Abstract
Induction of effective immune attack on cancer cells in patients requires conversion of weak tumor antigens into strong immunogens. Our strategy employs genetic technology to create DNA vaccines containing tumor antigen sequences fused to microbial genes. The fused microbial protein engages local CD4+ T cells to provide help for anti-tumor immunity, and to reverse potential regulation. In this review, we focus on induction of CD8+ T cells able to kill target tumor cells. The DNA vaccines incorporate tumor-derived peptide sequences fused to an engineered domain of tetanus toxin. In multiple models, this design induces strong CD8+ T-cell responses, able to suppress tumor growth. For clinical relevance, we have used “humanized” mice expressing HLA-A2, successfully inducing cytolytic T-cell responses against a range of candidate human peptides. To overcome physical restriction in translating to patients, we have used electroporation. Clinical trials of patients with cancer are showing induction of responses, with preliminary indications of suppression of tumor growth and evidence for clinically manageable concomitant autoimmunity.
Keywords: DNA vaccines, Cytotoxic T cells, Prostate cancer, Carcinoembryonic antigen, PIVAC 10
Introduction
Searching for effective cancer vaccines seems almost like seeking the Holy Grail. After many false trails, we have come to realize that successful therapeutic vaccination of patients with a weakened or tolerized immune system requires a level of ingenuity beyond that for prophylactic vaccines. Fortunately, modern molecular technology, combined with increasing knowledge of immunological pathways, offers the tools to achieve this goal. Those of us who chose to develop DNA vaccines are in strong position, since it is possible to incorporate multiple components to activate and direct selected immune effector pathways. The initial concern that promising data from mouse models would not apply to patients has been explained and has been largely overcome by modifications of vaccine delivery, especially using electroporation.
Essential components of DNA fusion vaccines
Plasmid backbone
Bacterial DNA is known to differ from mammalian DNA in the level and methylation status of CpG-containing motifs. The development of CpG-based oligonucleotides as adjuvants, in most cases stabilized as phosphorothioate analogs, led to the concept that the adjuvant activity of plasmid backbones also relied on these sequences and that their effect was mediated via TLR9 [1]. This conclusion was modified by the observation that TLR9−/− mice responded well to DNA vaccines [2]. It is now clear that plasmid DNA activates multiple pathways via endosomal and cytosolic sensors [3] with the outcome being stimulation of innate immunity. The pathways involved converge on induction of interferon α/β, together with activation of the inflammasome AIM2, generating IL-1β and IL-18 [4]. Clearly, double stranded DNA is a major activator of innate immunity, and DNA vaccines have a built-in adjuvant.
Tumor antigens
One of the frustrations of antibody therapy is the relative lack of target proteins expressed on the tumor cell surface and therefore susceptible to antibody attack. The majority of known target molecules are intracellular and are only displayed as MHC Class I-associated peptides. The main goal therefore has to be to induce peptide-specific CD8+ T cells able to recognize and kill tumor cells. Since ~40% of the Caucasian population carries the HLA-A0201* haplotype, the focus has been on finding peptides which bind to this, and algorithms have been developed for identification of candidate sequences within tumor antigens [5]. The other point, sometimes neglected, is that the peptide must be processed and presented by the tumor cell. Many clinical trials of peptides as vaccines have been carried out, with limited success, since responses, if induced, tended to be transient [6]. It became clear that exogenous peptides alone fail to activate effective levels of CD8+ T cells and are poor at inducing memory T cells.
T-cell help
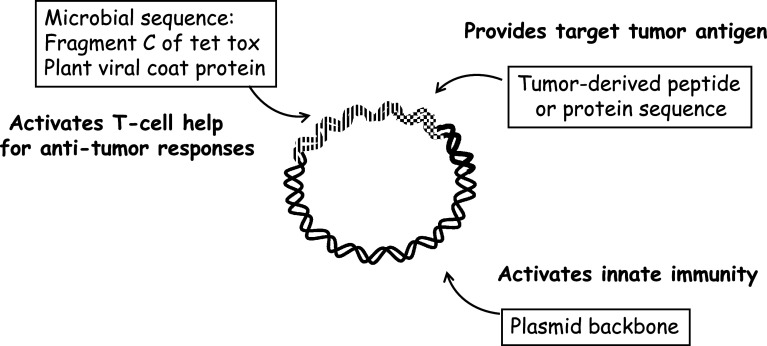
Pre-clinical studies revealed one reason for failure was a requirement for T-cell help [7]. This can be provided either by co-injecting a CD4+ T-cell inducing peptide such as PADRE or by using long peptides, which have the advantage of both potentially activating T-cell help and also being preferentially processed by dendritic cells [8]. Our approach was always to provide high levels of T-cell help, achieved in most of our designs by fusing a gene derived from tetanus toxin (TT) to the candidate peptide sequence [9]. This is highly effective but is not the only microbial sequence which can be used. We have also explored plant viral coat proteins in this setting [10] and, although not yet in clinical trials, in our view, these have considerable potential. The three essential components of our DNA fusion vaccines are shown in Fig. 1.
Fig. 1.
The p. DOM-epitope plasmid design incorporates three components: the backbone of bacterial DNA, the tumor-derived target peptide (or protein) sequence, and microbial sequences to activate T-cell help, derived from Tetanus Toxin or from plant viral coat proteins
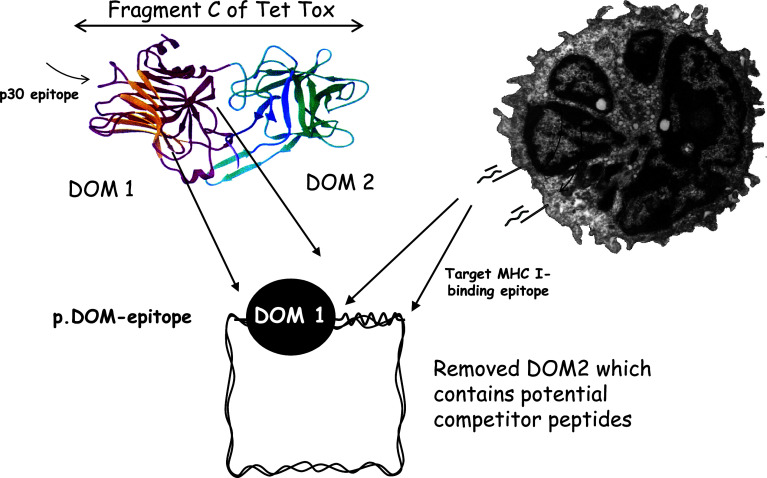
For induction of CD8+ cytolytic T cells, we engineered the Fragment C of TT to produce a single domain and fused the tumor peptide sequence to the C-terminus, thereby promoting its processing and presentation (Fig. 2). We selected the domain sequence on the basis of it contributing no identifiable HLA-0201*-binding peptides that could compete with the tumor peptide [11]. Immunodominance is a real concern for tumor vaccines, where target peptides may be weak. It appears that competitor peptides able to induce high levels of CD8+ T cells can lead to killing of antigen-presenting cells before a weaker peptide can activate a response [12]. Careful selection of molecules aimed to provide T-cell help is therefore required.
Fig. 2.
The design of the p.DOM-epitope vaccines aimed to induce anti-tumor CD8+ T cells. The Fragment C of tetanus toxin has been engineered to include only the N-terminal domain (DOM 1) which contains the promiscuous p30 epitope able to induce T-cell help. The second domain (DOM 2) that contains potentially competitive MHC Class I (HLA-A2)-binding peptides has been removed. The tumor-derived target peptide sequence is then placed at the C-terminus of DOM 1
Our main design named p.DOM-epitope (Fig. 2) has now been tested in ~20 models [9] with consistent success in inducing durable CD8+ T-cell responses able to suppress tumor. When compared with exogenous peptide (+PADRE and adjuvant), DNA delivery was superior [13]. The p.DOM-epitope design is also generally superior to the full length sequence ± added DOM [14] (and unpublished data). The vaccine design is also effective in inducing CD8+ T-cell responses in tolerant settings [15]. We have considered the question of whether there might be a need to induce tumor-specific CD4+ T cells, either for support for long-term CD8+ T-cell responses or to assist access to a tumor site. The pre-clinical models do not indicate that this is required, but we are developing vaccines to induce CD4+ T-cell responses for further testing. One possibility is that having a vaccine that induces a CD4+ T-cell response might have the undesirable outcome of activating regulatory T cells, and this will be investigated.
Vaccination of patients with cancer
Peptide selection and testing
Algorithms and testing of spontaneous T-cell responses in patients have identified many candidate peptides with potential for immune targeting [5]. We have selected a range of these and placed them in the p.DOM-epitope format. To test immunogenicity, we have used the invaluable HHD mice which carry a transgene encoding the human HLA-A2 restriction element [9]. While assessment of responding CD8+ T-cell responses is straightforward, it is more difficult to measure the ability of such T cells to kill human targets. The reason for this is that the mouse CD8 molecule does not interact with the human HLA molecule, and T cells have to be of very high affinity to overcome the lack of this interaction. Our approach has been to transduce the HHD molecule into the human target cell, and using this approach, we have been able to demonstrate effective killing.
Our initial focus has been on a protein expressed in prostate cancer, prostate-specific membrane antigen (PSMA), using the target peptide PSMA27 [16]. A second study has been of the Wilms tumor antigen (WT1), a protein expressed by several tumors, including chronic myeloid leukemia, using two target peptides, WT1-37 and WT1-126 [13]. In all cases, CD8+ T cells were readily induced in HHD mice and these were able to kill transduced target cells [17]. For each antigen, the p.DOM-epitope design was superior to the full length design.
Vaccine delivery by electroporation
Our pre-clinical data had shown that a critical influence on DNA vaccine performance is the volume injected [18]. In mice, 50 μl is the standard volume for intramuscular injection, and this cannot be reduced to <25 μl without a dramatic fall in response. Concentration of DNA is a less important variable. It appears that the disruption of muscle fibers by the hydrostatic pressure may be the key to either more efficient transfection or activation of inflammation. If calculated on a weight for weight basis, scaling up for patients would require >100 ml to be injected, clearly not feasible, but this requirement can be overcome by electroporation. Electroporation improves antibody and CD4+ T-cell responses [19]. We confirmed this and showed that a particularly effective combination was to prime with naked DNA and boost with electroporation [18]. Several groups have developed clinically acceptable electroporators, and we have worked with Inovio to test this strategy in the clinic.
Clinical trial of patients with prostate cancer
Building on our pre-clinical data, we began testing the pDOM-epitope vaccine concept in patients with solid tumors. We targeted the HLA A0201*-restricted peptide PSMA27 in a phase I/II study, recruiting patients who had progressed after radical treatment for prostate cancer [20]. Patients were identified by rising PSA levels in the absence of radiological evidence of metastatic disease. The trial recruited in two parallel arms, in which 3× monthly DNA injections were given as an intramuscular injection, with or without electroporation (EP). In both arms, escalating doses of DNA were tested in cohorts of 5 patients: 800, 1,600, and 3,200 μg/dose in the DNA alone arm, 400, 800, and 1,600 μg in the DNA/EP arm. At 6 and 12 months, boosting was given and cross-over was permitted. The vaccine was very well tolerated, and toxicity was mainly limited to local injection site reactions [20]. Electroporation also was well tolerated in our patients and with 150 applications no safety concerns were identified, in terms of either subjective tolerance or local or systemic toxicity. In particular, no significant changes in LDH or creatine kinase were observed either immediately after, at 3 or 5 days post-injection, or at repeated delivery.
We were able to assess three aspects of the immune response: antibody and CD4+ T-cell responses against the DOM protein, which indicate performance of the vaccine in each patient, and tumor-relevant CD8+ T-cell responses against the target PSMA27 peptide. Overall, humoral IgG responses to the DOM protein were substantially boosted by EP [20]. In patients who began vaccination with DNA alone, boosting with EP was able to rescue some of the humoral responses, but not to the same level as for patients who were given DNA with EP on each occasion. In total, 21/30 (70%) of patients developed significant humoral responses. Rather dramatically, all but one of the patients (30/31 (97%) evaluable patients) mounted significant increases in T-cell responses to DOM, readily detectable ex vivo. There was a suggestion for more rapid responses post electroporation, but no clear effect of either dose or delivery strategy was identified. Ideally, a large-scale clinical trial of the effects of EP on immune responses and on clinical outcome should be carried out, and this will be important for prophylactic vaccines. For cancer, the observation of stimulation of antibody and CD4+ T-cell responses, with a possible increase in the rate of response, may be sufficient to include EP in DNA vaccine delivery.
We considered that CD8+ T-cell responses at the time points used would most likely be of the central memory subset therefore all measurements were made following 1 week of restimulation ex vivo. Overall 17/31 (55%) patients showed significant and specific responses to PSMA27 peptide in terms of IFNγ production by ELISPOT. Similar to CD4+ T-cell responses, CD8+ T-cell responses appeared more quickly in the EP arm, though overall the frequency and level of CD8 T-cell responses were comparable between delivery strategies by week 16. Dose appeared again not to affect immune responses.
In terms of clinical effects, serum PSA levels proved to be erratic and less informative than expected, perhaps in line with other studies [21], [22]. The sample size is too small to assess effects on survival; however, a trend of delayed time to next treatment is emerging, compared with a cohort of HLA-A0201-negative patients who fulfilled other recruitment criteria but were not vaccinated. Our plan is to assess the effect in a randomized study, with increased numbers of patients.
A second study with the DOM-epitope design was conducted in patients with carcinoembryonic antigen (CEA)-expressing malignancies [23]. The peptide target was the HLA A0201*- restricted epitope of the CEA molecule, CAP-1 [24]. The study allowed recruitment based on immunohistochemical expression of CEA in the tissue, irrespective of tumor type. Twenty-seven patients were recruited, 15 with end stage disease, 12 without measurable disease. For the latter group, an estimated risk of recurrence at 5 years of about 50% was the key entry criterion. Six doses of vaccine were given at 1, 2, 4, 6, 8, and 12 weeks at a single dose of 1 mg.
Overall, the volume of disease affected induction of vaccine-specific immune responses against the DOM protein: 92% of patients without measurable disease developed cellular immune responses, but only 53% of those with end stage disease. CAP-1 responses were detected in about half of the overall responders, consistent with the data from the prostate study. In contrast to the prostate study, ~50% of patients developed an unexpectedly frequent but mild and self limiting side effects, mainly affecting the bowel. Diarrhea was associated with the frequency and durability of T-cell responses to vaccine-delivered DOM protein. Minor decreases in serum CEA levels were observed only in patients with diarrhea and, importantly, survival of patients with end stage disease with diarrhea was significantly prolonged compared with those without.
CD8+ T-cell responses to CAP-1 in the blood did not correlate either with immune responses to DOM or with outcome. In contrast, pre-clinical models have shown parallelism between CD4+ T-cell responses against DOM epitopes and CD8+ T-cell responses against tumor epitopes, even in tolerant settings (14). The apparent lack of this linkage in a few patients is likely to be due to the restriction of our assays to cells in the blood, with no information on T cells in the spleen or in tissue sites. No biopsy samples were available from the patients during this study. Further studies are ongoing to explore this observation, specifically of the bowel homing properties of the CAP-1-specific T cells. Detailed clinical data are being prepared for publication.
In summary, vaccination in CEA-expressing patients, with a range of organ involvement appears to have generated an unexpected diarrheal response, possibly due to autoimmunity against gut-associated CEA. A similar outcome was seen in a different context where vaccination was combined with removal of regulatory T cells [25] or where CEA specific T cells are given by adoptive transfer [26]. Further studies are obviously required but this is an intriguing indication that, at least for this antigen, transient autoimmunity might be a sign of an anti-tumor effect.
Acknowledgments
This work was supported by Cancer Research UK, Experimental Medicine Cancer Centre and Leukaemia and Lymphoma Research. We thank Lynsey Block for help with the preparation of the manuscript.
Footnotes
This paper is a Focussed Research Review based on a presentation given at the Tenth International Conference on Progress in Vaccination against Cancer (PIVAC 10), held in St. Catharine’s College, Cambridge, UK, on September 27–30th, 2010. It is part of a CII series of Focussed Research Reviews and meeting report.
References
- 1.Krieg AM. CpG motifs in bacterial DNA and their immune effects. Annu Rev Immunol. 2002;20:709–760. doi: 10.1146/annurev.immunol.20.100301.064842. [DOI] [PubMed] [Google Scholar]
- 2.Spies B, Hochrein H, Vabulas M, Huster K, Busch DH, Schmitz F, Heit A, Wagner H. Vaccination with plasmid DNA activates dendritic cells via toll-like receptor 9 (TLR9) but functions in TLR9-deficient mice. J Immunol. 2003;171(11):5908–5912. doi: 10.4049/jimmunol.171.11.5908. [DOI] [PubMed] [Google Scholar]
- 3.Wang Z, Choi MK, Ban T, Yanai H, Negishi H, Lu Y, Tamura T, Takaoka A, Nishikura K, Taniguchi T. Regulation of innate immune responses by DAI (DLM-1/ZBP1) and other DNA-sensing molecules. Proc Natl Acad Sci USA. 2008;105(14):5477–5482. doi: 10.1073/pnas.0801295105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burckstummer T, Baumann C, Bluml S, Dixit E, Durnberger G, Jahn H, Planyavsky M, Bilban M, Colinge J, Bennett KL, Superti-Furga G. An orthogonal proteomic-genomic screen identifies AIM2 as a cytoplasmic DNA sensor for the inflammasome. Nat Immunol. 2009;10(3):266–272. doi: 10.1038/ni.1702. [DOI] [PubMed] [Google Scholar]
- 5.Rammensee H, Bachmann J, Emmerich NP, Bachor OA, Stevanovic S. SYFPEITHI: database for MHC ligands and peptide motifs. Immunogenetics. 1999;50(3–4):213–219. doi: 10.1007/s002510050595. [DOI] [PubMed] [Google Scholar]
- 6.Rezvani K, Yong AS, Mielke S, Jafarpour B, Savani BN, Le RQ, Eniafe R, Musse L, Boss C, Kurlander R, Barrett AJ. Repeated PR1 and WT1 peptide vaccination in Montanide-adjuvant fails to induce sustained high-avidity, epitope-specific CD8+ T cells in myeloid malignancies. Haematologica. 2011;96(3):432–440. doi: 10.3324/haematol.2010.031674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Janssen EM, Lemmens EE, Wolfe T, Christen U, von Herrath MG, Schoenberger SP. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature. 2003;421(6925):852–856. doi: 10.1038/nature01441. [DOI] [PubMed] [Google Scholar]
- 8.Melief CJ, van der Burg SH. Immunotherapy of established (pre)malignant disease by synthetic long peptide vaccines. Nat Rev Cancer. 2008;8(5):351–360. doi: 10.1038/nrc2373. [DOI] [PubMed] [Google Scholar]
- 9.Rice J, Ottensmeier CH, Stevenson FK. DNA vaccines: precision tools for activating effective immunity against cancer. Nat Rev Cancer. 2008;8(2):108–120. doi: 10.1038/nrc2326. [DOI] [PubMed] [Google Scholar]
- 10.Savelyeva N, Munday R, Spellerberg MB, Lomonossoff GP, Stevenson FK. Plant viral genes in DNA idiotypic vaccines activate linked CD4+ T-cell mediated immunity against B-cell malignancies. Nat Biotechnol. 2001;19(8):760–764. doi: 10.1038/90816. [DOI] [PubMed] [Google Scholar]
- 11.Stevenson FK, Ottensmeier CH, Johnson P, Zhu D, Buchan SL, McCann KJ, Roddick JS, King AT, McNicholl F, Savelyeva N, Rice J. DNA vaccines to attack cancer. Proc Natl Acad Sci USA. 2004;101(Suppl 2):14646–14652. doi: 10.1073/pnas.0404896101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen W, McCluskey J. Immunodominance and immunodomination: critical factors in developing effective CD8+ T-cell-based cancer vaccines. Adv Cancer Res. 2006;95:203–247. doi: 10.1016/S0065-230X(06)95006-4. [DOI] [PubMed] [Google Scholar]
- 13.Chaise C, Buchan SL, Rice J, Marquet J, Rouard H, Kuentz M, Vittes GE, Molinier-Frenkel V, Farcet JP, Stauss HJ, Delfau-Larue MH, Stevenson FK. DNA vaccination induces WT1-specific T-cell responses with potential clinical relevance. Blood. 2008;112(7):2956–2964. doi: 10.1182/blood-2008-02-137695. [DOI] [PubMed] [Google Scholar]
- 14.Rice J, Dossett ML, Ohlen C, Buchan SL, Kendall TJ, Dunn SN, Stevenson FK, Greenberg PD. DNA fusion gene vaccination mobilizes effective anti-leukemic cytotoxic T lymphocytes from a tolerized repertoire. Eur J Immunol. 2008;38(8):2118–2130. doi: 10.1002/eji.200838213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rice J, Buchan S, Dewchand H, Simpson E, Stevenson FK. DNA fusion vaccines induce targeted epitope-specific CTLs against minor histocompatibility antigens from a normal or tolerized repertoire. J Immunol. 2004;173(7):4492–4499. doi: 10.4049/jimmunol.173.7.4492. [DOI] [PubMed] [Google Scholar]
- 16.Murphy G, Tjoa B, Ragde H, Kenny G, Boynton A. Phase I clinical trial: T-cell therapy for prostate cancer using autologous dendritic cells pulsed with HLA-A0201-specific peptides from prostate-specific membrane antigen. Prostate. 1996;29(6):371–380. doi: 10.1002/(SICI)1097-0045(199612)29:6<371::AID-PROS5>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 17.Stevenson FK, Ottensmeier CH, Rice J. DNA vaccines against cancer come of age. Curr Opin Immunol. 2010;22(2):264–270. doi: 10.1016/j.coi.2010.01.019. [DOI] [PubMed] [Google Scholar]
- 18.Buchan S, Gronevik E, Mathiesen I, King CA, Stevenson FK, Rice J. Electroporation as a “prime/boost” strategy for naked DNA vaccination against a tumor antigen. J Immunol. 2005;174(10):6292–6298. doi: 10.4049/jimmunol.174.10.6292. [DOI] [PubMed] [Google Scholar]
- 19.Gronevik E, Tollefsen S, Sikkeland LI, Haug T, Tjelle TE, Mathiesen I. DNA transfection of mononuclear cells in muscle tissue. J Gene Med. 2003;5(10):909–917. doi: 10.1002/jgm.416. [DOI] [PubMed] [Google Scholar]
- 20.Low L, Mander A, McCann K, Dearnaley D, Tjelle T, Mathiesen I, Stevenson F, Ottensmeier CH. DNA vaccination with electroporation induces increased antibody responses in patients with prostate cancer. Hum Gene Ther. 2009;20(11):1269–1278. doi: 10.1089/hum.2009.067. [DOI] [PubMed] [Google Scholar]
- 21.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB, Xu Y, Frohlich MW, Schellhammer PF. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363(5):411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 22.Small EJ, Schellhammer PF, Higano CS, Redfern CH, Nemunaitis JJ, Valone FH, Verjee SS, Jones LA, Hershberg RM. Placebo-controlled phase III trial of immunologic therapy with sipuleucel-T (APC8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer. J Clin Oncol. 2006;24(19):3089–3094. doi: 10.1200/JCO.2005.04.5252. [DOI] [PubMed] [Google Scholar]
- 23.Ottensmeier CHH, McCann KJ, et al. (2010) Clinical and immunological responses to a DNA fusion vaccine in patients with carcinoembryonic antigen–expressing tumors—a Cancer Research UK phase I/II study. American Society for Clinical Oncology (ASCO) annual meeting abstract 2579
- 24.Tsang KY, Zaremba S, Nieroda CA, Zhu MZ, Hamilton JM, Schlom J. Generation of human cytotoxic T cells specific for human carcinoembryonic antigen epitopes from patients immunized with recombinant vaccinia-CEA vaccine. J Natl Cancer Inst. 1995;87(13):982–990. doi: 10.1093/jnci/87.13.982. [DOI] [PubMed] [Google Scholar]
- 25.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbe C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parkhurst MR, Yang JC, Langan RC, Dudley ME, Nathan DA, Feldman SA, Davis JL, Morgan RA, Merino MJ, Sherry RM, Hughes MS, Kammula US, Phan GQ, Lim RM, Wank SA, Restifo NP, Robbins PF, Laurencot CM, Rosenberg SA. T cells targeting carcinoembryonic antigen can mediate regression of metastatic colorectal cancer but induce severe transient colitis. Mol Ther. 2011;19(3):620–626. doi: 10.1038/mt.2010.272. [DOI] [PMC free article] [PubMed] [Google Scholar]