cell line CFT1) (22, 38) on the molecular conversion of the viral genome or on the expression of the reporter gene. Differences in the transduction efficiencies of Ad vectors have been observed as a feature of cell differentiation (6, 7). CFT1 cells are more highly differentiated than CF/T43 cells, suggesting that differentiation status may directly or indirectly affect AAV transduction of airway cells. Another explanation could be related to the differences in the growth rates of these cell lines. CFT1 cells proliferate at a rate 50% slower than that of CF/T43 cells. Regardless of the mechanism, the evaluation of each of these CF cell lines will undoubtedly aid in our understanding of AAV-mediated gene transfer to CF patient airways. Since the more highly differentiated CFT1 cells are less efficiently transduced by AAV vectors than are the less-differentiated CF/T43 cells, the CFT1 cell line may be a better predictor of AAV transduction efficiency in differentiated airway epithelia of CF patients in vivo, an observation which has held true for Ad vectors (17).
AAV and Ad vectors were equally efficient at transferring genes to airway epithelial cells of CF patients (Fig. 2) unless AAV-mediated transduction was aided by the coadministration of Ad (Fig. 3). In the presence of Ad, AAV was more efficient at transducing airway epithelial cells than was Ad alone. However, the overall efficiency of transduction of airway cells was quite low, and the magnitude of the increase in transduction caused by Ad coinfection was much lower in the airway epithelial cells than that observed in the HEK 293 or HeLa cell line. Previously we have shown that Ad is able to enhance AAV transduction by overcoming a block in the conversion of single-stranded viral genomes into transcriptionally active, double-stranded molecules (12). A key issue relates to whether Ad-enhanced AAV transduction of airway cells is due to an increase in AAV uptake or to increased second-strand synthesis. In immortalized fibroblast cells, we have shown that the enhancement of AAV transduction by Ad is due to Ad E4 ORF6 gene expression (12). Several lines of evidence suggest that the Ad helper effect in the airway epithelium is also based on Ad E4 ORF6 gene expression. First, UV-psoralen treatment of Ad, which has been shown to inactivate gene expression but preserve entry functions (5), abolishes the effect of Ad on AAV transduction (Fig. 5 and 8). Second, the deletion of the E4 region from the Ad genome abrogates the helper effect. However, an Ad mutant with a partially deleted E4 region, containing only ORF6 and ORF6/7, retained the helper effect (Fig. 5 and 8). These data indicate that the E4 genes, specifically those in ORF6 or ORF6/7, are responsible for the enhancement of AAV transduction in airway epithelial cells of CF patients in a manner similar to that previously described for immortalized fibroblast cell lines (12, 13). The only difference is that the magnitude of the effect is considerably less in airway epithelial cells than in the previously studied cell lines. Transduction of HeLa and HEK 293 cells with AAV vectors increased 1,000-fold in the presence of Ad. Using the same rAAV vectors and helper Ad, transduction of airway epithelial cells increased only two- to sevenfold.
Since airway epithelial cells are less efficiently infected with Ad (Fig. 2 and 3A), it is possible that the effect of Ad on AAV transduction was limited by the inefficiency of the Ad infection. For this reason, we attempted to increase AAV transduction in the absence of Ad by treating AAV-infected cells with genotoxic agents. These compounds have previously been shown to increase AAV transduction in a manner analogous to Ad E4 ORF6 gene expression (1, 12, 30). Hydroxyurea and camptothesin were both able to increase rAAV transduction (Fig. 9). The maximal effect on AAV transduction with these agents was similar to that achieved with Ad (103 particles/cells), suggesting that Ad infection was not limiting in these determinations. Thus, agents which presumably act by increasing AAV second-strand DNA synthesis have only a small effect on vector transduction of human airway epithelial cells. This suggests the possibility that transduction of these cells is blocked at another level. However, it should be noted that histochemical staining for the detection of β-galactosidase activity may not be very sensitive in these cell types, and many more cells could have been positive than were scored by this method. Testing more-sensitive reporter genes, such as green fluorescent protein, may help resolve this potential concern.
Both AAV and Ad vectors exhibited incubation time-dependent increases in gene transduction (Fig. 6). These observations initially suggested that the nature of AAV vector transduction of airway cells was similar to that of Ad vectors. However, the need for a long AAV vector incubation time could be eliminated by increasing the time in culture prior to accessing gene transduction (Fig. 7) or by coinfecting with wild-type Ad (Fig. 8), whereas expression from Ad vectors declined steadily over time in culture and wild-type Ad had no effect on the time course of rAd transduction. This suggests that the factors governing effective transduction by each of these vectors are different. It seems likely that Ad is blocked at the level of vector entry (hence the requirement for prolonged exposure to the host cells) and that AAV may be blocked at a secondary intracellular event (hence the need for Ad coinfection or additional time in culture). It is intriguing that the block to efficient transduction alleviated by Ad is absent at 5 days postinfection. Presumably, airway epithelial cells are capable of supporting second-strand synthesis at a low level in the absence of Ad, such that AAV genomes become transcriptionally active slowly over time.
Finally, we tested the efficiency of gene transfer by AAV vectors to freshly excised human tracheal specimens. Typically, basal-like cells, rather than columnar ciliated cells, were transduced by AAV vectors. This phenomenon is very similar to Ad-mediated gene transfer to human airway tissue (17). There was no evidence of enhancement of AAV-mediated gene transfer to the human tracheal explants by Ad. However, this is not surprising due to the poor infectivity of ciliated epithelial cells by Ad (17). These preliminary results suggest that AAV vector transduction of the airway epithelium may be cell type dependent in a manner analogous to Ad vector transduction. Therefore, the efficiency of AAV transduction of human airway epithelia in vivo may not be predicted by the results of in vitro AAV transduction of cultured human primary cells, which exhibit a basal-cell-like phenotype. For this reason, the two CF airway epithelial cell lines used in this study, which exhibit very different states of differentiation (poorly differentiated [CF/T43] versus more highly differentiated [CFT1]), may be useful reagents for investigation of the rate-limiting steps in efficient transduction of airways of CF patients with recombinant AAV vectors. Our results of transductions of primary airway tissue also illustrate the need to perform parallel experiments in vivo in order to establish a complete analysis of vector behavior for efficient gene delivery.
ACKNOWLEDGMENTS
S. Teramoto and J. S. Bartlett contributed equally to this work.
This research was aided by NIH grants HL 51818 and HL 42384 to R.C.B. and 51880 to R.J.S. and by CFF grants R026 to R.C.B. and MARZLU96PO to J.S.B.
REFERENCES
- 1.Alexander I E, Russell D W, Miller A D. DNA-damaging agents greatly increase the transduction of nondividing cells by adeno-associated virus vectors. J Virol. 1994;68:8282–8287. doi: 10.1128/jvi.68.12.8282-8287.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartlett J S, Quattrocchi K B, Samulski R J. The development of adeno-associated virus as a vector for cancer gene therapy. In: Sobol R E, Scanlon K J, editors. The Internet book of gene therapy: cancer therapeutics. Stamford, Conn: Appleton & Lange; 1995. pp. 27–40. [Google Scholar]
- 3.Brody S L, Crystal R G. Adenovirus-mediated in vivo gene transfer. Ann N Y Acad Sci. 1994;31:90–101. doi: 10.1111/j.1749-6632.1994.tb21705.x. [DOI] [PubMed] [Google Scholar]
- 4.Conrad C K, Allen S S, Afione S A, Reynolds T C, Beck S E, Fee-Maki M, Barrazza-Ortiz X, Adams R, Askin F B, Carter B J, Guggino W B, Flotte T R. Safety of single-dose administration of an adeno-associated virus (AAV)-CFTR vector in the primate lung. Gene Ther. 1996;3:658–668. [PubMed] [Google Scholar]
- 5.Cotten M, Wagner E, Zatloukal K, Phillips S, Curiel D T, Birnstiel M L. High-efficiency receptor-mediated delivery of small and large (48 kilobase) gene constructs using the endosome-disruption activity of defective or chemically inactivated adenovirus particles. Proc Natl Acad Sci USA. 1992;89:6094–6098. doi: 10.1073/pnas.89.13.6094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Croyle M A, Walter E, Janich S, Roessler B J, Amidon G L. Role of integrin expression in adenovirus-mediated gene delivery to the intestinal epithelium. Hum Gene Ther. 1998;9:561–573. doi: 10.1089/hum.1998.9.4-561. [DOI] [PubMed] [Google Scholar]
- 7.Dupuit F, Zahm J M, Pierrot D, Brezillion S, Bonnet N, Imler J L, Pavirani A, Puchelle E. Regenerating cells in human airway surface epithelium represent preferential targets for recombinant adenovirus. Hum Gene Ther. 1995;6:1185–1193. doi: 10.1089/hum.1995.6.9-1185. [DOI] [PubMed] [Google Scholar]
- 8.Eissa N T, Chu C S, Danel C, Crystal R G. Evaluation of the respiratory epithelium of normals and individuals with cystic fibrosis for the presence of adenovirus E1a sequences relevant to the use of E1a-adenovirus vectors for gene therapy for the respiratory manifestations of cystic fibrosis. Hum Gene Ther. 1994;5:1105–1114. doi: 10.1089/hum.1994.5.9-1105. [DOI] [PubMed] [Google Scholar]
- 9.Engelhardt J F, Simon R H, Yang Y, Zepeda M, Weber-Pendleton S, Doranz B, Grossman M, Wilson J M. Adenovirus-mediated transfer of the CFTR gene to lung of nonhuman primates: biological efficacy study. Hum Gene Ther. 1993;4:759–769. doi: 10.1089/hum.1993.4.6-759. [DOI] [PubMed] [Google Scholar]
- 10.Engelhardt J F, Yang Y, Stratford-Perricaudet L D, Allen E D, Kozarsky K, Perricaudet M, Yankaskas J R, Wilson J. Direct gene transfer of human CFTR into human bronchial epithelia of xenografts with E1-deleted adenoviruses. Nat Genet. 1993;4:27–34. doi: 10.1038/ng0593-27. [DOI] [PubMed] [Google Scholar]
- 11.Engelhardt J F, Ye X, Doranz B, Wilson J M. Ablation of E2A in recombinant adenoviruses improves transgene persistence and decreases inflammatory response in mouse liver. Proc Natl Acad Sci USA. 1994;91:6196–6200. doi: 10.1073/pnas.91.13.6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferrari F K, Samulski T, Shenk T, Samulski R J. Second-strand synthesis is a rate-limiting step for efficient transduction by recombinant adeno-associated virus vectors. J Virol. 1996;70:3227–3234. doi: 10.1128/jvi.70.5.3227-3234.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fisher K J, Gao G-P, Weitzman M D, DeMatteo R, Burda J F, Wilson J M. Transduction with recombinant adeno-associated virus for gene therapy is limited by leading-strand synthesis. J Virol. 1996;70:520–532. doi: 10.1128/jvi.70.1.520-532.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flotte T R, Afione S A, Conrad C, McGrath S A, Solow R, Oka H, Zeitlin P L, Guggino W B, Carter B J. Expression of the cystic fibrosis transmembrane conductance regulator from a novel adeno-associated virus promoter. J Biol Chem. 1993;268:3781–3790. [PubMed] [Google Scholar]
- 15.Flotte T R, Afione S A, Conrad C, McGrath S A, Solow R, Oka H, Zeitlin P L, Guggino W B, Carter B J. Stable in vivo expression of the cystic fibrosis transmembrane regulator with an adeno-associated virus vector. Proc Natl Acad Sci USA. 1993;90:10613–10617. doi: 10.1073/pnas.90.22.10613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flotte T R, Solow R, Owens R A, Afione S, Zeitlin P L, Carter B J. Gene expression from adeno-associated virus vectors in airway epithelial cells. Am J Respir Cell Mol Biol. 1992;7:349–356. doi: 10.1165/ajrcmb/7.3.349. [DOI] [PubMed] [Google Scholar]
- 17.Grubb B R, Pickles R J, Ye H, Yankaskas J R, Vick R N, Engelhardt J F, Wilson J M, Johnson L G, Boucher R C. Inefficient gene transfer by adenovirus vector to cystic fibrosis airway epithelia of mice and humans. Nature. 1994;371:802–806. doi: 10.1038/371802a0. [DOI] [PubMed] [Google Scholar]
- 18.Halbert C L, Alexander I E, Wolgamot G M, Miller A D. Adeno-associated virus vectors transduce primary cells much less efficiently than immortalized cells. J Virol. 1995;69:1473–1479. doi: 10.1128/jvi.69.3.1473-1479.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Halbert D H, Cutt J R, Shenk T. Adenovirus early region 4 encodes functions required for efficient DNA replication, late gene expression, and host cell shutoff. J Virol. 1985;56:250–257. doi: 10.1128/jvi.56.1.250-257.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoggan M D, Blacklow N R, Rowe W P. Studies of small DNA viruses found in various adenovirus preparations: physical, biological, and immunological characteristics. Proc Natl Acad Sci USA. 1966;55:1457–1471. doi: 10.1073/pnas.55.6.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang M-M, Hearing P. Adenovirus early region 4 encodes two gene products with redundant effects in lytic infection. J Virol. 1989;63:2605–2615. doi: 10.1128/jvi.63.6.2605-2615.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jetten A M, Yankaskas J R, Stutts M J, Willumsen N J, Boucher R C. Persistence of abnormal chloride conductance regulation in transformed cystic fibrosis epithelia. Science. 1989;244:1472–1475. doi: 10.1126/science.2472008. [DOI] [PubMed] [Google Scholar]
- 23.Jones N, Shenk T. Isolation of adenovirus type 5 host range deletion mutants defective for transformation of rat embryo cells. Cell. 1979;17:683–689. doi: 10.1016/0092-8674(79)90275-7. [DOI] [PubMed] [Google Scholar]
- 24.Knowles M R, Hohnekker K W, Zhou Z, Olsen J C, Noah T L, Hu P-C, Leigh M W, Engelhardt J F, Edwards L J, Jones J R, Grossmann M, Wilson J M, Johnson L G, Boucher R C. A controlled study of transfer by adenoviral-vector-mediated gene transfer in the nasal epithelium of patients with cystic fibrosis. N Engl J Med. 1995;333:823–831. doi: 10.1056/NEJM199509283331302. [DOI] [PubMed] [Google Scholar]
- 25.Kotin R M. Prospects for the use of adeno-associated virus as a vector for human gene therapy. Hum Gene Ther. 1994;5:793–801. doi: 10.1089/hum.1994.5.7-793. [DOI] [PubMed] [Google Scholar]
- 26.Li J, Samulski R J, Xiao X. Role for highly regulated rep gene expression in adeno-associated virus vector production. J Virol. 1997;71:5236–5243. doi: 10.1128/jvi.71.7.5236-5243.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rich D P, Couture L A, Cardoza L M, Guiggio V M, Armentano D, Espino P C, Hehir K, Welsh M J, Smith A E, Gregory R J. Development and analysis of recombinant adenoviruses for gene therapy of cystic fibrosis. Hum Gene Ther. 1993;4:461–476. doi: 10.1089/hum.1993.4.4-461. [DOI] [PubMed] [Google Scholar]
- 28.Rosenfeld M A, Chu C S, Seth P, Danel C, Banks T, Yoneyama K, Yoshimura K, Crystal R G. Gene transfer to freshly isolated human respiratory epithelial cells in vitro using a replication-deficient adenovirus containing the human cystic fibrosis transmembrane conductance regulator cDNA. Hum Gene Ther. 1994;5:331–342. doi: 10.1089/hum.1994.5.3-331. [DOI] [PubMed] [Google Scholar]
- 29.Rosenfeld M A, Yoshimura K, Trapnell B C, Yoneyama K, Rosenthal E R, Dalemans W, Fukayama M, Bargon J, Stier L E, Stratford-Perricaudet L, et al. In vivo transfer of the human cystic fibrosis transmembrane conductance regulator gene to the airway epithelium. Cell. 1992;68:143–155. doi: 10.1016/0092-8674(92)90213-v. [DOI] [PubMed] [Google Scholar]
- 30.Russell D W, Miller A D, Alexander I E. DNA synthesis and topoisomerase inhibitors increase transduction by adeno-associated virus vectors. Proc Natl Acad Sci USA. 1995;92:5719–5723. doi: 10.1073/pnas.92.12.5719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Samulski R J, Chang L-S, Shenk T. Helper-free stocks of recombinant adeno-associated viruses: normal integration does not require viral gene expression. J Virol. 1989;63:3822–3828. doi: 10.1128/jvi.63.9.3822-3828.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sanes J R, Rubenstein J L R, Nicolas J F. Use of a recombinant retrovirus to study post-implantation cell lineage in mouse embryos. EMBO J. 1986;5:3133–3142. doi: 10.1002/j.1460-2075.1986.tb04620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simon R H, Engelhardt J F, Yang Y, Zepeda M, Pendelton S W, Grossman M, Wilson J M. Adenovirus-mediated transfer of the CFTR gene to lung of nonhuman primates: toxicity study. Hum Gene Ther. 1993;4:821–836. doi: 10.1089/hum.1993.4.6-771. [DOI] [PubMed] [Google Scholar]
- 34.Snyder R O, Xiao X, Samulski R J. Production of recombinant adeno-associated virus vectors. In: Dracopoli N, Haines J, Krof B, Moir D, Seidman C, Seidman J S, editors. Current protocols in human genetics. New York, N.Y: John Wiley & Sons Ltd.; 1996. pp. 12.1.1–12.2.23. [Google Scholar]
- 35.Wu R, Yankaskas J R, Cheng E, Knowles M R, Boucher R C. Growth and differentiation of human nasal epithelial cells in culture: serum-free, hormone-supplemented medium and proteoglycan synthesis. Am Rev Respir Dis. 1985;132:311–320. doi: 10.1164/arrd.1985.132.2.311. [DOI] [PubMed] [Google Scholar]
- 36.Yang Q, Chen F, Trempe J P. Characterization of cell lines that inducibly express the adeno-associated virus Rep proteins. J Virol. 1994;68:4847–4856. doi: 10.1128/jvi.68.8.4847-4856.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang Y, Nunes F A, Berencsi K, Gönczöl E, Engelhardt J F, Wilson J M. Inactivation of E2a in recombinant adenovirus improves the prospect for gene therapy in cystic fibrosis. Nat Genet. 1994;7:362–369. doi: 10.1038/ng0794-362. [DOI] [PubMed] [Google Scholar]
- 38.Yankaskas J R, Haizlip J P, Conrad M, Koval D, Lazarowski E, Paradiso A M, Rinehart C A, Jr, Sarkadi B, Schlegel R, Boucher R C. Papilloma virus immortalized tracheal epithelial cells retain a well-differentiated phenotype. Am J Physiol. 1993;264:C1219–C1230. doi: 10.1152/ajpcell.1993.264.5.C1219. [DOI] [PubMed] [Google Scholar]
- 39.Yei S, Mittereder N, Wert S, Whitsett J A, Wilmott R W, Trapnell B C. In vivo evaluation of the safety of adenovirus-mediated transfer of the human cystic fibrosis transmembrane conductance regulator cDNA to the lung. Hum Gene Ther. 1994;5:731–744. doi: 10.1089/hum.1994.5.6-731. [DOI] [PubMed] [Google Scholar]
- 40.Zabner J, Couture L A, Gregory R J, Graham S M, Smith A E, Welsh M J. Adenovirus-mediated gene transfer transiently corrects the chloride transport defect in nasal epithelia of patients with cystic fibrosis. Cell. 1993;75:207–216. doi: 10.1016/0092-8674(93)80063-k. [DOI] [PubMed] [Google Scholar]
- 41.Zabner J, Couture L A, Smith A E, Welsh M J. Correction of cAMP-stimulated fluid secretion in cystic fibrosis airway epithelia: efficiency of adenovirus-mediated gene transfer in vitro. Hum Gene Ther. 1994;5:585–593. doi: 10.1089/hum.1994.5.5-585. [DOI] [PubMed] [Google Scholar]