Abstract
One of the most important innate host defense mechanisms against viral infection is the induction of interferon (IFN)-stimulated genes (ISGs). Immediately upon entry, viruses activate interferon-regulatory factor 3 (IRF3), as well as nuclear factor κB (NF-κB), which transactivate a subset of ISGs, proinflammatory genes, as well as IFN genes. Most large DNA viruses exhibit countermeasures against induction of this response. However, whereas human cytomegalovirus (HCMV) inhibits IFN-dependent induction of ISGs, IFN-independent induction of ISGs is observed both in the presence and, even moreso, in the absence of viral gene expression. Rhesus CMV (RhCMV) is an emerging animal model for HCMV sharing important similarities in primary structure, epidemiology, and pathogenesis. To determine whether RhCMV would similarly induce ISGs, we performed DNA microarray and quantitative PCR analysis of ISG expression in rhesus fibroblasts infected with RhCMV or HCMV. In contrast to HCMV, however, RhCMV did not induce expression of ISGs or proinflammatory genes at any time after infection. Moreover, dimerization and nuclear accumulation of IRF3, readily observed in HCMV-infected cells, was absent from RhCMV-infected cells, whereas neither virus seemed to activate NFκB. RhCMV also blocked IRF3 activation by live or UV-inactivated HCMV, suggesting that RhCMV inhibits viral IRF3 activation and the resultant ISG induction with extraordinary efficiency. Since infection during inhibition of protein expression by cycloheximide or inactivation of viral gene expression by UV treatment did not trigger IRF3 activation or ISG expression by RhCMV, we conclude that RhCMV virions contain a novel inhibitor of IFN-independent viral induction of ISG expression by IRF3.
An essential and evolutionarily conserved component of the innate host response to viral infection is transcriptional induction of interferon (IFN)-stimulated genes (ISGs). Important ISGs include components of the double-stranded RNA (dsRNA)-dependent protein kinase (PKR), 2-5A oligoadenylate synthetase (2-5A), and Mx pathways. Intracellular inhibition of virus replication takes place through translation inhibition (PKR), single-stranded RNA cleavage (2-5A), and modulation of viral polymerase and protein trafficking (Mx). Viruses induce ISG expression via two separate but related mechanisms. First, the IFN-dependent pathway involves activation of the IFN receptor by exogenous IFN-α/β secreted from infected cells. Binding of IFN-α or -β to its receptor triggers JAK/STAT signal transduction that ultimately leads to the transcription of ISGs (24, 36). However, in recent years it has become increasingly clear that viral infection also triggers a subset of ISGs independent from secreted IFN. This takes place in response to specific molecular components of pathogens, called pathogen-associated molecular patterns (e.g., bacterial lipopolysaccharide or dsRNA and glycoproteins) that activate Toll-like receptors (TLRs) (16). TLR signaling activates nuclear factor κB (NF-κB) via the I kappa kinase (IKK) complex, thereby upregulating proinflammatory genes (19). Specific TLRs are also known to activate IFN regulatory factor 3 (IRF3). IRF3 is a constitutively expressed protein that normally shuttles freely between the cytoplasm and nucleus. It becomes activated via phosphorylation of carboxy-terminal serine and threonine residues, resulting in homodimerization and association with histone acetyltransferase nuclear proteins p300 and CREB-binding protein (CBP) (39, 42). The proteins act as transcriptional coactivators and cause IRF3 to become localized to the nucleus (23, 25, 39, 42). This complex stimulates transcription of a subset of ISGs and, together with NF-κB, IFN-β itself (16). Recent studies show that phosphorylation of IRF3 is performed by virus-induced IKK-related kinases (IKK-related IKKɛ and TANK-binding kinase 1 [TBK1]) (12, 35). Thus, viral infection results in NF-κB-dependent and IRF3-dependent induction of proinflammatory genes and a subset of ISGs, respectively. In addition, the induction of IFN-β gene in turn activates the IFN-dependent pathways and a second wave of ISG induction.
Evolutionary pressure exerted by ISG antiviral effects has given rise to viral countermeasures against either the induction or function of ISGs or both (13). In fact, recent data suggest that all enveloped DNA viruses, as well as diverse RNA viruses, trigger ISG expression through IRF3 when viral gene expression is inhibited (10). However, IRF3 was generally inactivated when cells were infected with live, translationally active virus. For instance, the alphaherpesvirus herpes simplex virus triggers a strong induction of ISGs when viral gene expression is inhibited by either UV treatment or in the presence of cycloheximide (CHX) (29, 33). Live virus, however, interferes with ISG induction through the activities of the viral proteins ICP0 and vhs (26). Similarly, the poxvirus protein E3L blocks IRF3-dependent ISG expression (38) and the gammaherpesvirus Kaposi's sarcoma associated virus encodes three IRF homologous that interfere with both in IFN-dependent and -independent pathways (18).
In contrast to these other large DNA viruses, however, the betaherpesvirus human cytomegalovirus (HCMV) triggers a strong induction of ISGs upon infection of cells in culture (45, 46) and activates IRF3 (30, 33). HCMV-mediated ISG induction requires membrane fusion events and is to a large extent triggered by the major viral glycoprotein gB (5). In fact, treatment of fibroblasts with purified gB triggers a strong ISG response similar to, but faster than, intact virus (5, 37), which is mediated through CD14/TLR-2 and IRF3 (4, 11). UV inactivation of virus or infection of cells in the presence of CHX exacerbates induction of ISGs and proinflammatory genes, suggesting that HCMV inhibits ISG induction, at least partially (8, 33). Indeed, the viral tegument protein pp65 was implicated in this partial inhibition of ISG induction, but the molecular mechanism is controversial, either involving interference with the nuclear translocation of NF-κB, IRF1 (7), or IRF3 (1).
Rhesus CMV (RhCMV) is an emerging animal model for HCMV that recapitulates many aspects of HCMV pathogenesis and epidemiology (28, 40). Similar to HCMV, RhCMV is highly prevalent in animals of a given colony of rhesus macaques. Moreover, RhCMV is an opportunistic infection during simian AIDS (34) making this model ideal for study of the interaction between the two viruses. The recent completion of the genomic sequence of RhCMV further revealed a high conservation of both genes that are essential for growth in tissue culture and nonessential genes, including many known or predicted immunomodulatory genes (15). In addition, RhCMV encodes its own unique genes that are of unknown function.
During an initial evaluation of the transcriptional reprogramming that occurs in rhesus fibroblasts during in vitro infection with RhCMV, we observed a complete absence of ISG induction during all stages of viral infection. In contrast, HCMV induced a strong ISG and proinflammatory gene response in rhesus fibroblasts, as observed previously in human fibroblasts. The lack of ISG induction was consistent with a lack of IRF3 activation in RhCMV-infected cells. Moreover, neither IRF3 activation nor ISG induction was observed upon treatment of infected cells with CHX or after UV inactivation of RhCMV. Thus, unlike all other enveloped viruses examined to date, RhCMV does not trigger ISG induction even in the absence of viral gene expression. Since it is unlikely that the virus lacks molecular signals that activate IRF3, we hypothesize that structural components of the RhCMV virion prevent ISG induction. This hypothesis is supported by the finding that RhCMV inhibits IRF3 activation by live or UV-inactivated HCMV.
MATERIALS AND METHODS
Cells, antibodies, and virus.
HCMV strain AD169 and RhCMV strain 68-1 were used in all experiments. Virus particles were purified by centrifugation through a 20% sorbitol cushion, resuspended in Dulbecco modified Eagle medium (DMEM), and stored at −80°C. Telomerized rhesus fibroblasts (TRFs) were cultured in DMEM supplemented with 10% fetal bovine serum (FBS), 1% glutamine, and 1% penicillin-streptomycin. Cells were grown to confluency in 35-mm plates and infected in 300 μl of serum-free DMEM at a multiplicity of infection of 3 PFU/cell. Infection in the presence of CHX and UV inactivation of virus were performed as described previously (8). Two separate antibodies to IRF3 were used. Rabbit anti-IRF3 used for immunofluorescence assays (IFAs) was a generous gift from Takashi Fujita (Tokyo Metropolitan Institute of Medical Science) (42). Rabbit anti-IRF3 used for Western blotting was obtained from Santa Cruz Biotechnology (catalog no. SC-9082). Rabbit anti-NF-κB used for both Western blotting and IFA was obtained from Santa Cruz Biotechnology (SC-372). Antibody to RhCMV IE1 was raised in the Vaccine and Gene Therapy Institute antibody core. Rabbit and mouse antibody to HCMV IE1 and rabbit anti-HCMV gB were obtained from Jay Nelson (Oregon Health and Science University).
Array hybridization.
At 8 and 24 h postinfection (hpi), inoculum was removed, cells were washed with phosphate-buffered saline (PBS), and total RNA isolated by using the Zymo Mini-RNA isolation kit according to the manufacturer's instructions (Zymo Research, Orange, CA). Gene expression was examined by using hybridization arrays displaying 8053 human cDNA clones manufactured by the OHSU Gene Microarray Shared Resource. Human genes were selected from a library of sequence verified IMAGE consortium clones (Invitrogen catalog no. 97001.V). Arrays were printed on aminosilane coated glass slides by using a Cartesian PixSys 5500 XL microarray printer (Genomic Solutions), and each cDNA was spotted twice to account for intrachip variation. Homology between rhesus macaque and human genes is high enough to allow cross species array hybridization to be an accurate technique for examining rhesus samples (9). mRNA was amplified to make cRNA from 1 μg of total RNA prior to probe synthesis according to the method of Phillips and Eberwine (32). Probes were synthesized from 1 μg of cRNA by randomly primed reverse transcription (RT) in the presence of biotin or fluorescein-conjugated dCTP. Probe-target hybridization of infected and mock-infected and cyanine-3 (Cy3) and cyanine-5 (Cy5) fluorophore deposition was performed by using the Micromax TSA labeling and detection kit according to the manufacturer's instructions (Perkin-Elmer, Wellesley, MA). Probe labeling and fluorophore deposition were reversed in each experiment to control for dye bias resulting in two arrays for each biological replicate. Arrays were scanned by using a confocal laser scanner (Perkin-Elmer) and intensities quantified by using ImaGene 5.0 (Biodiscovery).
Data analysis.
Subtraction of background signal was performed by using ImaGene 3.5.1. Intensity data were Loess normalized (smoothing parameter, 0.5; degree of fitness, 2) by using GeneSight (Biodiscovery) (41). Signals obtained from the same clone on an array were used to calculate the mean signal intensity. Differences in gene transcription between infected and uninfected samples were examined by using the ratio of signal intensity between fluorophores. Expression differences between infected and mock-infected samples were calculated by using the ratio of normalized intensities, and statistical significance was determined as described in reference 21. Genes were considered differentially regulated if they exhibited significant (P > 0.95) changes in the same direction for both Cy3/Cy5 orientations and both biological replicates and if the fold change was >1.5.
Quantitative real-time RT-PCR.
Quantitation of individual gene expression was performed by using quantitative real-time RT-PCR (qPCR) with the Applied Biosystems Sequence Detection System (Foster City, CA). This involves intercalation of SYBR green fluorescent molecules into DNA synthesized during PCR. Measurement of SYBR green occurs throughout the reaction and the cycle number at which fluorescence levels reach an arbitrary threshold during the exponential phase of amplification (cycle threshold) is recorded for each sample. According to the method of Livak and Schmittgen (27), this value is normalized within each sample to that of a housekeeping gene (GAPDH [glyceraldehyde-3-phosphate dehydrogenase]) and subsequently compared between mock-infected and infected samples which allows estimation of relative gene expression levels (i.e., fold change). Quantitation of expression of three ISGs was performed in this way: Viperin (nucleotide [nt] 765 to 879; GenBank accession no. NM_080657), Mx2 (nt 1811 to 1924; NM_002463), and IFI-15K (nt 327 to 446; NM_005101). PCR products were examined on a 1% agarose gel to ensure that specific amplification occurred.
Immunofluorescence.
Indirect immunofluorescence assay (IFA) was used to examine the subcellular localization of IRF3 and NF-κB in TRFs, as well as to monitor viral replication through examination of viral proteins. TRFs were grown on coverslips and infected or mock infected at subconfluency as described above. At room temperature, cells were washed twice with PBS, fixed for 30 min in 3.7% formaldehyde, washed, and quenched for 10 min with 50 mM NH4Cl. Cells were then permeabilized with 0.1% Triton X-100 for 7 min and washed three times with PBS containing 2% bovine serum albumin (BSA). Cells were incubated with primary antibody (diluted 1:500 in PBS containing 2% BSA) at 37°C for 60 min, washed three times in PBS containing 2% BSA (10 min each wash), and incubated with fluorescence-labeled secondary antibody (goat anti-mouse 488 and/or goat anti-rabbit 594; Molecular Probes) diluted 1:1,000 in PBS containing 2% BSA for 60 min. Cells were washed twice in PBS containing 2% BSA (10 min each) and once in PBS. Coverslips were mounted on a slide with Vectashield mounting medium (Vector Laboratories, Burlingame, CA) containing DAPI (4′,6′-diamidino-2-phenylindole).
Western blotting.
Examination of IRF3 dimerization by native polyacrylamide gel electrophoresis and Western blotting was performed as described in reference 17. TRFs infected and mock infected in 100-mm dishes were treated with trypsin, collected by using centrifugation, and resuspended in lysis buffer (50 mM Tris-Cl [pH 8.0], 1% Ipegal, 150 mM NaCl) supplemented with 1× protease inhibitor cocktail (Invitrogen) and phosphatase inhibitor cocktail (Sigma). Whole-cell lysate protein was quantified by using a BCA assay (Pierce), and equal amounts were added to loading buffer (125 mM Tris-Cl [pH 6.8], 30% glycerol, 0.1% bromophenol blue). Samples were subjected to 7.5% non-sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to ImmunoBlot polyvinylidene difluoride membranes (Bio-Rad Laboratories). After transfer, membranes were incubated in a blocking buffer (PBS containing 10% milk and 0.1% Tween 20), followed by incubation of the primary antibody diluted in PBS containing 5% milk and 0.1% Tween 20. Blots were washed with PBS containing 0.1% Tween 20 and incubated with a horseradish peroxidase-conjugated secondary antibody (Amersham Biosciences, Piscataway, N.J.) diluted in PBS containing 5% milk and 0.1% Tween 20. Blots were washed and developed by using the PicoPure chemiluminescence reagent (Pierce) according to the manufacturer′s protocol.
RESULTS
Rhesus fibroblasts display an innate immune response to HCMV but not to RhCMV.
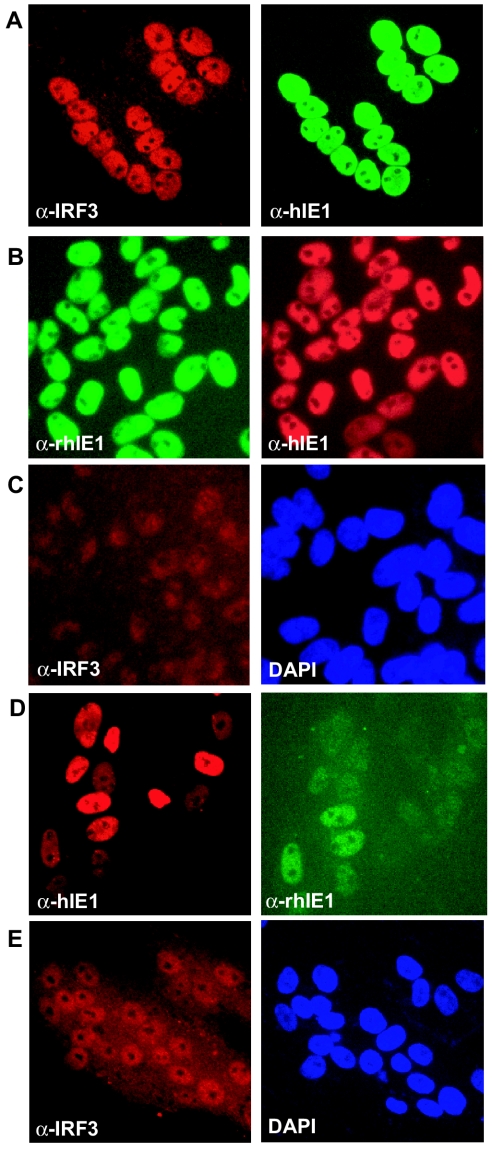
Expression profiling by DNA microarrays revealed previously that HCMV infection of human fibroblasts triggered massive changes in the transcriptional program of the infected cells mostly attributable to innate immune response genes such as ISGs and proinflammatory genes (1, 8, 37, 45). To determine whether infection with RhCMV would trigger a similar response, we compared the transcriptional response of rhesus fibroblasts (TRFs), telomerized as described previously by Bresnahan et al. (6), after infection with RhCMV or HCMV. To verify that HCMV was able to infect these nonhuman primate cells, we examined the expression of the immediate-early protein IE1 (UL123), as well as the late protein glycoprotein B (gB; UL55), at 24 hpi by using IFA. Figure 1 illustrates the expression in TRFs of RhCMV immediate-early open reading frame (ORF) Rh156 and HCMV proteins encoded by ORFs UL123 and UL55. Although the viral yield of HCMV from TRFs is lower than from human fibroblasts (not shown), our data are consistent with normal gene expression by HCMV in TRFs. To examine changes in host transcription, TRFs were infected with RhCMV or HCMV (MOI = 3) or mock infected, and total RNA was isolated at 8 and 24 hpi. After mRNA amplification, host cell gene expression was examined by using cDNA microarrays as described in Materials and Methods. Genes were considered differentially regulated between infected and mock-infected samples if statistically significant, similar direction fold changes of >1.5 were observed for both biological replicates and both dye label orientations.
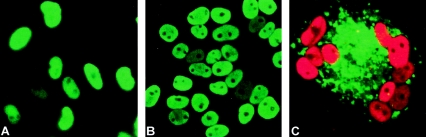
FIG. 1.
RhCMV and HCMV express viral proteins in TRFs. TRFs were infected with HCMV or RhCMV (MOI = 3) for 24 h as described in text, and indirect immunofluorescence was used to examine viral protein expression. (A) RhCMV immediate-early protein 1 (ORF Rh156); (B) HCMV immediate-early protein 1 (ORF UL123); (C) HCMV gB (ORF UL55) (green) and IE1 (red).
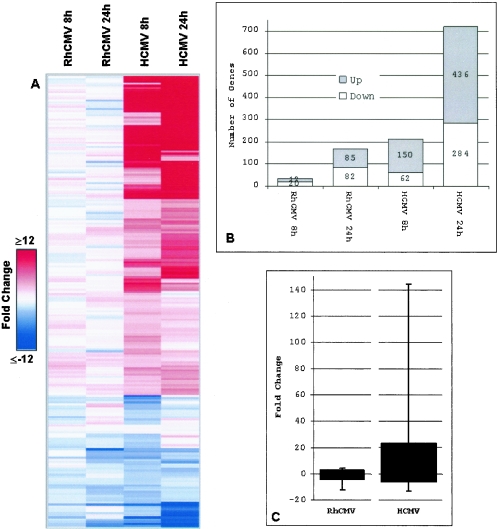
At 8 hpi HCMV infection induced transcriptional changes in 212 genes relative to mock-infected cells (150 upregulated, 62 downregulated), and RhCMV induced changes in 32 genes (12 upregulated, 20 downregulated) (see Tables S1 and S2 in the supplemental material). At 24 hpi HCMV induced changes in 720 genes (436 upregulated, 284 downregulated) and RhCMV induced changes in 167 genes (85 upregulated, 82 downregulated). Figure 2A graphically illustrates the differences in quantity and the fold changes of differentially expressed genes between the two viruses. Clearly, the changes in mRNA levels induced by HCMV infection are by far more numerous and of greater magnitude than those induced by RhCMV. Fold changes relative to mock infection were greater overall in HCMV-infected cells compared to RhCMV-infected cells. Figures 2B and C illustrate the number and average degrees of change for significantly regulated host genes across experiments. Fold changes of differentially regulated genes in RhCMV-infected cells at 24 hpi averaged 3.3 (standard deviation [SD] = 1.2) for upregulated genes and −4.5 (SD = 7.3) for downregulated genes, whereas upregulated genes at 24 hpi in HCMV-infected cells averaged 23.7 (SD = 120.8) for upregulated genes and −5.8 (SD = 7.1) for downregulated genes. Thus, infection of rhesus fibroblasts by RhCMV triggered a remarkably low host transcriptional response.
FIG. 2.
Differential gene regulation induced by RhCMV and HCMV infection of TRFs. (A) Comparative fold changes of host genes relative to mock-infected cells as measured by microarray hybridization. Displayed genes are those that were found to be significantly up- or downregulated in at least one of the four experiments. (B) Number of host genes whose expression was significantly changed relative to mock-infected cells after infection with RhCMV and HCMV at 8 and 24 h. (C) Averages and standard deviations of up- and downregulation (fold change) for genes significantly differentially regulated during RhCMV and HCMV infection at 24 h.
At 8 hpi, 7 genes and at 24 hpi 131 genes were differentially expressed in both HCMV- and RhCMV-infected cells (see Table S1 in the supplemental material). The majority of these genes are involved in general cellular metabolic, physiological processes or in cell-cell communication. In contrast, many innate immune response genes were induced by HCMV, but not RhCMV, including ISGs and proinflammatory cytokines and chemokines, as well as genes involved in prostaglandin synthesis. The induction of these genes is consistent with previous observations for human cells, suggesting that rhesus fibroblasts reacted very similarly to infection with HCMV. These genes were not induced, only minimally induced, or even repressed in TRFs infected with RhCMV. The dramatic response to HCMV infection ruled out the possibility that this low transcriptional response to RhCMV was caused by a general defect of innate immune responses in TRFs. Thus, we conclude that, in contrast to HCMV, RhCMV does not trigger an innate response to viral infection in vitro.
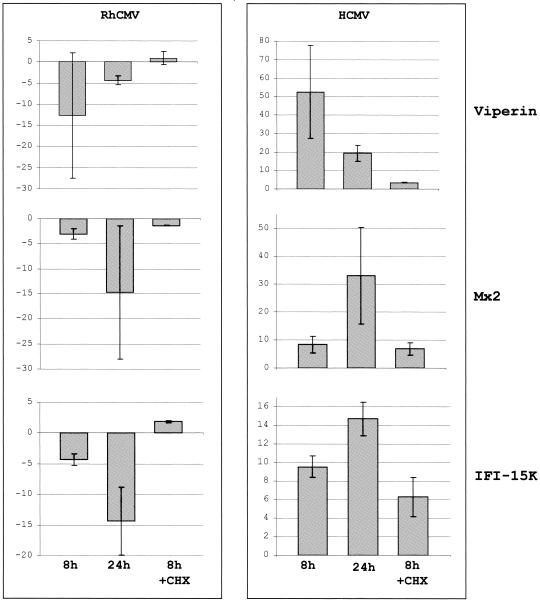
To independently confirm both expression levels measured by using microarray hybridization and the general disparity in ISG expression, we used qPCR to examine mRNA levels of three ISGs (viperin, IFI-15K, and Mx2) in two independent experiments. Gene-specific primers were selected based on the corresponding human sequence and successful amplification of a DNA band corresponding to the expected molecular size. In all cases, qPCR confirmed that ISGs were induced by HCMV but not by RhCMV (Fig. 3). Similar results were obtained after HCMV and RhCMV infection of TRFs in the presence of CHX at 8 hpi (Fig. 3), although magnitude of upregulation is distinctly lower presumably due to the absence of IFN secretion and subsequent JAK/STAT pathway stimulation. The apparent downregulation of ISGs in RhCMV-infected cells is the result of experimental variability in measurements obtained from very low mRNA copy numbers. The difference in ISG expression between RhCMV-infected and mock-infected cells is thus not significant. In contrast, the same transcripts were present in high copy numbers in HCMV-infected cells resulting in a significant difference to mock levels. Thus, we conclude that, unlike HCMV, RhCMV does not induce ISGs in infected fibroblasts.
FIG. 3.
Quantitative RT-PCR validates microarray results for three ISGs. The fold change versus mock-infected TRFs of transcript levels for three characterized ISGs, as measured by qPCR for duplicate experimental HCMV and RhCMV infections of TRFs at 8 hpi (including in the presence of CHX versus CHX-treated mock-infected TRFs) and 24 hpi, is shown.
Differential activation of IRF3, but not NF-κB, in HCMV-infected compared to RhCMV-infected cells.
The dramatic difference in gene expression profiles between HCMV- andRhCMV-infected cells, especially with respect to ISGs and proinflammatory genes, suggests that important step(s) in the activation of these genes are either absent or inhibited in RhCMV-infected cells. Many of the transcripts upregulated by HCMV but not RhCMV are known to be induced by the transcription factor NF-κB, IRF3, or both. Transcriptional activity of both factors upon HCMV entry has been demonstrated (4, 7, 22, 26, 42, 44). Therefore, we examined whether these pivotal transcription factors were differentially accumulated in fibroblast nuclei upon HCMV or RhCMV infection. Both transcription factors are constitutively present in fibroblasts.
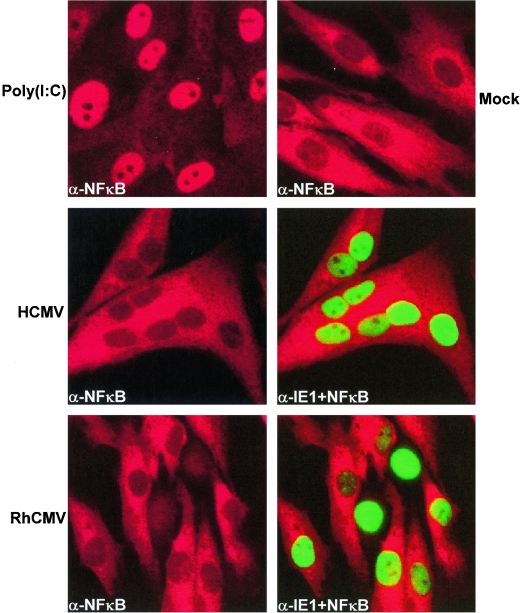
We examined the subcellular localization of NF-κB subunit p65 and IRF3 in TRFs infected with equivalent MOIs of either RhCMV or HCMV at 0.5, 1, 2, 4, 6, 8, and 24 hpi. We observed that neither HCMV nor RhCMV induced widespread redistribution of NF-κB from predominantly cytoplasmic to predominantly nuclear localization at any time point compared to mock-infected cells (Fig. 4). In contrast, cells transfected with poly(I-C) demonstrated a rapid nuclear accumulation of NF-κB. The lack of NF-κB accumulation in HCMV-infected cells is consistent with previous immunofluorescence observations for HCMV infection of human fibroblasts (7) but differ from other studies that demonstrated NF-κB activation by HCMV (22, 43, 44). It should be noted, however, that although the vast majority of infected cells primarily exhibited cytoplasmic NF-κB, cells were occasionally observed that contained weak cytoplasmic and slightly greater nuclear NF-κB staining at 2 to 24 hpi, a condition not observed in uninfected cells. Thus, our data do not rule out a low level of NF-κB activation that might be measurable in reporter gene or gel shift assays used in most studies (22, 43, 44). However, since we did not observe a substantial difference between HCMV and RhCMV in their ability to activate NF-κB at any time point, it seems highly unlikely that differential activation of NF-κB is responsible for the observed differential gene expression in cells infected with HCMV versus RhCMV.
FIG. 4.
Nuclear accumulation of NF-κB is induced by poly(I-C), but subcellular localization does not differ between RhCMV- and HCMV-infected TRFs. TRFs were either transfected with poly(I-C), mock infected, or infected with HCMV or RhCMV (MOI = 3) and then stained for NF-κB (red) and virus-specific IE1 (green) at 6 hpi.
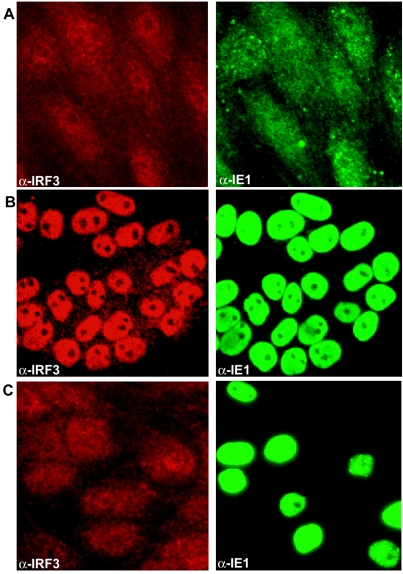
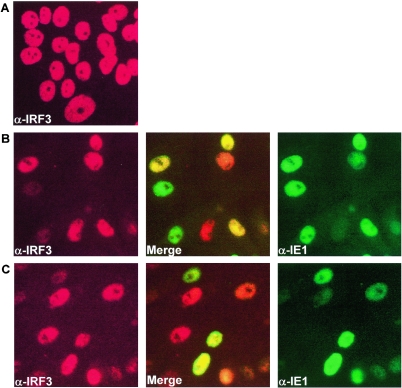
In contrast, a dramatic difference in the activation of IRF3 was observed between RhCMV- and HCMV-infected cells (Fig. 5). IRF3 accumulated in the nuclei of TRFs infected with HCMV beginning at 1 hpi and was maximal by 3 hpi, whereas diffuse subcellular localization of IRF3 was observed inRhCMV-infected cells similar to the cytoplasmic staining of IRF3 in mock-infected cells (Fig. 5). This differential activation of IRF3 correlated perfectly with the differential induction of ISGs and proinflammatory genes described above.
FIG. 5.
HCMV but not RhCMV induces nuclear accumulation of IRF3. TRFs were either mock infected (A) or infected with HCMV (B) or RhCMV (C) (MOI = 3) and stained for IRF3 (red) and type-specific IE1 (green) at 6 hpi.
RhCMV does not induce nuclear accumulation of IRF3 in the absence of host or viral gene expression.
Recently, Collins et al. (10) showed that the activation of IRF3 is a default reaction of mammalian cells to infection by enveloped DNA viruses unless the virus encodes inhibitors of this innate immune response pathway. Thus, diverse DNA and RNA viruses induced the phosphorylation of IRF3 upon inactivation of viral gene expression by either CHX or UV radiation. Therefore, we hypothesized that RhCMV would activate IRF3 and induce ISG expression upon UV-inactivation or CHX treatment.
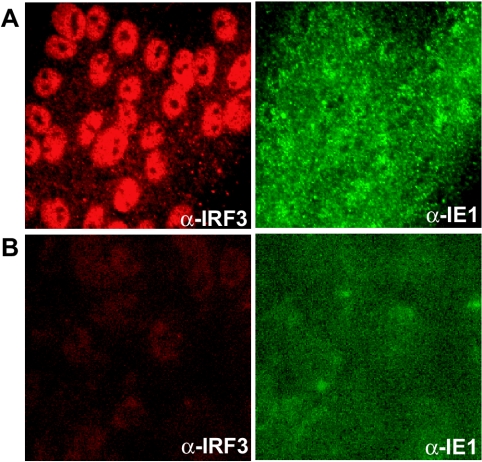
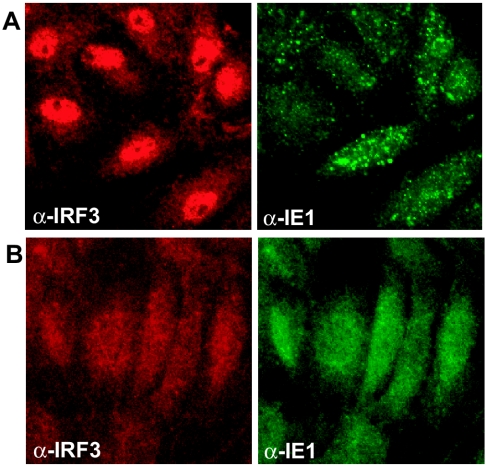
RhCMV and HCMV virus particles were UV inactivated, resulting in the absence of immediate-early gene expression in infected cells (Fig. 6). Similarly, infected cells treated with CHX did not demonstrate viral immediate-early gene expression (Fig. 7). As observed for the live virus, UV-inactivated HCMV (UV-HCMV) induced nuclear accumulation of IRF3 (Fig. 7). This was also observed upon treatment with CHX (Fig. 7). In contrast, neither UV inactivation of RhCMV nor RhCMV infection in the presence of CHX resulted in nuclear IRF3 accumulation (Fig. 6 and 7). Thus, RhCMV did not activate IRF3 even in the absence of viral or host gene expression.
FIG. 6.
HCMV but not RhCMV induces nuclear accumulation of IRF3 after UV inactivation. TRFs were treated with HCMV (A) or RhCMV (B) particles inactivated by UV as described in the text. At 6 hpi cells were stained for IRF3 (red) and IE1 (green).
FIG. 7.
HCMV but not RhCMV induces nuclear accumulation of IRF3 in the presence of cycloheximide. TRFs were treated with 100 μg of CHX/ml as described in text and infected with HCMV (A) or RhCMV (B) (MOI = 3). At 6 hpi cells were stained for IRF3 (red) and IE1 (green).
IRF3 does not dimerize after RhCMV infection.
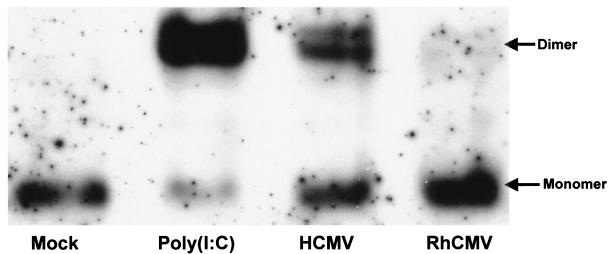
Nuclear accumulation of IRF3 first involves phosphorylation of C-terminal serine and threonine residues by IKKɛ or TBK1, followed by homodimerization and association with nuclear transcription factors CBP/p300. To determine whether IRF3 did not accumulate in the nucleus because it did not form homodimers due to lack of phosphorylation in RhCMV-infected cells, we studied the dimerization state of IRF3 in virus-infected cells. Infections were conducted in the presence of CHX to eliminate the effects of viral gene expression. As shown in Fig. 8, more slowly migrating dimerized IRF3 is detected in TRFs transfected with poly(I-C) or infected with HCMV. In contrast, the dimerization status of IRF3 in RhCMV-infected cells was similar to mock-infected cells. We therefore conclude that the lack of IRF3 nuclear accumulation results from the absence of IRF3 phosphorylation-mediated dimerization in RhCMV-infected cells.
FIG. 8.
HCMV but not RhCMV induces dimerization of IRF3. TRFs were left untreated, transfected with poly(I-C), or infected with HCMV or RhCMV (MOI = 3) in the presence of CHX (100 μg/ml). Whole-cell extracts were harvested at 6 hpi, and ca. 50 μg of sample was run on a 7.5% polyacrylamide gel, followed by blotting with an IRF3-specific antibody. Dimerized IRF3 migrates detectably slower than the monomer.
RhCMV inhibits HCMV-induced but not dsRNA-induced activation of IRF3.
Lack of IRF3 nuclear localization after RhCMV infection in the absence of viral or host protein synthesis indicates that RhCMV either lacks the ability to trigger the protein's activation or blocks activation in a manner that does not require virus gene expression, for example, via components of the virion. However, given the observation that diverse enveloped virus families (including HCMV) activate IRF3 upon cellular entry (10), it seems much more likely that RhCMV blocks IRF3 activation by a molecular mechanism that does not require viral or host gene expression. To examine whether RhCMV can inhibit IRF3 activation by a nonviral stimulus, we monitored IRF3 nuclear accumulation as induced by poly(I-C) after infection with RhCMV. TRFs were transfected with poly(I-C) for 2 to 3 h and subsequently infected with RhCMV (MOI = 3) for two to three more hours. The reverse sequence [RhCMV infection, followed by poly(I-C) transfection] was also performed. Significant IRF3 nuclear accumulation was detected under both conditions (Fig. 9), indicating that RhCMV neither blocks the signal transduction pathway activated by poly(I-C) nor reverses IRF3 nuclear accumulation induced by poly(I-C).
FIG. 9.
Treatment of TRFs with poly(I-C) and RhCMV induces nuclear accumulation of IRF3. (A) TRFs were transfected with poly(I-C) for 2 h and stained for IRF3; (B) TRFs were transfected with poly(I-C) for 2 h prior to infection with RhCMV (MOI = 3) for 2 to 3 h and stained for RhCMV IE1 and IRF3; (C) TRFs were infected with RhCMV (MOI = 3) for 2 to 3 h prior to transfection with poly(I-C) for 2 to 3 h and stained for RhCMV IE1 and IRF3.
Induction of IRF3 by poly(I-C) is known to take place via TLR3 (2). In contrast, HCMV induction of inflammatory cytokines via NF-κB has been shown to occur via TLR2 and CD14 (11). To determine whether RhCMV can inhibit IRF3 activation by HCMV, we examined IRF3 nuclear accumulation after treatment with both RhCMV and HCMV. Two experimental designs were used. (i) Cells were preinfected with HCMV (MOI = 3) for 3 h and then infected with RhCMV (MOI = 3) for 3 h, or (ii) cells were infected first with RhCMV followed by HCMV. Positive control cells were also treated with HCMV alone (MOI = 3) for 3 h.
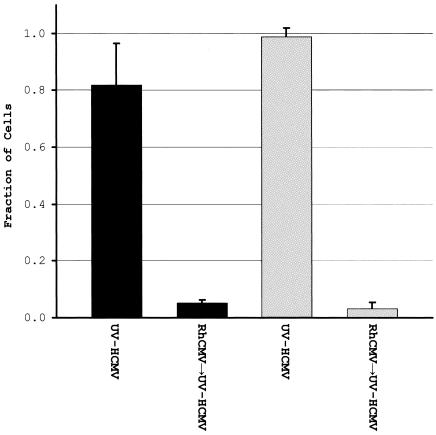
Coinfection of individual cells with live virus was verified by using antibodies that were specific for HCMV or RhCMV IE1 (Fig. 10). Preinfection with HCMV or UV-HCMV decreased the strength of RhCMV IE1 expression (Fig. 10). TRF cultures preinfected with HCMV or UV-HCMV and subsequently infected with RhCMV also seemed to exhibit additional cytopathic effects and increased syncytium formation (Fig. 10). However, the lower expression of RhCMV IE1 is presumably due to the action of the HCMV-induced innate antiviral response (4). Thus, HCMV probably induces an antiviral state that is antagonistic to RhCMV infection. In contrast, when TRFs were preinfected with RhCMV the vast majority of cells (>90%) expressed both RhCMV and HCMV specific IE1. Moreover, preinfection of cells with RhCMV inhibited or greatly diminished IRF3 induction by HCMV (Fig. 10). Since cells expressed immediate-early genes of both RhCMV and HCMV, we conclude that RhCMV actively prevented the activation of IRF3 by an HCMV-mediated trigger. UV-inactivated HCMV is known to be an even more potent inducer of ISG transcription than live virus (4, 7). Therefore, we also examined whether RhCMV could inhibit IRF3 activation by UV-inactivated HCMV. TRFs were (i) preinfected with RhCMV (MOI = 3) for 3 h, followed by treatment with UV-HCMV (MOI = 3), or (ii) pretreated with UV-HCMV for 3 h, followed by infection with RhCMV for 3 h. Positive control cells were treated only with UV-HCMV for 3 h. Cell cultures pretreated with UV-HCMV and subsequently infected with RhCMV generally displayed high IRF3 nuclear localization similar to cells treated with UV-HCMV only. In contrast, TRF cultures preinfected with RhCMV and subsequently treated with UV-HCMV exhibited greatly diminished IRF3 nuclear localization compared to cells treated with UV-HCMV only (Fig. 11). These data suggest that RhCMV is able to interfere with IRF3 activation and ultimately ISG induction triggered by HCMV entry and fusion events.
FIG. 10.
Preinfection of TRFs with RhCMV, followed by infection with HCMV, results in diminished nuclear accumulation of IRF3. (A) TRFs were infected with HCMV (MOI = 3) for 3 h and stained for HCMV IE1 and IRF3; (B) TRFs were infected with RhCMV (MOI = 3) for 3 h, followed by infection with HCMV (MOI = 3) for 3 h, and stained for RhCMV IE1 and HCMV IE1; (C) TRFs were infected with RhCMV (MOI = 3) for 3 h, followed by infection with HCMV (MOI = 3) for 3 h and stained for IRF3 and DAPI; (D) TRFs were infected with HCMV (MOI = 3) for 3 h, followed by infectionwith RhCMV (MOI = 3) for 3 h and stained for RhCMV IE1 and HCMV IE1; (E) TRFs were infected with HCMV (MOI = 3) for 3 h, followed by infection with RhCMV (MOI = 3) for 3 h and stained for IRF3 and DAPI.
FIG. 11.
Preinfection of TRFs with RhCMV, followed by infection with UV inactivated HCMV, results in diminished nuclear accumulation of IRF3. Cells were either treated with UV-HCMV for 3 h or preinfected with RhCMV for 3 h, followed by treatment with UV-HCMV for 3 h, and then stained for IRF3 and HCMV IE1, and nuclei were visualized by using DAPI. In duplicate experiments the fraction of cells exhibiting IRF3 nuclear localization after each treatment was determined by counting cells in multiple microscopic fields (≥400 cells each).
DISCUSSION
We report the finding that infection of fibroblasts with RhCMV does not result in induction of classic innate immune response gene expression. This result is surprising given the consistently observed strong HCMV-mediated induction of such pathways in different cell types. Overall, gene expression changes in RhCMV-infected cells were much fewer and of lower magnitude compared to those following HCMV infection. Importantly, the transcripts most strongly upregulated by HCMV, but unchanged by RhCMV, were largely innate immune response genes, signifying virus-specific differences in the induction of innate antiviral responses. Previously, it was speculated that induction of innate immune response genes was beneficial for the replication of HCMV (37, 45). This hypothesis was recently supported by the finding that the HCMV-induced upregulation of genes involved in prostaglandin synthesis, such as cyclooxygenase 2 (COX2), was required for virus replication (47). Interestingly, RhCMV encodes its own copy of COX2 (15) but does not induce cellular COX2 or other enzymes of the prostaglandin pathway. It is thus possible that some of the cellular genes induced by HCMV are indeed beneficial for virus replication and that RhCMV coped with this “deficiency” by copying cellular genes into its genome, a strategy well known among herpesviruses and poxviruses. However, many of the endpoint genes induced in the IFN pathway are clearly detrimental to viral replication since they interfere with general protein translation or transport (20). Furthermore, IFN-induced or proinflammatory cytokines secreted by infected cells are potent stimulators of the immune system. The resulting inflammatory response at the site of infection might or might not be beneficial for viral propagation in vivo. An alternative explanation for the difference between viruses could be that most studies involving HCMV have focused on tissue-culture-adapted laboratory strains. Unpublished observations from our laboratory and data in a recent report from by Compton and coworkers (4) indicate that clinical strains of HCMV are less active in inducing ISGs than the commonly used laboratory strain AD169. Thus, it is also possible that limiting ISG induction is important for a successful infection in vivo. However, we have thus far not found a clinical isolate that suppresses or does not induce ISGs, as observed for RhCMV. Therefore, we presume that both a species-specific component and an infectious-strain-specific component are responsible for the observed absence of ISG induction by RhCMV.
Two fundamentally different hypotheses may explain the absence of an innate immune response in infected cells to RhCMV. Either RhCMV fails to trigger IRF3 activation or RhCMV initially induces the signal transduction cascade leading to activation of IRF3 but then efficiently blocks this activation at one or more of the subsequent steps. We favor the latter hypothesis since we observed that RhCMV inhibits IRF3 activation by HCMV. Moreover, work by Mossman and coworkers showed that various herpesviruses, poxviruses, and diverse RNA viruses trigger IRF3 activation after infection, whereas the nonenveloped adenovirus failed to induce this pathway (10). In several instances (e.g., herpes simplex virus) it was necessary to inactivate viral gene expression before IRF3 phosphorylation was observed. The authors of that study therefore concluded that all enveloped DNA viruses trigger IRF3 activation. Since the only event shared by all of these viruses is their entry by membrane fusion, it seems likely that cells have sensors for such fusion events that trigger this pathway. For this reason it seems highly unlikely that a complex enveloped virus such as RhCMV would fail to induce this pathway. For HCMV it has been shown that the major surface gB is predominantly responsible for induction of ISGs (5, 37). Interestingly, an inhibitor of membrane fusion that binds specifically to gB inhibited ISG induction but did not prevent viral attachment to cells (31). It is thus likely that gB-mediated membrane fusion is the major event triggering ISG induction during entry by HCMV. Since ISG induction is also observed with purified gB, it is possible that the purified protein mimics steps involved in membrane fusion. gB is an essential protein that is highly conserved among CMVs, including RhCMV (15). Although we do not know whether isolated gB of RhCMV is able to trigger ISG induction, it seems highly likely given its high conservation and putative identical role for viral entry compared to HCMV. Moreover, gB from HCMV (a gift from T. Compton) triggered ISG induction in TRFs (not shown), suggesting that the same pathway exists in rhesus fibroblasts compared to human fibroblasts. The most likely explanation for our observations is therefore that, during entry, the gB of RhCMV initially induces the signaling pathway that ultimately leads to IRF3 activation, similarly to HCMV. In contrast to HCMV, however, RhCMV blocks this pathway very efficiently at some point of the signal transduction cascade. Since RhCMV failed to induce IRF3 or ISGs upon inhibition of viral gene expression, it is highly likely that this interfering factor is part of the virion.
At present we do not know whether the genes responsible are RhCMV specific or genes that are shared between RhCMV and HCMV. However, a possible candidate for the inhibitory function is the major tegument protein pp65 (ORF UL83). Studies from two groups reported that the HCMV-mediated induction of ISGs and proinflammatory genes was much more pronounced in cells infected with a pp65-deleted virus, suggesting that this tegument protein interferes with ISG induction (1, 7). However, the molecular mechanism of pp65 interference with ISG induction remains a point of contention. Although one study concluded that pp65 prevents the activation of NF-κB as well as IRF1, whereas IRF3 was activated (7), another study reported the contrasting result that pp65 was necessary and sufficient for HCMV-mediated IRF3 inhibition in human fibroblasts but did not contribute to NF-κB inhibition (1). Since the pathways leading to NF-κB and IRF1 activation overlap with that of IRF3 (12, 35), HCMV may interfere with a common event that is upstream of these innate response pathways. However, interference by HCMV seems to be inefficient, since HCMV ultimately does not prevent the transcriptional upregulation of hundreds of IFN and inflammatory response genes. In stark contrast, we observed the complete absence of this innate response in fibroblasts infected with RhCMV. Thus, interference as part of a reproductive strategy may be conserved between the viruses, but either RhCMV has developed a more efficient mechanism or HCMV has lost efficiency, or both. Intriguingly, RhCMV encodes two copies of pp65 (15) that could be more specialized and thus more efficient in blocking ISG induction. Since pp65 is an abundant tegument protein, this would also be consistent with the prediction that the IRF3 interfering protein is likely a component of the virion that can block this pathway even in the absence of viral protein synthesis. Viral tegument proteins are released into the cytoplasm upon viral entry where they could act on IRF3. We are currently in the process of testing this hypothesis.
The signal transduction pathways leading from viral entry to IRF3 activation are currently only poorly understood. However, HCMV was shown to trigger the innate immune response via a complex of TLR-2 and CD14 (11). In contrast, dsRNA and its mimic poly(I-C) act via TLR-3 (2). Since we observed that RhCMV was able to block IRF3 activation by HCMV, but not by poly(I-C), interference may take place within the signal transduction cascade leading from TLR-2/CD14 to IRF3 but not within TLR-3 signaling. This interference most likely occurs at events upstream of IRF3 activation since we did not observe inhibition of IRF3 phosphorylation or poly(I-C) induced IRF3 activation. Recent observations implicate the IKK complex protein IKKɛ and TANK binding-kinase in IRF3 phosphorylation (12, 35). Thus, RhCMV might prevent activation of these kinases via the TLR-2 pathway but not via the TLR-3 pathway. Further work will be required to elucidate this.
The observation that the host cell is transcriptionally relatively quiet in response to RhCMV infection is remarkable since CMVs are among the most complex viruses known. Interestingly, many of the genes induced by RhCMV independently of IRF3 were also induced by HCMV (Table S1 in the supplemental material). It is conceivable that induction of some of these genes might be required for cytomegalovirus replication in fibroblasts. Conversely, genes that were induced by HCMV, but not by RhCMV are likely linked to the activation of IRF3. We hypothesize that, at least in rhesus fibroblasts, activation of IRF3 is the central event in triggering the host response to HCMV since NF-κB, the other major factor involved in transcriptional induction of innate immune response genes, did not accumulate in the nucleus. Studies with constitutively active IRF3 identified many transcripts that are induced by IRF3 alone without the need of NF-κB activation (14), and a significant overlap exists between these genes and genes induced by HCMV (8). In contrast, genes that require the cooperation of both transcription factors, such as IFN-β, are not consistently activated by HCMV. For instance, IFN-β induction was observed in DNA microarray experiments only upon CHX treatment, whereas infection with live virus did not trigger IFN-β induction (8). However, it was shown that the concomitant administration of the TNF receptor ligand lymphotoxin induces IFN-β in HCMV-infected cells (3). These data suggest that HCMV interferes with IFN-β activation upstream of NF-κB activation by lymphotoxin. This is similar to our observation that RhCMV can block the activation of IRF3 by viral (i.e., HCMV) stimuli but is unable to prevent IRF3 activation by poly(I-C) in RhCMV-infected cells. Taken together, these observations suggest that the viral inhibitors of IRF3 and NF-κB-activation are tailored toward preventing viral stimuli from activating these important regulators of innate responses but are unable to prevent their activation by exogenous stimuli, e.g., by the cellular immune system.
In conclusion, we have identified an extraordinarily efficient pathway of inhibiting activation of the innate immune response by CMV. To our knowledge, this is the first description of an enveloped virus that fails to induce ISG expression upon entry. The identification of the viral genes responsible for this suppression is thus likely to yield excellent tools to dissect the as yet poorly defined pathway that leads from viral entry to induction of ISGs.
Supplementary Material
Acknowledgments
We thank Takahashi Fujita for generously providing the polyclonal IRF3 antibody and Jay Nelson for providing the mouse HCMV IE1 antibody. We also thank John Hiscott, Teresa Compton, and Karl Boehme for technical advice.
Footnotes
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Abate, D., S. Watanabe, and E. Mocarski. 2004. Major human cytomegalovirus structural protein pp65 (ppUL83) prevents interferon response factor 3 activation in the interferon response. J. Virol. 78:10995-11006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alexopoulou, L., A. C. Holt, R. Medzhitov, and R. A. Flavell. 2001. Recognition of double-stranded RNA and activation of NF-κB by Toll-like receptor 3. Nature 413:732-738. [DOI] [PubMed] [Google Scholar]
- 3.Benedict, C. A., T. A. Banks, L. Senderowicz, M. Ko, W. J. Britt, A. Angulo, P. Ghazal, and C. F. Ware. 2001. Lymphotoxins and cytomegalovirus cooperatively induce interferon-beta, establishing host-virus detente. Immunity 15:617-626. [DOI] [PubMed] [Google Scholar]
- 4.Boehme, K. W., J. Singh, S. T. Perry, and T. Compton. 2004. Human cytomegalovirus elicits a coordinated cellular antiviral response via envelope glycoprotein B. J. Virol. 78:1202-1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyle, K. A., R. L. Pietropaolo, and T. Compton. 1999. Engagement of the cellular receptor for glycoprotein B of human cytomegalovirus activates the interferon-responsive pathway. Mol. Cell. Biol. 19:3607-3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bresnahan, W. A., G. E. Hultman, and T. Shenk. 2000. Replication of wild-type and mutant human cytomegalovirus in life-extended human diploid fibroblasts. J. Virol. 74:10816-10818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Browne, E. P., and T. Shenk. 2003. Human cytomegalovirus UL83-coded pp65 virion protein inhibits antiviral gene expression in infected cells. Proc. Natl. Acad. Sci. USA 100:11439-11444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Browne, E. P., B. Wing, D. Coleman, and T. Shenk. 2001. Altered cellular mRNA levels in human cytomegalovirus-infected fibroblasts: viral block to the accumulation of antiviral mRNAs. J. Virol. 75:12319-12330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chismar, J. D., T. Mondala, H. S. Fox, E. Roberts, D. Langford, E. Masliah, D. R. Salomon, and S. R. Head. 2002. Analysis of result variability from high-density oligonucleotide arrays comparing same-species and cross-species hybridizations. BioTechniques 33:516-522. [DOI] [PubMed] [Google Scholar]
- 10.Collins, S. E., R. S. Noyce, and K. L. Mossman. 2004. Innate cellular response to virus particle entry requires IRF3 but not virus replication. J. Virol. 78:1706-1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Compton, T., E. A. Kurt-Jones, K. W. Boehme, J. Belko, E. Latz, D. T. Golenbock, and R. W. Finberg. 2003. Human cytomegalovirus activates inflammatory cytokine responses via CD14 and Toll-like receptor 2. J. Virol. 77:4588-4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fitzgerald, K. A., S. M. McWhirter, K. L. Faia, D. C. Rowe, E. Latz, D. T. Golenbock, A. J. Coyle, S. M. Liao, and T. Maniatis. 2003. IKKε and TBK1 are essential components of the IRF3 signaling pathway. Nat. Immunol. 4:491-496. [DOI] [PubMed] [Google Scholar]
- 13.Goodbourn, S., L. Didcock, and R. E. Randall. 2000. Interferons: cell signalling, immune modulation, antiviral response and virus countermeasures. J. Gen. Virol. 81:2341-2364. [DOI] [PubMed] [Google Scholar]
- 14.Grandvaux, N., M. J. Servant, B. tenOever, G. C. Sen, S. Balachandran, G. N. Barber, R. Lin, and J. Hiscott. 2002. Transcriptional profiling of interferon regulatory factor 3 target genes: direct involvement in the regulation of interferon-stimulated genes. J. Virol. 76:5532-5539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hansen, S. G., Strelow, L. I., Franchi, D. C., Anders, D. G., and S. W. Wong. 2003. Analysis of the complete DNA sequence of rhesus cytomegalovirus. J. Virol. 77:6620-6636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hertzog, P. J., L. A. O'Neill, and J. A. Hamilton. 2003. The interferon in TLR signaling: more than just antiviral. Trends Immunol. 24:534-539. [DOI] [PubMed] [Google Scholar]
- 17.Iwamura, T., M. Yoneyama, K. Yamaguchi, W. Suhara, W. Mori, K. Shiota, Y. Okabe, H. Namiki, and T. Fujita. 2001. Induction of IRF-3/-7 kinase and NF-κB in response to double-stranded RNA and virus infection: common and unique pathways. Genes Cells 6:375-388. [DOI] [PubMed] [Google Scholar]
- 18.Jenner, R. G., and C. Boshoff. 2002. The molecular pathology of Kaposi's sarcoma-associated herpesvirus. Biochim. Biophys. Acta 1602:1-22. [DOI] [PubMed] [Google Scholar]
- 19.Karin, M., and M. Delhase. 2000. The IκB kinase (IKK) and NF-κB: key elements of proinflammatory signalling. Semin. Immunol. 12:85-98. [DOI] [PubMed] [Google Scholar]
- 20.Katze, M. G., Y. He, and M. Gale, Jr. 2002. Viruses and interferon: a fight for supremacy. Nat. Rev. Immunol. 2:675-687. [DOI] [PubMed] [Google Scholar]
- 21.Kerr, M. K., M. Martin, and G. A. Churchill. 2000. Analysis of variance for gene expression microarray data. J. Comput. Biol. 7:819-837. [DOI] [PubMed] [Google Scholar]
- 22.Kowalik, T. F., B. Wing, S. Haskill, J. C. Azizkhan, A. S. Baldwin, and E. H. Huang. 1993. Multiple mechanisms are implicated in the regulation of NF-κB activity during human cytomegalovirus infection. Proc. Natl. Acad. Sci. USA 90:1107-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumar, K. P., K. M. McBride, B. K. Weaver, C. Dingwall, and N. C. Reich. 2000. Regulated nuclear-cytoplasmic localization of interferon regulatory factor 3, a subunit of double-stranded RNA-activated factor 1. Mol. Cell. Biol. 20:4159-4168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leonard, W. J. 2001. Role of Jak kinases and STATs in cytokine signal transduction. Int. J. Hematol. 73:271-277. [DOI] [PubMed] [Google Scholar]
- 25.Lin, R., C. Heylbroeck, P. M. Pitha, and J. Hiscott. 1998. Virus-dependent phosphorylation of the IRF-3 transcription factor regulates nuclear translocation, transactivation potential, and proteasome-mediated degradation. Mol. Cell. Biol. 18:2986-2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin, R., R. S. Noyce, S. E. Collins, R. D. Everett, and K. L. Mossman. 2004. The herpes simplex virus ICP0 RING finger domain inhibits IRF3- and IRF7-mediated activation of interferon-stimulated genes. J. Virol. 78:1675-1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Livak, K. J., and T. D. Schmittgen. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25:402-408. [DOI] [PubMed] [Google Scholar]
- 28.Lockridge, K. M., G. Sequar, S. S. Zhou, Y. Yue, C. P. Mandell, and P. A. Barry. 1999. Pathogenesis of experimental rhesus cytomegalovirus infection. J. Virol. 73:9576-9583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mossman, K. L., P. F. Macgregor, J. J. Rozmus, A. B. Goryachev, A. M. Edwards, and J. R. Smiley. 2001. Herpes simplex virus triggers and then disarms a host antiviral response. J. Virol. 75:750-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Navarro, L., K. Mowen, S. Rodems, B. Weaver, N. Reich, D. Spector, and M. David. 1998. Cytomegalovirus activates interferon immediate-early response gene expression and an interferon regulatory factor 3-containing interferon-stimulated response element-binding complex. Mol. Cell. Biol. 18:3796-3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Netterwald, J. R., T. R. Jones, W. J. Britt, S. J. Yang, I. P. McCrone, and H. Zhu. 2004. Postattachment events associated with viral entry are necessary for induction of interferon-stimulated genes by human cytomegalovirus. J. Virol. 78:6688-6691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Phillips, J., and J. H. Eberwine. 1996. Antisense RNA amplification: a linear amplification method for analyzing the mRNA population from single living cells. Methods 10:283-288. [DOI] [PubMed] [Google Scholar]
- 33.Preston, C. M., A. N. Harman, and M. J. Nicholl. 2001. Activation of interferon response factor-3 in human cells infected with herpes simplex virus type 1 or human cytomegalovirus. J. Virol. 75:8909-8916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sequar, G., W. J. Britt, F. D. Lakeman, K. M. Lockridge, R. P. Tarara, D. R. Canfield, S. S. Zhou, M. B. Gardner, and P. A. Barry. 2002. Experimental coinfection of rhesus macaques with rhesus cytomegalovirus and simian immunodeficiency virus: pathogenesis. J. Virol. 76:7661-7671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sharma, S., B. R. tenOever, N. Grandvaux, G. P. Zhou, R. Lin, and J. Hiscott. 2003. Triggering the interferon antiviral response through an IKK-related pathway. Science 300:1148-1151. [DOI] [PubMed] [Google Scholar]
- 36.Shuai, K., and B. Liu. 2003. Regulation of JAK-STAT signalling in the immune system. Nat. Rev. Immunol. 3:900-911. [DOI] [PubMed] [Google Scholar]
- 37.Simmen, K. A., J. Singh, B. G. Luukkonen, M. Lopper, A. Bittner, N. E. Miller, M. R. Jackson, T. Compton, and K. Fruh. 2001. Global modulation of cellular transcription by human cytomegalovirus is initiated by viral glycoprotein B. Proc. Natl. Acad. Sci. USA 98:7140-7145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith, E. J., I. Marie, A. Prakash, A. Garcia-Sastre, and D. E. Levy. 2001. IRF3 and IRF7 phosphorylation in virus-infected cells does not require double-stranded RNA-dependent protein kinase R or IκB kinase but is blocked by vaccinia virus E3L protein. J. Biol. Chem. 276:8951-8957. [DOI] [PubMed] [Google Scholar]
- 39.Suhara, W., M. Yoneyama, I. Kitabayashi, and T. Fujita. 2002. Direct involvement of CREB-binding protein/p300 in sequence-specific DNA binding of virus-activated interferon regulatory factor-3 holocomplex. J. Biol. Chem. 277:22304-22313. [DOI] [PubMed] [Google Scholar]
- 40.Tarantal, A. F., M. S. Salamat, W. J. Britt, P. A. Luciw, A. G. Hendrickx, and P. A. Barry. 1998. Neuropathogenesis induced by rhesus cytomegalovirus in fetal rhesus monkeys (Macaca mulatta). J. Infect. Dis. 177:446-450. [DOI] [PubMed] [Google Scholar]
- 41.Yang, Y. H., S. Dudoit, P. Luu, D. M. Lin, V. Peng, J. Ngai, and T. P. Speed. 2002. Normalization for cDNA microarray data: a robust composite method addressing single and multiple slide systematic variation. Nucleic Acids Res. 30:e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yoneyama, M., W. Suhara, Y. Fukuhara, M. Fukuda, E. Nishida, and T. Fujita. 1998. Direct triggering of the type I interferon system by virus infection: activation of a transcription factor complex containing IRF-3 and CBP/p300. EMBO J. 17:1087-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yurochko, A. D., E. S. Hwang, L. Rasmussen, S. Keay, L. Pereira, and E. S. Huang. 1997. The human cytomegalovirus UL55 (gB) and UL75 (gH) glycoprotein ligands initiate the rapid activation of Sp1 and NF-κB during infection. J. Virol. 71:5051-5059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yurochko, A. D., T. F. Kowalik, S. M. Huong, and E. S. Huang. 1995. Human cytomegalovirus upregulates NF-κB activity by transactivating the NF-κB p105/p50 and p65 promoters. J. Virol. 69:5391-5400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhu, H., J. P. Cong, G. Mamtora, T. Gingeras, and T. Shenk. 1998. Cellular gene expression altered by human cytomegalovirus: global monitoring with oligonucleotide arrays. Proc. Natl. Acad. Sci. USA 95:14470-14475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhu, H., J. P. Cong, and T. Shenk. 1997. Use of differential display analysis to assess the effect of human cytomegalovirus infection on the accumulation of cellular RNAs: induction of interferon-responsive RNAs. Proc. Natl. Acad. Sci. USA 94:13985-13990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhu, H., J. P. Cong, D. Yu, W. A. Bresnahan, and T. E. Shenk. 2002. Inhibition of cyclooxygenase 2 blocks human cytomegalovirus replication. Proc. Natl. Acad. Sci. USA 99:3932-3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.