Abstract
Background
Ipilimumab can result in durable clinical responses among patients with advanced melanoma. However, no predictive marker of clinical activity has yet been identified. We provide preliminary data describing the correlation between immunological parameters and response/survival among patients with advanced melanoma who received ipilimumab 10 mg/kg in an expanded access programme.
Methods
Patients received ipilimumab 10 mg/kg every 3 weeks (Q3W) for four doses (induction) and Q12W from week 24 (W24) as maintenance therapy. Tumor assessments were conducted Q12W. Expression of inducible T cell costimulator (ICOS) on CD4+ and CD8+ T cells was assessed at baseline, W7, W12 and W24, and the ratio between absolute neutrophils (N) and lymphocytes (L) determined at baseline, W4, W7 and W10.
Results
Median overall survival among 27 patients was 9.6 months (95 % CI 3.2–16.1), with 3- and 4-year survival rates of 20.4 %. Five patients survived >4 years. Patients with an increase in the number of circulating ICOS+ T cells at W7 were more likely to experience disease control and have improved survival. An N/L ratio below the median at W7 and W10 was also associated with better survival compared with an N/L ratio above the median.
Conclusions
Ipilimumab can induce long-term survival benefits in heavily pretreated patients with metastatic melanoma. Changes in the number of circulating ICOS+ T cells or N/L ratio during ipilimumab treatment may represent early markers of response. However, given the limited sample size, further investigation is required.
Keywords: Biomarker, Expanded access programme, Ipilimumab, Long-term survival, Metastatic melanoma
Introduction
Melanoma is a highly aggressive malignancy and the leading cause of skin cancer-related deaths worldwide [1, 2]. Patients with advanced stage melanoma have a poor prognosis, and median survival with standard chemotherapy ranges from 6 to 9 months, with a 1-year mortality rate of around 75 % [3, 4]. Historically, treatments for advanced melanoma were limited to cytotoxic chemotherapy agents such as dacarbazine (DTIC) or interleukin-2. Although objective responses have been reported with these agents, no systemic therapy was previously shown to provide a significant survival benefit over palliative care [5, 6].
A recent breakthrough in the treatment for advanced melanoma was the development of ipilimumab, a fully human monoclonal antibody against the inhibitory surface molecule cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4) [7]. By blocking CTLA-4, ipilimumab enables continued T cell activation and proliferation, thereby enhancing endogenous antitumor responses [3, 7]. Ipilimumab was approved in the European Union in 2011 for pretreated adult patients with advanced (unresectable or metastatic) melanoma and in the United States for both first- and second-line treatment for advanced melanoma. In phase III trials, ipilimumab nearly doubled the 1- and 2-year survival rates of patients with advanced melanoma compared with the comparator arm (gp100 vaccine) [8] and combination therapy with ipilimumab and DTIC significantly improved overall survival (OS) compared with DTIC alone [9].
Because ipilimumab has a novel immunotherapeutic mechanism of action (MoA), few markers have been identified that can be used to guide patient selection or serve as early markers of response and/or survival. Identifying prognostic biomarkers remains an important goal of immuno-oncology to maximize treatment benefits and minimize potential risks. Putative biomarkers for response to ipilimumab therapy have been investigated prospectively in clinical trials [10] and retrospectively using data from clinical studies [11, 12] and expanded access programmes (EAPs) [13–15]. Most of these studies have examined immune response parameters in peripheral blood samples, including absolute lymphocyte counts (ALCs) [13–15], tumor antigen NY-ESO-1 responses [12], and the expression of immune markers such as inducible T cell co-stimulator (ICOS) and transcription factors eomesodermin and forkhead box protein P3 on CD4+ and CD8+ T cells [11, 15]. The identification and validation of putative markers that can predict treatment outcomes with ipilimumab remain an important focus of research.
The European EAP is a valuable source of information regarding ipilimumab treatment administered outside of the clinical trial setting [13, 16–18]. Initially designed to provide patients with ipilimumab at a dose of 10 mg/kg, the EAP was amended in 2008 to administer a 3 mg/kg dose based on the current label [8].We previously reported significant clinical efficacy with ipilimumab in 27 heavily pretreated patients with advanced melanoma who were enrolled in the earlier EAP and received ipilimumab at a dose of 10 mg/kg at the University Hospital of Siena. With a median 8.5-month follow-up, median OS was 9 months and 1- and 2-year survival rates were 34.8 and 23.5 %, respectively. In this interim analysis, increases in ALC during ipilimumab treatment were associated with improved prognosis [13]. Here, we report long-term survival data from these 27 patients and provide further analyses of immunological parameters potentially associated with clinical response and survival. These data were first presented, in part, at the 2012 European Society of Medical Oncology Congress [19].
Methods
Patients
This was a retrospective analysis of data from 27 patients who received ipilimumab treatment at the University Hospital of Siena through the European EAP. Patients eligible for the programme had life-threatening unresectable Stage III or Stage IV melanoma and had failed or were intolerant to at least one prior systemic treatment. Compassionate use ipilimumab was available on physicians’ request where no alternative treatment option was available. An Eastern Co-operative Oncology Group (ECOG) performance status of 0, 1 or 2 was required, and an interval of at least 28 days since treatment with chemotherapy, biochemotherapy, surgery, radiation or immunotherapy recommended. The EAP was approved by a local ethics committee, and all participating patients provided signed informed consent before enrollment.
Study design
Patients included in this report were treated with ipilimumab through the European EAP as previously described [13]. During the induction phase, ipilimumab 10 mg/kg was administered intravenously over 90 min at weeks 1, 4, 7 and 10. Eligible patients received maintenance therapy every 12 weeks from week 24 (W24), as tolerated, for as long as the treating physician believed that the patient would benefit from treatment. Tumor assessments were conducted at baseline, W12, W24 and every 12 weeks thereafter, and classified according to modified World Health Organization criteria [8]. Clinical response was defined as follows: a complete response (CR; disappearance of all index lesions), partial response [PR; ≥50 % decrease from baseline in the sum of the product of diameters (SPD) of defined index lesions], progressive disease (PD; ≥25 % increase from the smallest recorded SPD of defined index lesions) or stable disease (SD; criteria not met for CR or PR, in the absence of PD). Disease control was defined as SD lasting ≥3 months, or a PR, or CR. Patients were considered for retreatment with ipilimumab in the EAP if they demonstrated disease progression following initial disease control in response to induction therapy. All patients were monitored continuously for safety, and adverse events (AEs), including immune-related AEs, were graded according to the Common Terminology Criteria for Adverse Events, version 3.0.
Peripheral blood mononuclear cells were collected and available from a subset of patients at baseline and at weeks 7, 12 and 24, and analyzed by flow cytometry for ICOS expression on CD4+ and CD8+ T lymphocytes. The ratio between absolute neutrophil (N) and lymphocyte (L) counts was determined at baseline and at weeks 4, 7 and 10.
Statistical analysis
Patient and disease characteristics were analyzed using descriptive statistics. Discrete variables were expressed as relative frequencies (percentages) and continuous variables as median and range. OS was estimated using Kaplan–Meier analysis and expressed as a median value with corresponding two-sided 95 % confidence interval (CI), and standard error was calculated for survival rates. Paired t tests were used to compare ICOS+ lymphocyte counts at W7, W12 and W24 with baseline. Survival distributions according to circulating ICOS+ T cells at W7, and for patients with N/L ratios above or below the median value at W7 and W10, were compared using the log-rank (Mantel–Cox) test.
Results
Patient characteristics and treatment
Of the 27 patients treated with ipilimumab 10 mg/kg at the University Hospital of Siena, 2 patients (7 %) had Stage III and 25 (93 %) had Stage IV metastatic melanoma, of which 21 patients had M1c stage disease. All patients had an ECOG status of either 0 or 1 and had received a median of 3 prior systemic therapies (range 1–5) for advanced disease. The study population included 23 patients (85 %) with cutaneous melanoma, three patients with uveal melanoma and 1 patient with mucosal melanoma. All patients received at least one dose of ipilimumab 10 mg/kg, and 21 patients (78 %) completed all four doses of induction therapy. Eleven patients (41 %) went on to receive maintenance therapy with ipilimumab 10 mg/kg. Baseline patient and disease characteristics are provided in Table 1.
Table 1.
Baseline patient characteristics among all patients (N = 27)
| Characteristic | N = 27 |
|---|---|
| Median age, years (range) | 55 (23–77) |
| Gender, n (%) | |
| Male | 14 (52) |
| Female | 13 (48) |
| Melanoma diagnosis | |
| Cutaneous | 23 (85) |
| Uveal | 3 (11) |
| Mucosal | 1 (4) |
| M stage, n (%) | |
| M0 Stage III | 2 (7) |
| M1a | 3 (11) |
| M1b | 1 (4) |
| M1c | 21 (78) |
| ECOG performance status, n (%) | |
| 0 | 15 (56) |
| 1 | 12 (44) |
| LDH >1 × ULN, N (%) | 11 (41) |
| Number of prior therapies, median (range) | 3 (1–5) |
| Types of prior therapy, n (%)a | |
| Surgery | 12 (44) |
| Radiotherapy | 9 (33) |
| Chemotherapy | 25 (93) |
| Immunotherapy | 11 (41) |
| Chemotherapy + immunotherapy | 2 (7) |
aPatients may have received more than one type of prior therapy
LDH lactate dehydrogenase, ULN upper limit of normal
Long-term efficacy and safety
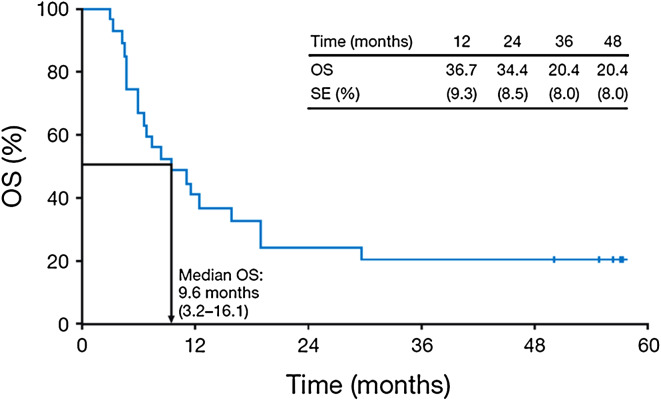
With a median follow-up of 9.6 months, median OS for all 27 patients was 9.6 months (95 % CI 3.2–16.1; Fig. 1). The 3- and 4-year survival rates were both 20.4 %, and 5 patients had long-term survival of at least 4 years (maximum: 57.2 months at the time of manuscript preparation). Among the 5 patients with long-term survival, 4 were still receiving maintenance treatment with ipilimumab 10 mg/kg as of December 2012 including 2 patients who were retreated following tumor progression (Fig. 2). Baseline characteristics of these 5 patients are provided in Table 2.
Fig. 1.
Kaplan–Meier analysis of OS for 27 patients with metastatic melanoma treated with ipilimumab 10 mg/kg. SE standard error
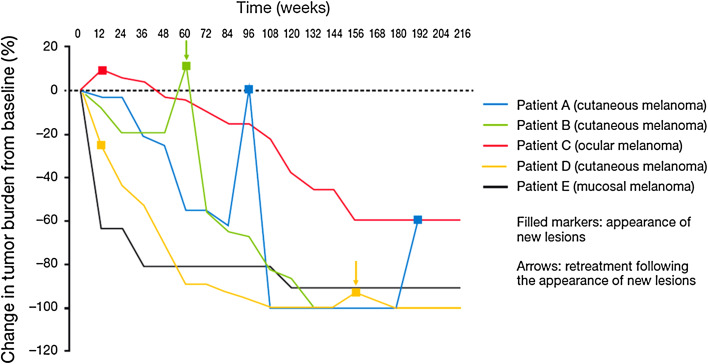
Fig. 2.
Tumor response patterns among five patients with long-term survival (>4 years) following treatment with ipilimumab 10 mg/kg. Patient A had a PR at W48 then developed new subcutaneous lesions at W96. These lesions had resolved by W108 when the patient reached a CR that lasted until W192, at which point a further subcutaneous lesion was identified. Patient B had durable SD until W60 when new subcutaneous lesions were detected. The patient was retreated with ipilimumab 10 mg/kg and subsequently achieved a CR, ongoing at W204. Patient C had PD at W12, with the appearance of subcutaneous and soft tissue lesions. By W24, the patient’s disease had stabilized, culminating in a PR at W120, ongoing at W216. Patient D had PD at W12 after developing new liver lesions. Shrinkage of existing lesions resulted in a pathologic CR of cutaneous lesions at W56 and of liver lesions at W102 [13]. Patient D subsequently developed new cutaneous lesions and was retreated with ipilimumab 3 mg/kg within the amended EAP from W156, achieving an eventual CR at W180, ongoing at W216 in the absence of further treatment. Patient E had a PR in lung lesions at W12 [13] with a continued slow, steady shrinkage of tumor load until W120, after which there were no further changes
Table 2.
Baseline characteristics of 5 patients with long-term survival
| Characteristic | Patient A | Patient B | Patient C | Patient D | Patient E |
|---|---|---|---|---|---|
| Age | 36 | 49 | 47 | 61 | 50 |
| Gender | Female | Female | Male | Male | Female |
| Melanoma diagnosis | Cutaneous | Cutaneous | Ocular | Cutaneous | Mucosal |
| M Stage | M1c | M0 Stage III | M1a | M1a | M1c |
| ECOG performance status | 0 | 0 | 1 | 1 | 1 |
| LDH >1 × ULN | No | No | Yes | Yes | No |
| Number of prior therapies | 3 | 2 | 3 | 3 | 3 |
| Types of prior therapy | Chemotherapy, immunotherapy | Surgery, chemotherapy | Chemotherapy, immunotherapy | Chemotherapy | Surgery, radiotherapy, chemotherapy, immunotherapy |
All 5 patients achieved prolonged survival despite initial disease progression and/or the appearance of new lesions in 4 of them (Fig. 2). Ipilimumab-related patterns of response comprised response in the presence of new, late-onset lesions (Patient A), durable SD followed by a CR upon retreatment (Patient B), response after an early increase in total tumor burden (Patient C), slowly building response following initial development of new lesions (Patient D) and a slow, steady shrinkage of baseline lesions without new lesions (Patient E).
Overall safety data for the 27 patients included in this analysis have been described previously [13]. With regard to safety among long-term survivors, Patients A and C both developed grade 2 thyroiditis during the early stages of ipilimumab treatment, which was effectively managed with thyroid hormone replacement therapy (levothyroxine). Patient A was subsequently diagnosed with late-onset, grade 2 diarrhea following the 15th dose of ipilimumab (W144), which gradually worsened. Following a diagnosis of grade 4 diarrhea at W192, ipilimumab treatment was permanently discontinued.
Immunological parameters
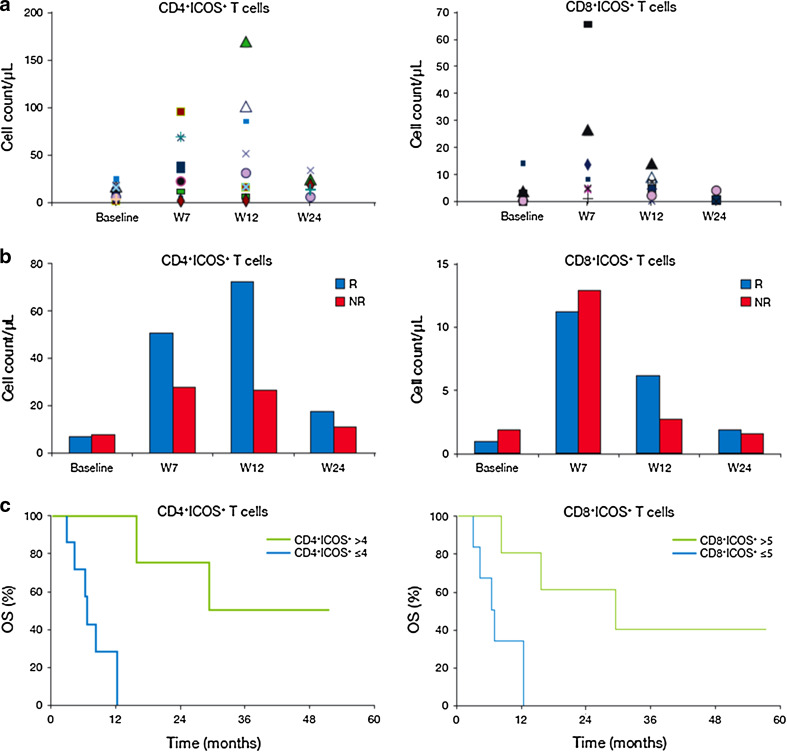
ICOS expression was analyzed in CD4+ and CD8+ T cells isolated from 17 patients. Compared with baseline, there was a significant increase in the number of circulating CD4+ICOS+ T cells at weeks 7 (P < 0.001), 12 (P < 0.003) and 24 (P < 0.02), and in CD8+ICOS+ T cell counts at weeks 7 (P < 0.003) and 12 (P < 0.02) (Fig. 3a). Among patients who achieved disease control, the increase in both CD4+ICOS+ and CD8+ICOS+ T cell counts at weeks 7 and 12 was more than fivefold. By comparison, patients with PD as their best response (non-responders) had a less than threefold increase in ICOS+ T cells (Fig. 3b). Significantly better rates of survival were observed in patients with an increase at W7 in CD4+ICOS+ T cells [hazard ratio for death (HR) with median >fourfold increase: 0.14; 95 % CI 0.02–1.25; P = 0.009] or CD8+ICOS+ T cells (HR with median >fivefold increase: 0.11; 95 % CI 0.01–1.01; P = 0.02) than in those with smaller increases in ICOS+ T cell counts (Fig. 3c).
Fig. 3.
Changes in ICOS+ circulating T cells counts and clinical outcome with ipilimumab treatment. a Increase in absolute CD4+ICOS+ or CD8+ICOS+ circulating T cells from baseline at W7, W12 and W24. Each symbol represents 1 patient. b Absolute counts of CD4+ICOS+ or CD8+ICOS+ circulating T cells in peripheral blood samples from responders (R patients who achieved disease control) or non-responders (NR patients with PD). c Kaplan–Meier analysis of OS according to increases from baseline in circulating CD4+ICOS+ and CD8+ICOS+ T cell counts at W7
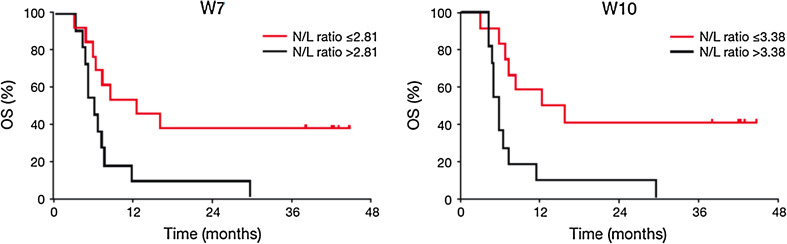
The N/L ratio was determined at baseline (for all 27 patients), W4 (27 patients), W7 (24 patients) and W10 (23 patients). Median N/L ratios at W7 and W10 were 2.81 and 3.38, respectively. Patients with an N/L ratio lower than the median values at W7 and W10 had significantly improved survival compared with those with N/L ratios greater than the median value at these time points (HR for N/L ratio >2.81 at W7: 3.86, 95 % CI 1.43–10.41, P < 0.004; HR for N/L ratio >3.38 at W10: 6.57, 95 % CI 2.22–19.50, P < 0.0001; Fig. 4).
Fig. 4.
Correlation between N/L ratio at W7 and W10 and patient survival
Discussion
The durable responses and long-term survival benefits demonstrated with ipilimumab treatment are encouraging for patients with metastatic melanoma [9, 13, 20, 21]. However, despite its clinical efficacy in clinical trials and in the EAP setting, further research is needed to identify and validate predictive markers of response or survival with ipilimumab. In the present study, we report long-term follow-up data from 27 heavily pretreated patients with advanced melanoma who received ipilimumab at the University Hospital of Siena as part of the European EAP. Retrospective analysis of immunological parameters was also performed to identify putative biomarkers for clinical benefit with ipilimumab treatment.
We previously reported 1- and 2-year survival rates of 34.8 and 23.5 % in this study population [13]. Our follow-up data reveal that most patients alive at 2 years continued to survive for 4 years and beyond, indicating long-term survival benefits with ipilimumab 10 mg/kg in the EAP setting. The survival rate of 20.4 % remained unchanged between 3 and 4 years and is similar to survival rates reported with extended treatment or long-term follow-up in phase II and III clinical trials of ipilimumab 10 mg/kg [9, 20, 21]. This is particularly important, as it shows that ipilimumab treatment can provide long-term survival benefits to patients who have failed several prior systemic therapies.
The kinetics of response with ipilimumab differ from those observed with targeted agents or conventional chemotherapy, and some patients who initially progress or appear to progress may still experience long-term survival benefits with ipilimumab [22]. Indeed, most of the patients who survived >4 years in this analysis developed new lesions during the course of ipilimumab treatment. Two patients achieved durable objective responses following retreatment with ipilimumab, highlighting the potential benefit of reactivating an immune response in patients who initially respond to treatment and then progress. Another patient, diagnosed with PD at W12, subsequently experienced SD lasting almost 2 years and a PR that was ongoing >4 years after treatment initiation.
We previously reported that the safety profile among this patient population was similar to that observed in clinical trials [13, 23]. AEs were predominantly immune related, manageable and generally reversible. Ipilimumab is associated with inflammatory AEs resulting from increased or excessive immune activity, likely to be related to its MoA. These most commonly affect the gastrointestinal system or skin, but can also involve other organ systems, including endocrine-related events [24, 25]. Among the 5 patients with long-term survival in this analysis, 2 developed thyroiditis, which was effectively managed with hormone replacement therapy as per protocol-specific treatment guidelines [24, 25]. One of these patients subsequently developed diarrhea that progressively increased in severity, resulting in the permanent discontinuation of ipilimumab. These data indicate that AEs can be managed with prompt recognition and treatment per defined algorithms; however, ongoing vigilance is necessary to prevent complications or exacerbation.
Predictive biomarkers would be a valuable tool in patient selection for ipilimumab treatment and could potentially be used as surrogate markers of efficacy in clinical trials. Furthermore, changes in certain parameters during the early stages of ipilimumab treatment may help physicians to predict which patients are likely to experience favorable outcomes. Among the immunological parameters investigated here, baseline ICOS+ T cell counts and N/L ratios did not appear to be predictive of clinical activity. However, early changes in these parameters between weeks 7 and 12 of the ipilimumab induction phase were associated with clinical response and survival.
The costimulatory molecule ICOS constitutes a third member of the CD28/CTLA-4 family, which is expressed at low levels on naïve T cells but is rapidly upregulated during an immune response [26–28]. In line with this, we found a substantial increase in CD4+ICOS+ and CD8+ICOS+ T cell counts following ipilimumab treatment, particularly in patients who experienced disease control. Retrospective analyses from a phase II extended dosing study of ipilimumab with or without a peptide vaccine also revealed a significant increase in T cell ICOS expression following ipilimumab treatment although in this previous study the increase did not correlate with clinical response. These earlier data, however, were derived from comparisons of ICOS+ expression between baseline and 3 or 6 months [11]. It may be that increased CD4+ ICOS+ and CD8+ ICOS+ T cell counts only serve as markers of efficacy during the early course of ipilimumab treatment. Importantly, we were able to identify median values of fold increase (fourfold increase for CD4+ICOS+ and fivefold increase for CD8+ICOS+ T cells) above which OS was significantly improved. If validated as an early marker of response in larger prospective studies, ICOS expression may prove extremely valuable for predicting survival outcomes with ipilimumab treatment in daily practice.
The N/L ratio provides an important indication of the immune system’s ability to recognize and eliminate malignant tissue and is negatively correlated with survival in several malignancies, including colorectal, pancreatic and non-small-cell lung cancers [29–31]. Our results suggest this parameter should also be explored as a potential predictive biomarker for survival in melanoma patients treated with ipilimumab. The link between N/L ratio and prognosis in other cancers was observed following various local and systemic treatments, which suggests this parameter is a general measure of antitumor immune activity rather than being treatment specific. Nevertheless, the association between N/L ratio and survival reported here is consistent with ipilimumab’s proposed MoA [7], suggesting this parameter can specifically be applied to ipilimumab treatment in patients with metastatic melanoma. In addition, the link between N/L ratio and treatment outcome is consistent with previous reports of a correlation between ALC and disease control and/or OS in ipilimumab-treated patients [13–15]. Further investigation is needed to determine whether changes in either ALC or N/L ratio following CTLA-4 blockade can be used as prognostic biomarkers in melanoma patients treated with ipilimumab.
Overall, these results are noteworthy and highlight the importance of performing peripheral blood tests during the induction phase of ipilimumab therapy to enable analyses of putative prognostic markers. Although the study population described here is too small to draw any definitive conclusions, we identified ICOS+ T cell counts and N/L ratio as potential immunological parameters for investigation in larger prospective studies which should incorporate an appropriate control dataset to ensure validation.
Conclusions
The follow-up data reported here reveal long-term survival benefits with ipilimumab 10 mg/kg in heavily pretreated patients with advanced melanoma. Our preliminary data on immunological correlates suggest increased numbers of circulating CD4+ICOS+ and CD8+ICOS+ T cells within the first 12 weeks of treatment, and a low N/L ratio may be associated with disease control and improved survival in patients with metastatic melanoma treated with ipilimumab 10 mg/kg. The results suggest these immunological parameters could be used as potential biomarkers that may help predict clinical outcome with ipilimumab treatment; along this line, further investigations are warranted to ensure validation.
Acknowledgments
This work was supported in part by the Associazione Italiana per la Ricerca sul Cancro, the Italian Ministry of Health, via the Ricerca Finalizzata 2010, and by an investigational grant from Bristol-Myers Squibb. The EAP was sponsored by Bristol-Myers Squibb. Editorial and writing assistance was provided by StemScientific, funded by Bristol-Myers Squibb. The authors would like to thank the patients and investigators who participated in the European EAP.
Conflict of interest
Michele Maio has had an advisory role for and has received honoraria from Bristol-Myers Squibb. All other authors declared that they had no conflict of interest.
References
- 1.Ferlay J, Parkin DM, Steliarova-Foucher E. Estimates of cancer incidence and mortality in Europe in 2008. Eur J Cancer. 2010;46:765–781. doi: 10.1016/j.ejca.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 3.Korn EL, Liu PY, Lee SJ, Chapman JA, Niedzwiecki D, Suman VJ, Moon J, Sondak VK, Atkins MB, Eisenhauer EA, Parulekar W, Markovic SN, Saxman S, Kirkwood JM. Meta-analysis of phase II cooperative group trials in metastatic stage IV melanoma to determine progression-free and overall survival benchmarks for future phase II trials. J Clin Oncol. 2008;26:527–534. doi: 10.1200/JCO.2007.12.7837. [DOI] [PubMed] [Google Scholar]
- 4.Tarhini AA, Agarwala SS. Cutaneous melanoma: available therapy for metastatic disease. Dermatol Ther. 2006;19:19–25. doi: 10.1111/j.1529-8019.2005.00052.x. [DOI] [PubMed] [Google Scholar]
- 5.Eggermont AM. Advances in systemic treatment of melanoma. Ann Oncol. 2010;21(Suppl 7):vii339–vii344. doi: 10.1093/annonc/mdq364. [DOI] [PubMed] [Google Scholar]
- 6.Serrone L, Zeuli M, Sega FM, Cognetti F. Dacarbazine-based chemotherapy for metastatic melanoma: thirty-year experience overview. J Exp Clin Cancer Res. 2000;19:21–34. [PubMed] [Google Scholar]
- 7.Peggs KS, Quezada SA, Korman AJ, Allison JP. Principles and use of anti-CTLA4 antibody in human cancer immunotherapy. Curr Opin Immunol. 2006;18:206–213. doi: 10.1016/j.coi.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 8.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbe C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robert C, Thomas L, Bondarenko I, O’Day S, Garbe JWC, Lebbe C, Baurain JF, Testori A, Grob JJ, Davidson N, Richards J, Maio M, Hauschild A, Miller WH, Jr, Gascon P, Lotem M, Harmankaya K, Ibrahim R, Francis S, Chen TT, Humphrey R, Hoos A, Wolchok JD. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–2526. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 10.Hamid O, Schmidt H, Nissan A, Ridolfi L, Aamdal S, Hansson J, Guida M, Hyams DM, Gomez H, Bastholt L, Chasalow SD, Berman D. A prospective phase II trial exploring the association between tumor microenvironment biomarkers and clinical activity of ipilimumab in advanced melanoma. J Transl Med. 2011;9:204. doi: 10.1186/1479-5876-9-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang W, Yu D, Sarnaik AA, Yu B, Hall M, Morelli D, Zhang Y, Zhao X, Weber JS. Biomarkers on melanoma patient T cells associated with ipilimumab treatment. J Transl Med. 2012;10:146. doi: 10.1186/1479-5876-10-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yuan J, Adamow M, Ginsberg BA, Rasalan TS, Ritter E, Gallardo HF, Xu Y, Pogoriler E, Terzulli SL, Kuk D, Panageas KS, Ritter G, Sznol M, Halaban R, Jungbluth AA, Allison JP, Old LJ, Wolchok JD, Gnjatic S. Integrated NY-ESO-1 antibody and CD8 + T-cell responses correlate with clinical benefit in advanced melanoma patients treated with ipilimumab. Proc Natl Acad Sci USA. 2011;108:16723–16728. doi: 10.1073/pnas.1110814108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Di Giacomo AM, Danielli R, Calabro L, Bertocci E, Nannicini C, Giannarelli D, Balestrazzi A, Vigni F, Riversi V, Miracco C, Biagioli M, Altomonte M, Maio M. Ipilimumab experience in heavily pretreated patients with melanoma in an expanded access program at the university hospital of Siena (Italy) Cancer Immunol Immunother. 2011;60:467–477. doi: 10.1007/s00262-010-0958-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ku GY, Yuan J, Page DB, Schroeder SE, Panageas KS, Carvajal RD, Chapman PB, Schwartz GK, Allison JP, Wolchok JD. Single-institution experience with ipilimumab in advanced melanoma patients in the compassionate use setting: lymphocyte count after 2 doses correlates with survival. Cancer. 2010;116:1767–1775. doi: 10.1002/cncr.24951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simeone E, Gentilcore G, Romano A, Daponte A. Immunological and biological changes during ipilimumab (Ipi) treatment and their correlation with clinical response and survival. J Clin Oncol. 2012;30(Suppl):8573. [Google Scholar]
- 16.Berrocal A, Lopez-Martin JA, Arance AM, Soriano VEE (2012) Spanish experience with the ipilimumab Expanded Access Program. J Clin Oncol 30(Suppl): abstract e19023
- 17.Danielli R, Ridolfi R, Chiarion-Sileni V, Queirolo P, Testori A, Plummer R, Boitano M, Calabro L, Rossi CD, Di Giacomo AM, Ferrucci PF, Ridolfi L, Altomonte M, Miracco C, Balestrazzi A, Maio M. Ipilimumab in pretreated patients with metastatic uveal melanoma: safety and clinical efficacy. Cancer Immunol Immunother. 2012;61:41–48. doi: 10.1007/s00262-011-1089-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilgenhof S, Four SD, Everaert H, Neyns B. Patterns of response in patients with pretreated metastatic melanoma who received ipilimumab 3 mg/kg in a European expanded access program: five illustrative case reports. Cancer Invest. 2012;30:712–720. doi: 10.3109/07357907.2012.727934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Di Giacomo AM, Calabrò L, Danielli R, Fonsatti E, Bertocci E, Pesce I, Giannarelli D, Biagioli M, Altomonte M, Maio M (2012) Long term survival and immunological correlates in metastatic melanoma treated with ipilimumab at 10 mgs within an expanded access program. Ann Oncol 23(Suppl 9):abstract 1117PD
- 20.Lebbe C, Weinberg AD, Maio M, Neyns B, Harmankaya K, Chin K, Opatt McDowell D, Cykowski L, McHenry MB, Wolchok JD (2012) Five-year survival rates for patients (Pts) with metastatic melanoma (MM) treated with ipilimumab (Ipi) in phase II trials. Ann Oncol 23(Suppl 9):abstract 1116PD
- 21.Prieto PA, Yang JC, Sherry RM, Hughes MS, Kammula US, White DE, Levy CL, Rosenberg SA, Phan GQ. CTLA-4 blockade with ipilimumab: long-term follow-up of 177 patients with metastatic melanoma. Clin Cancer Res. 2012;18:2039–2047. doi: 10.1158/1078-0432.CCR-11-1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wolchok JD, Hoos A, O’Day S, Weber JS, Hamid O, Lebbe C, Maio M, Binder M, Bohnsack O, Nichol G, Humphrey R, Hodi FS. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15:7412–7420. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 23.Weber J. Review: anti-CTLA-4 antibody ipilimumab: case studies of clinical response and immune-related adverse events. Oncologist. 2007;12:864–872. doi: 10.1634/theoncologist.12-7-864. [DOI] [PubMed] [Google Scholar]
- 24.YERVOY™ Summary of Product Characteristics (2012) Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_Product_Information/human/002213/WC500109299.pdf. Accessed December 2012
- 25.Weber JS, Kahler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30:2691–2697. doi: 10.1200/JCO.2012.41.6750. [DOI] [PubMed] [Google Scholar]
- 26.Hutloff A, Dittrich AM, Beier KC, Eljaschewitsch B, Kraft R, Anagnostopoulos I, Kroczek RA. ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature. 1999;397:263–266. doi: 10.1038/16717. [DOI] [PubMed] [Google Scholar]
- 27.Schenk AD, Gorbacheva V, Rabant M, Fairchild RL, Valujskikh A. Effector functions of donor-reactive CD8 memory T cells are dependent on ICOS induced during division in cardiac grafts. Am J Transplant. 2009;9:64–73. doi: 10.1111/j.1600-6143.2008.02460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoshinaga SK, Whoriskey JS, Khare SD, Sarmiento U, Guo J, Horan T, Shih G, Zhang M, Coccia MA, Kohno T, Tafuri-Bladt A, Brankow D, Campbell P, Chang D, Chiu L, Dai T, Duncan G, Elliott GS, Hui A, McCabe SM, Scully S, Shahinian A, Shaklee CL, Van G, Mak TW, Senaldi G. T-cell co-stimulation through B7RP-1 and ICOS. Nature. 1999;402:827–832. doi: 10.1038/45582. [DOI] [PubMed] [Google Scholar]
- 29.An X, Ding PR, Li YH, Wang FH, Shi YX, Wang ZQ, He YJ, Xu RH, Jiang WQ. Elevated neutrophil to lymphocyte ratio predicts survival in advanced pancreatic cancer. Biomarkers. 2010;15:516–522. doi: 10.3109/1354750X.2010.491557. [DOI] [PubMed] [Google Scholar]
- 30.Sarraf KM, Belcher E, Raevsky E, Nicholson AG, Goldstraw P, Lim E. Neutrophil/lymphocyte ratio and its association with survival after complete resection in non-small cell lung cancer. J Thorac Cardiovasc Surg. 2009;137:425–428. doi: 10.1016/j.jtcvs.2008.05.046. [DOI] [PubMed] [Google Scholar]
- 31.Walsh SR, Cook EJ, Goulder F, Justin TA, Keeling NJ. Neutrophil-lymphocyte ratio as a prognostic factor in colorectal cancer. J Surg Oncol. 2005;91:181–184. doi: 10.1002/jso.20329. [DOI] [PubMed] [Google Scholar]