Abstract
In patients with Kaposi’s sarcoma (KS), human herpesvirus 8 (HHV-8) can invariably be detected in KS tumor tissue and, at a lower frequency, in prostate tissue and peripheral blood B lymphocytes. Whereas the majority of KS spindle cells are latently infected by HHV-8, linear HHV-8 genomes characteristic for lytic infection are found predominantly in the peripheral blood cells of KS patients. In this study, we show that HHV-8 can stably infect B lymphocytes in vitro in the presence of Epstein-Barr virus (EBV). We were able to generate immortalized HHV-8+/EBV+ lymphoblastoid cell lines (LCLs) derived from peripheral blood mononuclear cells (PBMC) of EBV− and EBV+ donors. In HHV-8+/EBV+ LCLs, which have the phenotype of activated B lymphocytes (CD19+, surface immunoglobulin M, CD23+, CD30+, CD80+), HHV-8 was still present after more than 25 passages (more than 9 months of culture). Latent viral transcripts and proteins were present in nonstimulated HHV-8+/EBV+ LCLs. After induction by phorbol ester and n-butyrate, HHV-8+/EBV+ LCLs expressed lytic HHV-8 transcripts and proteins. Moreover, HHV-8 could be serially passaged from HHV-8+/EBV+ LCLs to fresh PBMC.
Human herpesvirus 8 (HHV-8) was recently detected in Kaposi’s sarcoma (KS) (11, 18, 30) and B-cell lymphomas (5, 39, 45), as well as in other malignancies and in healthy individuals. HHV-8 is a type 2 gammaherpesvirus (genus Rhadinovirus) with sequence similarity to Epstein-Barr virus (EBV), herpesvirus saimiri (HVS), equine herpesvirus 2, murine herpesvirus 68, and two recently identified herpesviruses causing retroperitoneal fibromatosis in macaques (34, 40, 41, 48). Although seroprevalence data indicated that HHV-8 is more widespread and not restricted to KS, HHV-8 is suspected of possessing transforming activity similar to EBV and HVS, which cause tumors in their natural hosts and in experimental animals (2, 15, 16, 19, 24, 27, 44). In most cell lines established from KS tissue, HHV-8 is lost during continuous propagation. Several cell lines derived from primary effusion lymphoma (PEL) and KS, however, are stably infected by latent HHV-8 and can be induced to undergo lytic viral replication and virus production (6, 38, 42). Serial propagation of HHV-8 on primary cells or cultured cell lines has not been possible thus far. In vitro, HHV-8 could be transferred to several cell lines and primary cells including 293 (13, 37) and B lymphocytes (25); however, this did not result in stable virus infection. In vivo, HHV-8 was detected in biopsy specimens of KS lesions (11, 18, 30) but also in prostate tissue (28), semen (28), saliva (47), peripheral blood B lymphocytes (1, 10), and lymphoid tissue (4) of KS patients. Human B lymphocytes can also be infected by EBV, the nearest relative of HHV-8 that is pathogenic to humans. EBV is able to transform B cells in vitro to permanent lymphoblastoid cell lines (LCLs) (20). EBV-induced B-cell transformation leads to a sequential expression of at least 11 latent EBV genes (EBNAs, LMPs, and EBERs), entry into the cell cycle and continuous cell proliferation, RNA synthesis, and expression of activation (e.g., transferrin receptor, major histocompatibility complex class II, CD21, CD23, CD39, CD40 and CD44) and adhesion (e.g., ICAM1, LFA1, and LFA3) molecules, resulting in a phenotype similar to stimulated B cells. Thus far, there has been no experimental evidence that infectious HHV-8 particles can stably infect and transform either primary cells or cell lines in vitro. In this study, we present evidence that HHV-8 persistently infects peripheral blood B lymphocytes and that LCLs continuously infected with HHV-8 can be established.
MATERIALS AND METHODS
Blood donors.
Peripheral blood mononuclear cells (PBMC) were isolated from EDTA-treated blood of four EBV-negative and EBV-positive healthy individuals by Ficoll-Hypaque discontinuous gradient centrifugation (Lymphoflot; Biotest AG, Dreieich, Germany) as specified by the manufacturer. Blood donors were not from HHV-8 risk groups. EBV serologic testing was performed with the following commercial kits detecting virus capsid antigen (VCA) immunoglobulin G (IgG) (Fresenius, Bad Homburg, Germany) and IgM (Viramed, Martinsried, Germany) and Epstein-Barr virus nuclear antigen (EBNA) IgG antibodies (Biotest, Dreieich, Germany). EBV-positive individuals were positive for VCA IgG and EBNA IgG antibodies but negative for VCA IgM antibodies. EBV-negative individuals were negative for VCA IgG, VCA IgM, and EBNA IgG.
Cell culture and EBV and/or HHV-8 infection.
BCBL-1 (38), BC-1 (35), B95-8 (CRL1612), Raji (CCL 86), and LCLs were cultured in RPMI (Life Technologies, Paisley, Scotland) supplemented with 20% heat-inactivated fetal calf serum (FCS; Life Technologies), 100 IU of penicillin (Life Technologies) per ml, 100 mg of streptomycin (Life Technologies) per ml, 2 mM l-glutamine (Life Technologies), and 0.05 mM 2-mercaptoethanol (Sigma, St. Louis, Mo.). For cells at low density, the 2-mercaptoethanol was replaced by 20 mM bathocuproine disulfonic acid (Sigma), 50 μm α-thioglycerol (Sigma), and 1 mM sodium pyruvate (Life Technologies). To induce the expression of lytic viral proteins, cells were treated with 20 ng of phorbol 12-myristate 13-acetate (TPA; Sigma) per ml and 3 mM sodium n-butyrate (Sigma) for 24 h.
Supernatants of either BCBL-1, BC-1, B95-8, or Raji cells grown at very high densities (5 × 105 per ml) were used to infect PBMC. Supernatants were filtered through 0.4-μm filters and serially diluted with cell culture medium in 96-well plates, and PBMC were added to 104 cells per well. For serial virus passage, supernatants of ABEH-1, ABE-1, GMEH-1, and GME-1 cells were generated similarly and added to 104 PBMC from an EBV+ donor.
Isolation of cellular DNA and detection of viral DNA by PCR.
Total cellular DNA was prepared from 106 cells by the method of Miller et al. (26). The primer used to amplify HHV-8 DNA flanked the K12 open reading frame (K12for, 5′ cggaattcatggatagaggcttaacg 3′; K12rev, 5′ cgctcgagtcagtgcgcgcccgttgc 3′). The length of the expected amplified product is 196 bp. The primers used to amplify EBV DNA from the BALF5 open reading frame were Epolfor (5′ aggttggcggggctcagggc 3′) and Epolrev (5′ agcacaggctagccggcctg 3′), and the amplified product was 343 bp in size. Each reaction mixture contained 500 ng of cellular DNA, 100 ng of each primer, and 5 U of Taq polymerase (Perkin-Elmer Cetus, Branchburg, N.J.). The mixture was cycled in a DNA thermal cycler 2400 (Perkin-Elmer) for 30 cycles of amplification (96°C for 1 min; 60°C for 1 min; 72°C for 1 min). One-tenth of the PCR products were analyzed on 2% agarose–40 mM Tris acetate–1 mM EDTA and visualized after ethidium bromide staining. As negative and positive PCR controls, water and plasmid DNAs of EBV polymerase and K12 cloned into pBluescript KS II+ were used, respectively.
Preparation of RNA and RT-PCR.
RNA was extracted from 106 cells with Tri Reagent (Molecular Research Center, Cincinnati, Ohio) as specified by the manufacturer. A 5-μg portion of total RNA in 10 μl of water and 1 μg of oligo(dT)18 primer were heated to 70°C for 10 min and then cooled to 4°C on ice. A 10-μl volume of reaction mixture (100 mM Tris-HCl [pH 8.3], 6 mM MgCl2, 150 mM KCl, 1 mM each deoxynucleoside triphosphate (Boehringer, Mannheim, Germany), 30 U of RNase inhibitor [MBI Fermentas, Vilnius, Lithuania], 200 U of Superscript [Life Technologies]) was added, and the mixture was incubated at 37°C for 1.5 h and heated to 67°C for 15 min. A 2-μl volume of the reverse transcription reaction mixture was amplified with gene-specific primers in a 100-μl PCR mixture containing 10 mM Tris-HCl (pH 8.3), 1.5 mM MgCl2, 0.2 mM each deoxynucleoside triphosphate, 100 ng of each primer, and 5 U of Taq polymerase. The mixture was cycled in a DNA thermal cycler 2400 for 30 cycles of amplification (96°C for 1 min; 60°C for 1 min; 72°C for 1 min). One-tenth of the PCR mixtures were analyzed on 1 or 2% agarose–40 mM Tris-acetate–1 mM EDTA and visualized after ethidium bromide staining.
The oligonucleotide primers used in this study were K12for, K12rev, VP23for (5′ cgcgggtctagaatcgcactcgacaagagtata 3′), VP23rev (5′ cgcggggaattctttagcgtggggaataccaacagga 3′), Actfor (5′ cgcgaatccccccagtgtgacatgg 3′), and Actrev (5′ cgcggatcccagccaggtccagacg 3′). As negative controls for these experiments, reverse transcription-PCR (RT-PCR) was performed with 1 μg of total RNA. As negative and positive PCR controls, water and plasmid DNAs of full-length cDNA of VP23, actin, and K12 cloned into pBluescript KS II+ were used, respectively.
Flow cytometry.
To analyze the surface phenotype of LCLs, BC-1 and BCBL-1 cells (5 × 105) were washed in phosphate-balanced saline (PBS) and FACS buffer (PBS containing 2.5% FCS and 0.02% sodium azide) and stained directly with anti-CD3-phycoerythrin (PE), anti-CD14-PE, anti-CD16-PE, anti-CD19-PE, anti-CD56-PE (Pharmingen, Hamburg, Germany), anti-IgG-fluorescein isothiocynate (FITC), and anti-IgM-FITC (Sigma). Isotype controls were included in each assay. For indirect staining, cells were incubated first with mouse anti-CD19, anti-CD20, anti-CD23, or anti-CD30 (Dako Diagnostika, Hamburg, Germany) and then with goat anti-mouse IgG-FITC (Dianova, Hamburg, Germany). As the negative control, only goat anti-mouse IgG-FITC was used in each assay. The cells were washed three times and analyzed on a FACScan with Cellquest software (Becton Dickinson, San Jose, Calif.).
Immunofluorescence and confocal microscopy.
After stimulation for 24 h with TPA and n-butryate as described above, the cells were dried on poly-l-lysine-coated coverslips (Marienfeld, Bad Mergentheim, Germany), fixed with acetone, and blocked for 1 h with 5% FCS in PBS. After incubation for 1 h with monoclonal antibody (MAb) vp4G2 (anti-HHV-8 VP23), kap5C4 (anti-HHV-8 K12), or 817 (anti-EBV VCA) (Chemicon, Temecula, Calif.) diluted in 5% FCS in PBS, the cells were incubated for 2 h with Cy3-conjugated goat anti-rat IgG or goat anti-mouse IgG (Sigma) diluted 1:200 in 5% FCS in PBS. MAbs vp4G2 (23a) and kap5C4 (21a) were generated as described elsewhere. The cells were washed and examined under a TNT confocal microscope DMIRB (Leica, Bensheim, Germany).
RESULTS
Immortalization of PBMC and persistent viral infection by EBV and HHV-8.
Supernatants of BCBL-1 (HHV-8+ PEL cell line), BC-1 (HHV-8+ EBV+ PEL cell line), B95-8 (EBV+ marmoset cell line), and Raji (human EBV-transformed cell line with a deletion in the EBV genome resulting in an inability to release virus) cells were used to infect PBMC from four EBV− and four EBV+ individuals. Incubation of PBMC with control supernatant or supernatant of Raji cells did not result in the outgrowth of LCLs, independent of EBV status, indicating that the EBV present in PBMC from EBV+ individuals was not sufficient to transform B cells under the conditions used (Table 1). Incubation with supernatants of B95-8 resulted in the outgrowth of LCLs in PBMC of each individual, again independent of EBV status. Supernatants of HHV-8+ BCBL-1 cells resulted in the outgrowth of LCLs if PBMC were derived from EBV+ but not from EBV− individuals. Interestingly, supernatants of HHV-8+ and EBV+ BC-1 cells resulted in the immortalization of PBMC from both EBV− and EBV+ individuals. Since supernatants from BCBL-1 cells (HHV-8+ EBV−) immortalized PBMC from EBV+ but not EBV− donors, we hypothesized that HHV-8 either provides a cofactor promoting the immortalization of EBV-transformed B cells or itself has transforming activity if EBV or EBV-derived cofactors are present. Since supernatants of BCBL-1 cells did not promote the immortalization of B lymphocytes derived from EBV− donors in the assay system we used, we thus far have no hints for the latter.
TABLE 1.
Establishment of HHV-8+ EBV+ LCLs from PBMC of EBV− and EBV+ donors
| Supernatant | LCL outgrowtha after incubation of PBMC from:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| EBV+ donor:
|
EBV− donor:
|
|||||||
| A | B | C | D | E | F | G | I | |
| Control medium | —b | — | — | — | — | — | — | — |
| Raji | — | — | — | — | — | — | — | — |
| B95-8 | 14 | 17 | 15 | 15.7 | 26 | 19 | 22.5 | 24.6 |
| BCBL-1 | 0.3 | 1.5 | 0.3 | 0.75 | Lc | L | L | L |
| BC-1 | 1.2 | 4.3 | 10 | 4.8 | 12 | 9.6 | 10.4 | 13.2 |
Outgrowth is expressed as 103 outgrowing LCLs at the highest dilution.
—, no growth.
L, initial limited growth.
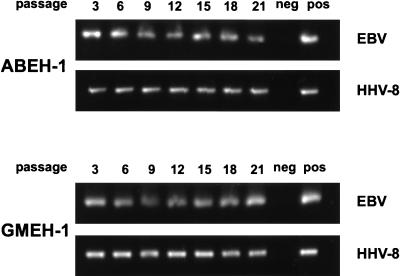
Initially, six cell lines were established from PBMC of each donor and supernatant. PBMC from one donor were used twice, with a similar outcome. Thus, a total of 54 cell lines were established by using either BCBL-1 or BC-1 supernatant. HHV-8+ EBV+ LCLs were tested after various passages for the presence of EBV and HHV-8 DNA by PCR (Fig. 1). Both EBV and HHV-8 were present in all cell lines derived from PBMC of EBV+ donors infected with supernatant of the HHV-8+ cell lines BC-1 and BCBL-1, as well as in cell lines derived from EBV− donors infected with supernatant of BC-1 cells. In the experiment in Fig. 1, two LCLs, ABEH-1 and GMEH-1, were tested after 3, 6, 9, 12, 15, 18, and 21 passages and found to be positive for both EBV and HHV-8 with no apparent decline in viral load. Four immortalized HHV-8+ EBV+ cell lines are continuously kept in culture for currently more than 25 passages and longer than 9 months. The other HHV-8+ EBV+ cell lines were frozen in liquid nitrogen since they appeared to be similar. Once the HHV-8+ EBV+ cell lines were established, there was no difference in terms of the efficiency of long-term culture in comparison to EBV+ LCLs. All four cell lines kept in continuous cell culture for more than 9 months were found to be positive for both EBV and HHV-8. Furthermore, none of the HHV-8+ EBV+ LCLs have become negative for either HHV-8 or EBV thus far.
FIG. 1.
Persistent infection of LCLs by HHV-8 and EBV. Viral DNAs in the LCLs ABEH-1 and GMEH-1 were detected by PCR from passages 3 to 21 by using either EBV (BALF5, 343 bp)- or HHV-8 (K12, 196 bp)-specific primers. Cellular DNA was extracted after the indicated number of passages, and 500 ng of each DNA preparation was used in an unnested PCR of 30 cycles. One-tenth of the PCR mixture was applied to a 2% agarose gel and visualized after ethidium bromide staining.
Phenotypic analysis of EBV+ HHV-8+ LCLs.
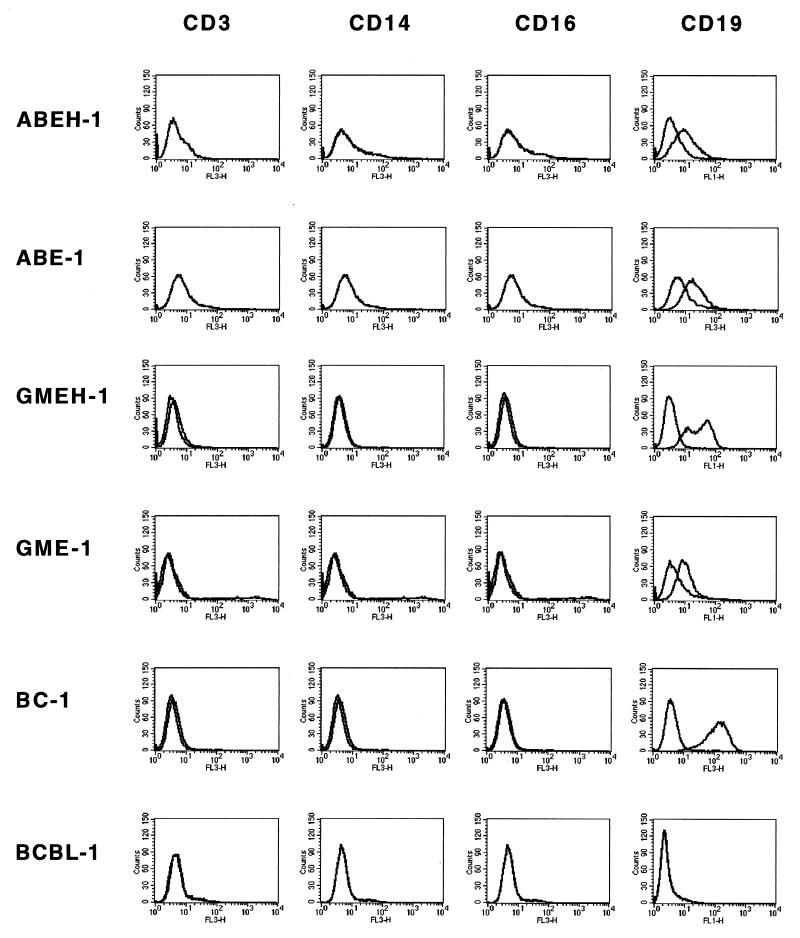
Next, we determined the phenotype of the immortalized cell lines. All HHV-8+ EBV+ LCLs tested were positive for CD19 (B-lymphocyte antigen) but negative for CD3 (T-lymphocyte marker), CD14 (monocytes/macrophages), and CD16 (NK cells) (Fig. 2), indicating that these cells are of B-lymphocyte origin. Moreover, HHV-8+ EBV+ LCLs expressed surface IgM, as well as B-cell activation markers CD23, CD30, and CD80 similarly to EBV-transformed LCLs (Table 2). Surface IgG, CD20, and CD56 were not found on these cells. The phenotype differed from BCBL-1 and BC-1 cell lines by some markers; e.g., BCBL-1 cells were negative for CD80, and BC-1 cells were negative for CD30. Intriguingly, the surface phenotype as well as growth characteristics varied between LCLs from different donors. Whereas most of the HHV-8+ EBV+ LCLs grow in large conglomerates in suspension similar to EBV-transformed LCLs, cells of the HHV-8+ EBV+ LCL COEH-1 show semiadherent growth and are spindle shaped (data not shown).
FIG. 2.
Expression of the B-cell antigen CD19 on HHV-8+ EBV+ LCLs. HHV-8+ EBV+ LCLs (ABEH-1 and GMEH-1), EBV+ HHV-8− LCLs (ABE-1 and GME-1), BC-1, and BCBL-1 were stained with anti-CD3, anti-CD14, anti-CD16, and anti-CD19 MAbs. Isotype controls were included in each assay. Flow cytometry was performed by gating on 10,000 living cells.
TABLE 2.
Surface phenotype of HHV-8+ EBV+ and EBV+ LCLs
| Cell line | Presence ofa:
|
||||||
|---|---|---|---|---|---|---|---|
| IgG | IgM | CD20 | CD23 | CD30 | CD56 | CD80 | |
| ABEH-1 (HHV-8+ EBV+) | − | + | − | + | + | − | + |
| ABE-1 (HHV-8− EBV+) | + | + | − | + | + | − | + |
| GMEH-1 (HHV-8+ EBV+) | − | + | − | + | + | − | + |
| GME-1 (HHV-8− EBV+) | + | + | − | + | + | − | + |
| BC-1 (HHV-8+ EBV+) | − | + | − | + | − | − | + |
| BCBL-1 (HHV-8+ EBV−) | − | + | − | + | + | − | − |
CD20, mature B cells and pre-B cells, not plasma cells (B1); CD23, mature and/or activated B cells (low-affinity IgE receptor); CD30, activated B and T cells (TNF receptor superfamily); CD56, interleukin-2-dependent T-cell clones, NK cells (NCAM); CD80, activated B and T cells (B7-1 costimulatory molecule). Expression of surface molecules was determined by immunofluorescence with either FITC-conjugated polyclonal antibodies (IgG and IgM) or PE-conjugated MAbs (CD20, CD23, CD30, CD56, and CD80).
Latent infection of transformed B-cell lines by HHV-8.
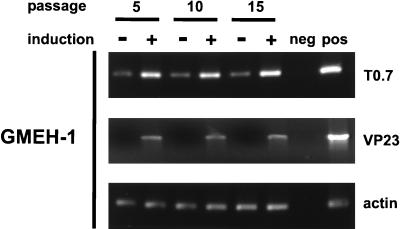
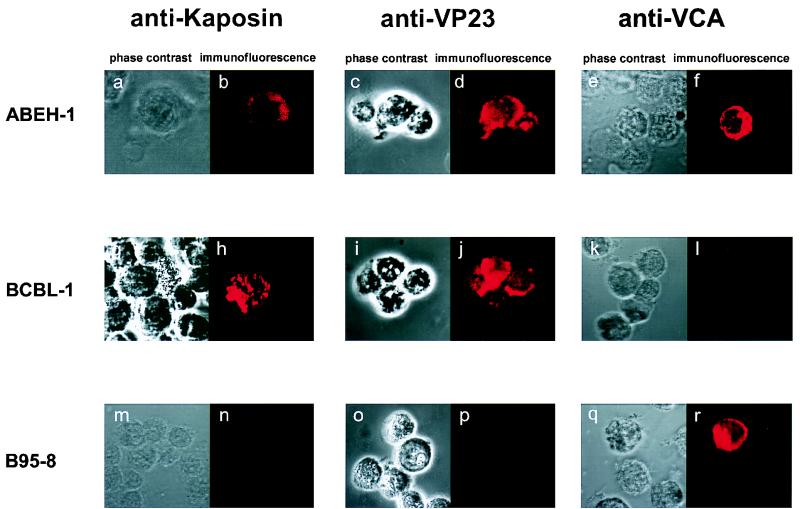
We next tested HHV-8+ EBV+ LCLs for expression of the latent viral transcript T0.7, which codes for kaposin. T0.7 was detected in the noninduced HHV-8+ EBV+ LCL GMEH-1 after 5, 10, and 15 passages by RT-PCR, and expression could be increased by induction with phorbol ester and n-butyrate (Fig. 3). The signal resulting from the amplification of actin mRNA was similar for all samples tested, indicating that the RNA content was comparable. The RT-PCR result was confirmed by Northern blot analysis, which revealed the presence of T0.7 in unstimulated ABEH-1 and GMEH-1 cells after more than 25 passages. In accordance with the RT-PCR results, kaposin protein was detected in the noninduced HHV-8+ EBV+ LCLs GMEH-1 and ABEH-1 by immunofluorescence with the MAb kap5C4 (Fig. 4). Kaposin was also detected on BCBL-1 cells but not on B95-8 cells.
FIG. 3.
Detection of latent HHV-8 transcripts in noninduced, and lytic HHV-8 transcripts in induced, HHV-8+ EBV+ LCLs. RT-PCR analysis of EBV+ HHV-8+ LCL GMEH-1 was performed after 5, 10, and 15 passages either with or without induction by TPA and sodium butyrate. The RT reaction was performed by oligo(dT) priming followed by PCR with gene-specific primers for the latent T0.7 transcript (196 bp), the lytic viral mRNA VP23 (917 bp), and actin mRNA (245 bp) as a control. As negative and positive PCR controls, water and plasmid DNAs of full-length cDNA of VP23, actin, and K12 were used, respectively.
FIG. 4.
Detection of the HHV-8 protein kaposin in noninduced, lytic HHV-8 minor capsid protein VP23 and the lytic VCA of EBV in induced HHV-8+ EBV+ ABEH-1 LCLs. EBV+ HHV-8+ LCL ABEH-1 (a to f), BCBL-1 (g to l), and B95-8 (m to r) were either stained with MAb kap5C4 (b, h, and n), MAb Vp234G2 (d, j, and p) or MAb directed to VCA of EBV (f, l, and r) after fixation with acetone. Cells stained with kap5C4 were noninduced, whereas cells stained with MAb VP234G2 or anti-VCA of EBV were induced with TPA and sodium butyrate. Bound antibodies were visualized with Cy3-conjugated goat anti-rat IgG or goat anti-mouse IgG and examined under a Leica TNT confocal microscope.
Induction of lytic viral proteins in HHV-8+ EBV+ LCLs.
We next investigated whether latent HHV-8 can be reactivated in HHV-8+ EBV+ LCLs. GMEH-1 and ABEH-1 LCLs after 5, 10, and 15 culture passages were stimulated with phorbol ester and n-butyrate and subsequently tested for lytic viral transcripts coding for VP23 by RT-PCR (Fig. 3). In all immortalized HHV-8+ EBV+ LCLs tested, lytic HHV-8 mRNA was detected after induction by phorbol ester and n-butyrate, similar to BC-1 and BCBL-1 cells. In accordance with the RT-PCR results, the viral capsid protein VP23 was detected by immunofluorescence with the MAb vp4G2 in cells of the HHV-8+ EBV+ LCL ABEH-1 and in BCBL-1 cells. No reactivity was seen with B95-8 (Fig. 4) and EBV+ LCLs of the same donors (data not shown), indicating that there was no cross-reactivity with EBV proteins. Reactivity against EBV VCA was detected in B95-1 and in ABEH-1 cells but not in BCBL-1 cells.
Serial passage of HHV-8 from HHV-8+ EBV+ LCLs.
Since lytic HHV-8 genes could be induced in HHV-8+ EBV+ LCLs, we hypothesized that HHV-8+ EBV+ LCLs are able to produce infectious viral particles and infect new cells. PBMC of an EBV+ donor were incubated with supernatants of HHV-8+ EBV+ and EBV+ LCLs (Table 3). As anticipated, supernatants of the EBV+ LCLs ABE-1 and GME-1 immortalized B cells. Intriguingly, incubation with supernatants of HHV-8+ EBV+ LCLs also promoted the outgrowth of cell lines, whereas control supernatant of Raji cells or culture medium had no such effect. Six second-generation cell lines were established for each supernatant, and one second-generation cell line of each supernatant was tested by immunofluorescence analysis after two passages. Cell lines that were derived from PBMC incubated with supernatant of ABE-1 and GME-1 cells were found to be positive for EBV VCA and negative for HHV-8 kaposin. Cell lines that were derived from PBMC incubated with supernatant of cells of the HHV-8+ EBV+ LCLs ABEH-1 and GMEH-1 were positive for both HHV-8 kaposin and EBV VCA, indicating that HHV-8 has been transferred to these cells.
TABLE 3.
Serial passage of HHV-8 from HHV-8+ EBV+ LCLs to PBMC of an EBV+ donor
| Supernatanta | LCL outgrowthb | Expression ofc:
|
|
|---|---|---|---|
| HHV-8 kaposin | EBV VCA | ||
| Control | − | NDd | ND |
| Raji | − | ND | ND |
| ABEH-1 | 0.18 | + | + |
| ABE-1 | 45 | − | + |
| GMEH-1 | 1.4 | + | + |
| GME-1 | 35 | − | + |
Supernatants were prepared from ABEH-1 LCLs at passage 18, ABE-1 LCLs at passage 14, GMEH-1 LCLs at passage 23, and GME-1 LCLs at passage 14 as described in Materials and Methods.
Expressed as 102 outgrowing LCLs at the highest dilution.
Expression of HHV-8 kaposin and EBV VCA on outgrowing cells was determined by immunofluorescence with the MAbs kap5C4 and 817, respectively.
ND, not done.
DISCUSSION
In this study, we were able to show that (i) peripheral blood B lymphocytes can be persistently infected by HHV-8 in vitro, (ii) HHV-8 supports transforming activity of EBV on B cells, and (iii) infection leads to the outgrowth of permanent HHV-8+ EBV+ LCLs. Infection of PBMC with EBV in vitro results in the immortalization of B cells and the generation of EBV+ LCLs. In vivo, EBV infection causes a primary infection known as mononucleosis followed by a lifelong persistence of EBV in a latent state in B lymphocytes. The frequency of EBV-infected B cells in the peripheral blood was reported to be between 1 in 104 and 1 in 106 (43). EBV present in isolated PBMC can lead to the spontaneous outgrowth of immortalized cells without the addition of EBV-containing supernatants; however, a minimal number of cells must be used (22, 23). In the experiments shown here, the number of cells used was not sufficient for spontaneous outgrowth of EBV+ LCLs (Table 1). In PBMC of none of the eight donors tested did culture medium or Raji cell supernatant (Raji cells contain EBV genomes but cannot produce infectious EBV) alone lead to the outgrowth of immortalized B-cell lines. In contrast, supernatants of BCBL-1 cells containing HHV-8 induced the outgrowth of LCLs in PBMC of all four EBV+ donors but not in PBMC of EBV− donors.
The LCLs were persistently HHV-8 and EBV infected and possessed the phenotype of activated B cells, similar to EBV+ LCLs. HHV-8 could also be transferred to PBMC from EBV− donors, but only if BC-1 supernatants containing HHV-8 and EBV were used. Thus, HHV-8 infects EBV+ B cells and favors the outgrowth of immortalized LCLs containing both viruses. Intriguingly, infection of PBMC with BC-1 supernatant did not result in the outgrowth of EBV+ but, rather, resulted in the outgrowth of HHV-8+ EBV+ LCLs, which might indicate that there is a selection for infection with both viruses. Moreover, we cloned two HHV-8+ EBV+ cell lines and tested eight clones of one of them for the presence of HHV-8 and EBV by PCR. All eight clones were HHV-8+ EBV+, indicating that none of the clones had lost either virus. Thus, there are several lines of evidence suggesting that cells infected with both viruses possess a growth advantage and that HHV-8 perhaps has an intrinsic transforming potential. This is supported by the fact that many BCBL cell lines are infected with both viruses. It is formally possible that either of the two viruses is the primary transforming agent in HHV-8+ EBV+ LCLs whereas the other virus provides an essential or nonessential (growth-promoting) cofactor. If HHV-8 is a transforming virus for B lymphocytes, it appears to depend on an EBV-derived cofactor, at least under the conditions we used. An alternative explanation might be a two-step transformation process, divided into an initiating step and a maintaining step. In this case, the virus supplying the initiating step should be lost at least in some cell lines, since there is no selection for it. At the moment, however, we have no evidence for this explanation. Interestingly, HHV-8 has no homologs to EBNAs, LMPs, and EBERs; however, there are several other candidates for transforming genes like v-IL6 (29, 33), cc-chemokines (21), G-protein-coupled receptor (3, 12), v-cyclin (7, 8, 17), K1 (14), interferon regulatory factor (29, 31), kaposin (32), v-FLIP (46), LANA (36), and v-bcl2 (9). Thus, the mechanism by which HHV-8 supports transformation might be different from that for EBV.
It is known that peripheral blood B lymphocytes can be infected by HHV-8 in vivo in KS patients and healthy individuals (1, 10, 25). At present, there is no evidence that these cells are doubly infected by HHV-8 and EBV in vivo (4). In contrast, almost all of the PEL tumors are positive for both EBV and HHV-8, implying that double infection could play a causal role in this entity. In KS, only a minority of tumors are positive for EBV. Thus, it is unlikely that both viruses in concert play a role in tumorigenesis of KS. The primary target cell of HHV-8 infection is not yet known. This study provides some evidence that B cells could be the primary reservoir and the place of latency in HHV-8-infected individuals with and without KS. It presents a model system of HHV-8 infection in vitro and might give some clues about the role of B-cell infection in the pathogenesis of HHV-8.
ACKNOWLEDGMENTS
We acknowledge the expert technical assistance of C. Atzler. Cell lines BC-1 and BCBL-1 were kindly provided by Patrick Moore and Don Ganem, respectively.
S.K. was supported in part by a scholarship from the Graduiertenkolleg “Infektion und Immunität” of the Deutsche Forschungsgemeinschaft.
REFERENCES
- 1.Ambroziak J A, Blackbourn D J, Herndier B G, Glogau R G, Gullett J H, McDonald A R, Lennette E T, Levy J A. Herpes-like sequences in HIV-infected and uninfected Kaposi’s sarcoma patients. Science. 1995;268:582–583. doi: 10.1126/science.7725108. . (Letter.) [DOI] [PubMed] [Google Scholar]
- 2.Andre S, Schatz O, Bogner J R, Zeichhardt H, Stoffler M M, Jahn H U, Ullrich R, Sonntag A K, Kehm R, Haas J. Detection of antibodies against viral capsid proteins of human herpesvirus 8 in AIDS-associated Kaposi’s sarcoma. J Mol Med. 1997;75:145–152. doi: 10.1007/s001090050099. [DOI] [PubMed] [Google Scholar]
- 3.Arvanitakis L, Geras R E, Varma A, Gershengorn M C, Cesarman E. Human herpesvirus KSHV encodes a constitutively active G-protein-coupled receptor linked to cell proliferation. Nature. 1997;385:347–350. doi: 10.1038/385347a0. [DOI] [PubMed] [Google Scholar]
- 4.Bigoni B, Dolcetti R, de Lellis L, Carbone A, Boiocchi M, Cassai E, di Luca D. Human herpesvirus 8 is present in the lymphoid system of healthy persons and can reactivate in the course of AIDS. J Infect Dis. 1996;173:542–549. doi: 10.1093/infdis/173.3.542. [DOI] [PubMed] [Google Scholar]
- 5.Cesarman E, Chang Y, Moore P S, Said J W, Knowles D M. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N Engl J Med. 1995;332:1186–1191. doi: 10.1056/NEJM199505043321802. [DOI] [PubMed] [Google Scholar]
- 6.Cesarman E, Moore P S, Rao P H, Inghirami G, Knowles D M, Chang Y. In vitro establishment and characterization of two acquired immunodeficiency syndrome-related lymphoma cell lines (BC-1 and BC-2) containing Kaposi’s sarcoma-associated herpesvirus-like (KSHV) DNA sequences. Blood. 1995;86:2708–2714. [PubMed] [Google Scholar]
- 7.Cesarman E, Nador R G, Bai F, Bohenzky R A, Russo J J, Moore P S, Chang Y, Knowles D M. Kaposi’s sarcoma-associated herpesvirus contains G protein-coupled receptor and cyclin D homologs which are expressed in Kaposi’s sarcoma and malignant lymphoma. J Virol. 1996;70:8218–8223. doi: 10.1128/jvi.70.11.8218-8223.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang Y, Moore P S, Talbot S J, Boshoff C H, Zarkowska T, Godden K, Paterson H, Weiss R A, Mittnacht S. Cyclin encoded by KS herpesvirus. Nature. 1996;382:410. doi: 10.1038/382410a0. . (Letter.) [DOI] [PubMed] [Google Scholar]
- 9.Cheng E H, Nicholas J, Bellows D S, Hayward G S, Guo H G, Reitz M S, Hardwick J M. A Bcl-2 homolog encoded by Kaposi sarcoma-associated virus, human herpesvirus 8, inhibits apoptosis but does not heterodimerize with Bax or Bak. Proc Natl Acad Sci USA. 1997;94:690–694. doi: 10.1073/pnas.94.2.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Decker L L, Shankar P, Khan G, Freeman R B, Dezube B J, Lieberman J, Thorley L D. The Kaposi sarcoma-associated herpesvirus (KSHV) is present as an intact latent genome in KS tissue but replicates in the peripheral blood mononuclear cells of KS patients. J Exp Med. 1996;184:283–288. doi: 10.1084/jem.184.1.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dupin N, Grandadam M, Calvez V, Gorin I, Aubin J T, Havard S, Lamy F, Leibowitch M, Huraux J M, Escande J P, et al. Herpesvirus-like DNA sequences in patients with Mediterranean Kaposi’s sarcoma. Lancet. 1995;345:761–762. doi: 10.1016/s0140-6736(95)90642-8. [DOI] [PubMed] [Google Scholar]
- 12.Eliopoulos A G, Dawson C W, Mosialos G, Floettmann J E, Rowe M, Armitage R J, Dawson J, Zapata J M, Kerr D J, Wakelam M J, Reed J C, Kieff E, Young L S. CD40-induced growth inhibition in epithelial cells is mimicked by Epstein-Barr virus-encoded LMP1: involvement of TRAF3 as a common mediator. Oncogene. 1996;13:2243–2254. [PubMed] [Google Scholar]
- 13.Foreman K E, Friborg J, Kong W P, Woffendin C, Polverini P J, Nickoloff B J, Nabel G J. Propagation of a human herpesvirus from AIDS-associated Kaposi’s sarcoma. N Engl J Med. 1997;336:163–171. doi: 10.1056/NEJM199701163360302. [DOI] [PubMed] [Google Scholar]
- 14.Ganem D. KSHV and Kaposi’s sarcoma: the end of the beginning? Cell. 1997;91:157–160. doi: 10.1016/s0092-8674(00)80398-0. [DOI] [PubMed] [Google Scholar]
- 15.Gao S J, Kingsley L, Hoover D R, Spira T J, Rinaldo C R, Saah A, Phair J, Detels R, Parry P, Chang Y, Moore P S. Seroconversion to antibodies against Kaposi’s sarcoma-associated herpesvirus-related latent nuclear antigens before the development of Kaposi’s sarcoma. N Engl J Med. 1996;335:233–241. doi: 10.1056/NEJM199607253350403. [DOI] [PubMed] [Google Scholar]
- 16.Gao S J, Kingsley L, Li M, Zheng W, Parravicini C, Ziegler J, Newton R, Rinaldo C R, Saah A, Phair J, Detels R, Chang Y, Moore P S. KSHV antibodies among Americans, Italians and Ugandans with and without Kaposi’s sarcoma. Nat Med. 1996;2:925–928. doi: 10.1038/nm0896-925. [DOI] [PubMed] [Google Scholar]
- 17.Godden-Kent D, Talbot S J, Boshoff C, Chang Y, Moore P, Weiss R A, Mittnacht S. The cyclin encoded by Kaposi’s sarcoma-associated herpesvirus stimulates cdk6 to phosphorylate the retinoblastoma protein and histone H1. J Virol. 1997;71:4193–4198. doi: 10.1128/jvi.71.6.4193-4198.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang Y Q, Li J J, Kaplan M H, Poiesz B, Katabira E, Zhang W C, Feiner D, Friedman K A. Human herpesvirus-like nucleic acid in various forms of Kaposi’s sarcoma. Lancet. 1995;345:759–761. doi: 10.1016/s0140-6736(95)90641-x. [DOI] [PubMed] [Google Scholar]
- 19.Kedes D H, Operskalski E, Busch M, Kohn R, Flood J, Ganem D. The seroepidemiology of human herpesvirus 8 (Kaposi’s sarcoma-associated herpesvirus): distribution of infection in KS risk groups and evidence for sexual transmission. Nat Med. 1996;2:918–924. doi: 10.1038/nm0896-918. . (Erratum, 2:1041.) [DOI] [PubMed] [Google Scholar]
- 20.Kieff E. Epstein Barr virus and its replication. In: Fields B N, Knipe D M, Howley P M, Chanock R M, Melnick J L, Monath T P, Roizman B, Straus S E, editors. Virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven; 1998. pp. 2343–2396. [Google Scholar]
- 21.Kledal T N, Rosenkilde M M, Coulin F, Simmons G, Johnsen A H, Alouani S, Power C A, Luttichau H R, Gerstoft J, Clapham P R, Clark L I, Wells T C, Schwartz T W. A broad-spectrum chemokine antagonist encoded by Kaposi’s sarcoma-associated herpesvirus. Science. 1997;277:1656–1659. doi: 10.1126/science.277.5332.1656. [DOI] [PubMed] [Google Scholar]
- 21a.Kliche, S., et al. Unpublished data.
- 22.Kohler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256:495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- 23.Kremmer E, Ohst K, Kiefer J, Brewis N, Walter G. Separation of PP2A core enzyme and holoenzyme with monoclonal antibodies against the regulatory A subunit: abundant expression of both forms in cells. Mol Cell Biol. 1997;17:1692–1701. doi: 10.1128/mcb.17.3.1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23a.Kremmer, E., et al. Unpublished data.
- 24.Lennette E T, Blackbourn D J, Levy J A. Antibodies to human herpesvirus type 8 in the general population and in Kaposi’s sarcoma patients. Lancet. 1996;348:858–861. doi: 10.1016/S0140-6736(96)03240-0. [DOI] [PubMed] [Google Scholar]
- 25.Mesri E A, Cesarman E, Arvanitakis L, Rafii S, Moore M A, Posnett D N, Knowles D M, Asch A S. Human herpesvirus-8/Kaposi’s sarcoma-associated herpesvirus is a new transmissible virus that infects B cells. J Exp Med. 1996;183:2385–2390. doi: 10.1084/jem.183.5.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller G, Heston L, Grogan E, Gradoville L, Rigsby M, Sun R, Shedd D, Kushnaryov V M, Grossberg S, Chang Y. Selective switch between latency and lytic replication of Kaposi’s sarcoma herpesvirus and Epstein-Barr virus in dually infected body cavity lymphoma cells. J Virol. 1997;71:314–324. doi: 10.1128/jvi.71.1.314-324.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller G, Rigsby M O, Heston L, Grogan E, Sun R, Metroka C, Levy J A, Gao S J, Chang Y, Moore P. Antibodies to butyrate-inducible antigens of Kaposi’s sarcoma-associated herpesvirus in patients with HIV-1 infection. N Engl J Med. 1996;334:1292–1297. doi: 10.1056/NEJM199605163342003. [DOI] [PubMed] [Google Scholar]
- 28.Monini P, de Lellis L L, Fabris M, Rigolin F, Cassai E. Kaposi’s sarcoma-associated herpesvirus DNA sequences in prostate tissue and human semen. N Engl J Med. 1996;334:1168–1172. doi: 10.1056/NEJM199605023341805. [DOI] [PubMed] [Google Scholar]
- 29.Moore P S, Boshoff C, Weiss R A, Chang Y. Molecular mimicry of human cytokine and cytokine response pathway genes by KSHV. Science. 1996;274:1739–1744. doi: 10.1126/science.274.5293.1739. [DOI] [PubMed] [Google Scholar]
- 30.Moore P S, Chang Y. Detection of herpesvirus-like DNA sequences in Kaposi’s sarcoma in patients with and without HIV infection. N Engl J Med. 1995;332:1181–1185. doi: 10.1056/NEJM199505043321801. [DOI] [PubMed] [Google Scholar]
- 31.Moorthy R K, Thorley L D. Biochemical, genetic, and functional analyses of the phosphorylation sites on the Epstein-Barr virus-encoded oncogenic latent membrane protein LMP-1. J Virol. 1993;67:2637–2645. doi: 10.1128/jvi.67.5.2637-2645.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muralidhar S, Pumfery A M, Hassani M, Sadaie M R, Azumi N, Kishishita M, Brady J N, Doniger J, Medveczky P, Rosenthal L J. Identification of kaposin (open reading frame K12) as a human herpesvirus 8 (Kaposi’s sarcoma-associated herpesvirus) transforming gene. J Virol. 1998;72:4980–4988. doi: 10.1128/jvi.72.6.4980-4988.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Neipel F, Albrecht J C, Ensser A, Huang Y Q, Li J J, Friedman K A, Fleckenstein B. Human herpesvirus 8 encodes a homolog of interleukin-6. J Virol. 1997;71:839–842. doi: 10.1128/jvi.71.1.839-842.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Neipel F, Albrecht J C, Fleckenstein B. Cell-homologous genes in the Kaposi’s sarcoma-associated rhadinovirus human herpesvirus 8: determinants of its pathogenicity? J Virol. 1997;71:4187–4192. doi: 10.1128/jvi.71.6.4187-4192.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Orenstein J M, Alkan S, Blauvelt A, Jeang K T, Weinstein M D, Ganem D, Herndier B. Visualization of human herpesvirus type 8 in Kaposi’s sarcoma by light and transmission electron microscopy. AIDS. 1997;11:F35–F45. doi: 10.1097/00002030-199705000-00001. [DOI] [PubMed] [Google Scholar]
- 36.Rainbow L, Platt G M, Simpson G R, Sarid R, Gao S J, Stoiber H, Herrington C S, Moore P S, Schulz T F. The 222- to 234-kilodalton latent nuclear protein (LNA) of Kaposi’s sarcoma-associated herpesvirus (human herpesvirus 8) is encoded by orf73 and is a component of the latency-associated nuclear antigen. J Virol. 1997;71:5915–5921. doi: 10.1128/jvi.71.8.5915-5921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Renne R, Blackbourn D, Whitby D, Levy J, Ganem D. Limited transmission of Kaposi’s sarcoma-associated herpesvirus in cultured cells. J Virol. 1998;72:5182–5188. doi: 10.1128/jvi.72.6.5182-5188.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Renne R, Zhong W, Herndier B, McGrath M, Abbey N, Kedes D, Ganem D. Lytic growth of Kaposi’s sarcoma-associated herpesvirus (human herpesvirus 8) in culture. Nat Med. 1996;2:342–346. doi: 10.1038/nm0396-342. [DOI] [PubMed] [Google Scholar]
- 39.Rettig M B, Ma H J, Vescio R A, Pold M, Schiller G, Belson D, Savage A, Nishikubo C, Wu C, Fraser J, Said J W, Berenson J R. Kaposi’s sarcoma-associated herpesvirus infection of bone marrow dendritic cells from multiple myeloma patients. Science. 1997;276:1851–1854. doi: 10.1126/science.276.5320.1851. [DOI] [PubMed] [Google Scholar]
- 40.Rose T M, Strand K B, Schultz E R, Schaefer G, Rankin G W, Jr, Thouless M E, Tsai C C, Bosch M L. Identification of two homologs of the Kaposi’s sarcoma-associated herpesvirus (human herpesvirus 8) in retroperitoneal fibromatosis of different macaque species. J Virol. 1997;71:4138–4144. doi: 10.1128/jvi.71.5.4138-4144.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Russo J J, Bohenzky R A, Chien M C, Chen J, Yan M, Maddalena D, Parry J P, Peruzzi D, Edelman I S, Chang Y, Moore P S. Nucleotide sequence of the Kaposi sarcoma-associated herpesvirus (HHV8) Proc Natl Acad Sci USA. 1996;93:14862–14867. doi: 10.1073/pnas.93.25.14862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Said J W, Chien K, Takeuchi S, Tasaka T, Asou H, Cho S K, de Vos S, Cesarman E, Knowles D M, Koeffler H P. Kaposi’s sarcoma-associated herpesvirus (KSHV or HHV-8) in primary effusion lymphoma: ultrastructural demonstration of herpesvirus in lymphoma cells. Blood. 1996;87:4937–4943. [PubMed] [Google Scholar]
- 43.Sarid R, Flore O, Bohenzky R A, Chang Y, Moore P S. Transcription mapping of the Kaposi’s sarcoma-associated herpesvirus (human herpesvirus 8) genome in a body cavity-based lymphoma cell line (BC-1) J Virol. 1998;72:1005–1012. doi: 10.1128/jvi.72.2.1005-1012.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Simpson G R, Schulz T F, Whitby D, Cook P M, Boshoff C, Rainbow L, Howard M R, Gao S J, Bohenzky R A, Simmonds P, Lee C, de Ruiter A, Hatzakis A, Tedder R S, Weller I V, Weiss R A, Moore P S. Prevalence of Kaposi’s sarcoma associated herpesvirus infection measured by antibodies to recombinant capsid protein and latent immunofluorescence antigen. Lancet. 1996;348:1133–1138. doi: 10.1016/S0140-6736(96)07560-5. [DOI] [PubMed] [Google Scholar]
- 45.Soulier J, Grollet L, Oksenhendler E, Cacoub P, Cazals H D, Babinet P, d’Agay M F, Clauvel J P, Raphael M, Degos L, et al. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman’s disease. Blood. 1995;86:1276–1280. [PubMed] [Google Scholar]
- 46.Thome M, Schneider P, Hofmann K, Fickenscher H, Meinl E, Neipel F, Mattmann C, Burns K, Bodmer J L, Schroter M, Scaffidi C, Krammer P H, Peter M E, Tschopp J. Viral FLICE-inhibitory proteins (FLIPs) prevent apoptosis induced by death receptors. Nature. 1997;386:517–521. doi: 10.1038/386517a0. [DOI] [PubMed] [Google Scholar]
- 47.Vieira J, Huang M L, Koelle D M, Corey L. Transmissible Kaposi’s sarcoma-associated herpesvirus (human herpesvirus 8) in saliva of men with a history of Kaposi’s sarcoma. J Virol. 1997;71:7083–7087. doi: 10.1128/jvi.71.9.7083-7087.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Virgin H W, Latreille P, Wamsley P, Hallsworth K, Weck K E, Dal C A, Speck S H. Complete sequence and genomic analysis of murine gammaherpesvirus 68. J Virol. 1997;71:5894–5904. doi: 10.1128/jvi.71.8.5894-5904.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]