Escherichia coli is the most abundant facultative anaerobic gram-negative bacterium of the intestinal microflora, naturally colonizing the mucous layer of the colon. A conserved core genomic structure is common to both commensal and pathogenic strains, providing the microorganisms with mechanisms required for survival under the competitive conditions in the gut, as well as the ability to spread among hosts and survive in the environment (90). In pathogenic bacteria, the core genome framework is enhanced with novel genetic islands and small clusters of genes often associated with increased virulence (74-76). These novel genes provide the bacteria with a higher level of adaptation, leading to specific tissue targeting that can open up new ecological niches and facilitate efficient dissemination to new hosts. A striking example of highly adapted enteric bacteria that have acquired, by horizontal gene transfer, superior fitness and a competitive edge within the gut is a group of extracellular intestinal pathogens that have evolved to use attaching and effacing (A/E) lesion formation as a major mechanism of tissue targeting and infection. Among these pathogenic bacteria are the diarrheal agents enteropathogenic E. coli (EPEC) and enterohemorrhagic E. coli (EHEC), highly successful pathogens that have adapted to cause infections in different hosts via related, but distinct, mechanisms of transmission.
EPEC and EHEC cause acute gastroenteritis in humans (22, 135). EPEC, the first type of E. coli to be associated with human disease, is a frequent cause of infantile diarrhea in the developing world, and EHEC, an emerging zoonotic pathogen, causes a wide spectrum of illnesses ranging from mild diarrhea to severe diseases such as hemorrhagic colitis and hemolytic uremic syndrome. Hemolytic uremic syndrome is the leading cause of acute pediatric renal failure in developed countries and is associated with the production of potent Shiga toxins (135). Strains of EHEC belonging to serogoup O157 are most commonly associated with severe human diseases.
EHEC and EPEC are sophisticated pathogens that display several virulence-associated traits, some of which are also found in other bacterial pathogens (82). The mechanisms by which EPEC and EHEC intimately adhere to epithelial cells represent the most studied feature in their pathogenesis. By adhering to intestinal epithelial cells, these bacteria subvert cytoskeletal processes to produce a histopathological feature known as an A/E lesion (reviewed in reference 64), which is characterized by localized destruction of brush border microvilli and intimate attachment of the bacteria to the plasma membranes of the host epithelial cells.
The capacity to form A/E lesions is encoded mainly by the locus of enterocyte effacement (LEE) pathogenicity island (PAI) (55, 125). The fact that the LEE is inserted into diverse chromosomal loci among various EPEC and EHEC serotypes suggests that it was acquired multiple times during the evolution of these pathogens (178).
Regulation of LEE gene expression is complex, dependent on environmental conditions, quorum sensing, and several regulators, including the EPEC adherence factor plasmid-carried per (plasmid-encoded regulator) locus and the LEE-encoded Ler (LEE-encoded regulator), GrlA (global regulator of LEE activator), and GrlR (global regulator of LEE repressor) (90).
In additon to Ler, GrlR, and GrlA (47, 128), the 5′ end of the main coding strand of the LEE region encodes structural components of a type III secretion system (TTSS) commonly found in pathogenic gram-negative bacteria (82). The central part of the LEE encodes the outer membrane adhesin intimin (65, 85) and the translocated intimin receptor (Tir) (43, 97), and the 3′ end encodes additional TTSS structural, translocator, and effector proteins. Important recent discoveries have highlighted the fact that the LEE-encoded TTSS is being employed to translocate effector proteins that are encoded at different locations in the genome and play an essential role in infection and disease. In this review, we summarize the current state of knowledge about EPEC and EHEC infections, concentrating on the mechanisms that the EPEC and EHEC pathogens employ, while remaining extracellular, to subvert cellular functions for the benefit of the bacteria.
THE FILAMENTOUS TTSS
The TTSS pathway is associated with the virulence of many gram-negative pathogens which infect humans, animals, insects, and plants. It is utilized by the pathogens to directly translocate virulence factors from the bacteria into the targeted host cells in a single step. The TTSS apparatus is a multicomponent organelle assembled from the products of approximately 20 genes (Fig. 1 and 2).
FIG. 1.
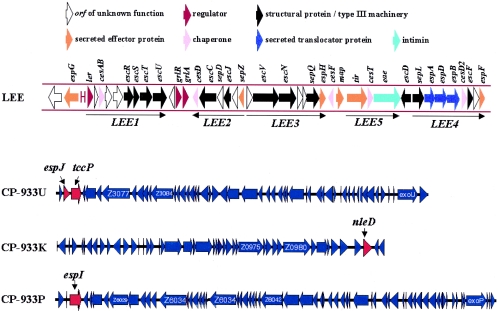
Genetic organization of the EPEC/EHEC LEE and EHEC prophages CP-933U, CP-933K, and CP-933P. The image was generated using coliBase (http://colibase.bham.ac.uk/) (21).
FIG. 2.
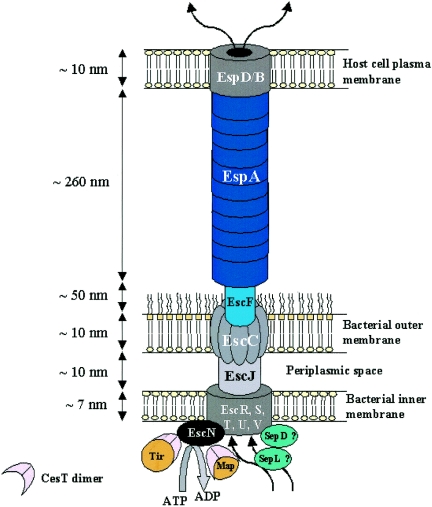
Schematic representation of the EPEC/EHEC type III secretion apparatus. The basal body of the TTSS is composed of the secretin EscC, the inner membrane proteins EscR, EscS, EscT, EscU, and EscV, and the EscJ lipoprotein, which connects the inner and outer membrane ring structures. EscF constitutes the needle structure, whereas EspA subunits polymerize to form the EspA filament. EspB and EspD form the translocation pore in the host cell plasma membrane, connecting the bacteria with the eukaryotic cell via EspA filaments. The cytoplasmic ATPase EscN provides the energy to the system by hydrolyzing ATP molecules into ADP. SepD and SepL have been represented as cytoplasmic components of the TTSS.
Many components are broadly conserved among both virulence and flagellar TTSSs (3, 117, 164). The two structures consist of a succession of protein rings spanning both bacterial membranes with a needle-like extension or a flagellar hook (3, 10). The type III secretion organelles, termed the needle complexes (NC), of Salmonella and Shigella species and EPEC have been visualized by electron microscopy, revealing overall similar structures and shapes (9, 40, 100, 108, 109, 123, 151, 162, 180). The assembly of the TTSS machinery is sequential. First, the membrane-bound components, which harbor a cleavable sec-dependent signal sequence, are exported through the sec pathway to form the foundation of the NC. Cytosolic components are then added to the basal structure. The second phase of NC assembly occurs in a sec-independent manner, through the previously assembled needle base. The type III export machinery is then utilized for the secretion of components constituting the more distal elements of the apparatus. Cytoplasmic ATPases, related to the catalytic subunits of bacterial F0F1 ATPase, are believed to energize the type III export machinery and promote protein secretion (4).
In EPEC and EHEC, EscC and EscV are the main components of the outer and inner membrane ring structures, respectively (69). EscC (a mature protein of 54 kDa) belongs to the secretin family of proteins, which are found in all TTSSs (e.g., YscC in Yersinia spp. [106, 144], InvG in Samonella spp. [28, 108], HrcC in Pseudomonas spp. [46], and MxiD in Shigella spp. [11, 150, 162]) and are involved in the transport of molecules across the outer membrane by formation of a large homomultimeric annular complex (82). Although EscC has a cleavable signal sequence, which suggests that the protein is secreted through the sec pathway, its correct localization seems to be dependent on both sec and type III secretion systems as it was found in the periplasm of escN and escV mutants (69). EscV (a mature protein of 72 kDa) is predicted to have seven transmembrane domains and to form the EPEC/EHEC inner membrane ring structures. The presence of a putative signal sequence indicates that the protein is likely to be directed to the inner membrane through the sec pathway, as observed for EscV homologues in other TTSSs (e.g., PrgH in Salmonella [100, 108, 109], MxiG in Shigella [11, 150, 162], and YscV in Yersinia [26]).
The EPEC/EHEC TTSS protein EscJ, which also contains a sec-dependent signal sequence, is highly conserved within TTSSs (e.g., PrgK in Salmonella [100, 160], MxiJ in Shigella [5, 150], and YscJ in Yersinia [5]). EscJ and its homologues are lipoproteins proposed to span the periplasm, serving as a bridge between the inner and outer membrane protein rings (35, 46, 150). Those proteins share sequence similarity within the domain of the flagellar protein FliF that is believed to line the central pore of the inner membrane ring and proposed to form part of the proximal rod structure (35, 169). EscJ is required for the secretion of more distal apparatus components across the outer membrane, as an escJ mutant failed to assemble functional type III machinery. The solution structure of the EPEC EscJ has recently been determined by nuclear magnetic resonance and shows that EscJ is composed of two structured domains connected by a linker, creating a molecule that can stretch up to 10 nm in length, which approximates the width of the periplasmic spaces of gram-negative bacteria (35). The covalent linkage between the two domains is required to retain EscJ biological activity (35). This suggests that EscJ could form a cylindrical structure functioning as a bridge across the periplasmic space, connecting the outer and inner membrane rings of the NC. Structural analysis of the Salmonella NC recently revealed that PrgK is associated with the inner membrane rings and that the periplasmic rod is mainly composed of PrgJ (123). As no PrgJ homologue is present in EPEC, it is suggested that PrgK/J function is combined in a single EPEC protein, EscJ. In addition, cell fractionation has shown that, unlike PrgK, EscJ is associated mainly with the outer membrane, although small amounts of EscJ were cofractionated with inner membrane markers (35).
The EPEC/EHEC TTSS needle is likely to be composed of a single protein, EscF (180); EscF homologues are PrgI in Salmonella (123, 159), YscF in Yersinia (122), and MxiH in Shigella (86). The EscF needle forms a projection channel required for TTSS-dependent protein secretion, as an escF mutation abolishes secretion of the translocator and effector proteins (151, 180). In situ, EPEC needle length appears to be tightly regulated (180). InvJ in Salmonella (146), Spa32 in Shigella (163), and YscP in Yersinia (87) spp. were all shown to control the needle lengths in their respective TTSSs. Such a regulator has yet to be identified in EPEC/EHEC TTSSs.
The unique feature of EPEC/EHEC TTSSs is the presence of a filamentous extension to the NC-associated EscF needle, called the EspA filament (40, 51, 103, 151, 180), defining a new class of filamentous TTSS (FTTSS). The EspA filament is a polymer of the translocator protein EspA, which is likely to be the sole constituent of these hollow filamentous conduits (39, 45). Polymerization of the EspA filaments is mediated by coiled-coil interactions between EspA subunits (45). EspA filaments have been shown to bind directly to the needle protein EscF (180). Daniell and collaborators have determined the three-dimensional structure of the EspA filament (39). It consists of a helical tube with an outer diameter of ca. 120 Å and a continuous hollow central channel of ca. 2.5 nm in diameter. Although the two systems differ in size, comparisons can easily be established between the EspA filaments and the flagellar filaments in terms of helical symmetry and packing of the subunits to form the filamentous structure. In a process similar to flagellar elongation, which occurs at the distal end of the filamentous structure (56), newly synthesized EspA subunits are incorporated at the tip of the growing filament (35a). Like the NC, the EspA filament has a defined length in situ. Increasing the intracellular concentration of EspA subunits results in significantly longer EspA filaments (35a), suggesting that the amount of monomeric EspA produced in bacteria is the limiting factor and is set for a defined filament length. The EspA filament has been identified as a hollow tube, suggesting that proteins are delivered from the bacteria to the host cell through this channel. Recent studies showing that the effector protein Tir is secreted from the filament tip to the extracellular medium have provided the first experimental evidence that proteins that are secreted through the FTTSS travel along the hollow EspA filament (35a). Mature EspA filaments are important adhesion factors (23, 51), establishing a transient link between the bacterium and the host cell (103) which enables effector protein translocation. Following effector protein translocation, EspA filaments and the NC are eliminated from the bacterial cell surface; this is necessary to allow intimate bacterial attachment through intimin-Tir interactions (64, 103). Elimination of surface TTSS components parallels down-regulation of LEE gene expression in EHEC attached to eukaryotic plasma membranes (36).
Effector proteins are delivered to the host cell cytoplasm from the extremity of the EspA filament through a translocation pore formed in the plasma membrane of the host cell by the translocator proteins EspB and EspD (13, 38, 83, 107, 110). In addition, EspD is required for the biogenesis of the EspA filaments (103). This suggests that EspD could have a dual role, first as a capping protein of the EspA filament and second as an anchor connecting the filament to the host cell plasma membrane (45, 59). Translocator proteins among TTSSs present low homology. However, they share similar topologies and structural properties such as hydrophobic transmembrane domains, predicted coiled-coil domains, and the capacity to homo- and/or heteromultimerize to form a functional translocation pore (38, 44, 61, 172). EspD has been shown to interact with itself (33, 38) and with EspB (83); both EspD and EspB can be purified from eukaryotic cell membranes following EPEC infection (153, 172, 181). Functionality of the translocation pore can be demonstrated by its ability to mediate hemolysis of red blood cells (RBCs) (77, 104, 153, 174). This system has shown that EspD is the major component of the translocation pore and that EspB is required to achieve full hemolytic activity (153).
An additional protein called SepL, a homologue of SsaL in Salmonella (25), has recently been reported to play a role in the formation of the translocation apparatus (138). SepL is a soluble cytoplasmic protein which interacts with SepD (33). SepD is predicted to be cytosolic and was identified as a component of the TTSS, as a sepD mutant strain is unable to secrete translocator or effector proteins or to induce A/E lesions. In contrast, a sepL mutant secretes normal levels of effector proteins. However, as it secretes markedly reduced quantities of those proteins involved in translocation (EspA, EspB, and EspD) and is deficient in EspA filament biosynthesis, it is incapable of A/E lesion formation (47, 138). SepL is the first protein reported to have a selective activity towards effector and translocator proteins of the EPEC/EHEC TTSS. These data suggest that SepL and SepD could be involved in the “switch” from secretion of translocator proteins to secretion of effector proteins through the type III machinery.
TYPE III SECRETION CHAPERONES
Chaperones are essential to assure efficient secretion and translocation of many type III secreted proteins (82, 177). The absence of chaperones can be detrimental for their specific substrates, impairing their secretion and/or stability (14, 27, 176). Type III chaperones have little sequence homology, although they show structural similarities (44, 177). Type III chaperones have been divided into four classes (IA, IB, II, and III) based on sequence homology and functional properties (140).
Up to now, five type III chaperones have been described for EPEC/EHEC. CesF belongs to class IA and chaperones the effector protein EspF (54). CesT, also a member of class IA, is a bivalent chaperone required for translocation of the effector proteins Tir and Map (1, 32, 52). Accordingly, we propose to rename CesT as CesMT. CesMT has been shown to function as a homodimer to chaperone Tir (114). In addition, CesMT binds the EscN type III ATPase, suggesting a role for the chaperone in bringing Tir into physical contact with the type III secretion apparatus (68).
CesD is a member of class II, which shares sequence similarities with other type III chaperones such as Shigella IpgC and Yersinia SycD (139, 176). CesD is a bivalent chaperone, has for substrates EspD and EspB, and localizes in both the cytoplasm and the inner membranes (173). It plays an important role in the secretion of its substrates, although a CesD mutant does not completely abolish effector protein translocation or A/E lesion formation. This is likely to be related to the existence of auxiliary chaperones: CesD2 for EspD, which also localizes to the cytoplasm and the inner membrane compartments (136), and CesAB, which is likely to be the primary chaperone for EspB. In addition, CesAB chaperones EspA and is essential for the stabilization of cytoplasmic EspA and for EspA filament biogenesis (34).
OUTCOMES OF EFFECTOR PROTEIN TRANSLOCATION
The hallmark of EPEC and EHEC infection is induction of A/E lesions, characterized by local effacement of the brush border microvilli and intimate attachment of the bacterium to the plasma membrane of the host cell (102). Importantly, while remaining extracellular, EPEC and EHEC subvert the host cell cytoskeletal microtubule, intermediate filament (IF), and actin microfilament networks.
Other alterations to the host epithelial cells caused by EPEC and EHEC infections include (i) disruption of intestinal barrier function, including increased tight-junction permeability in infected polarized cells (127, 133, 170) paralleled by a decrease in transepithelial resistance (TER); (ii) loss of mitochondrial membrane potential, triggering the formation of misshapen mitochondria, indicative of mitochondrial swelling and damage (98, 99); (iii) inhibition of the cell cycle G2/M phase transition (121); and (iv) induction of cellular apoptosis (29, 30, 60). The physiological consequences of the infection include (i) production of interleukin-8 and transmigration of acute inflammatory cells, primarily polymorphonuclear cells, to the site of infection (149) and (ii) diarrhea.
INTIMIN: THE OUTER MEMBRANE EPEC AND EHEC ADHESION MOLECULE
Intimin is exported via the general secretory pathway to the periplasm, where it is inserted into the outer membrane by a putative autotransport mechanism (167). Intimin has two functional regions: the N-terminal region, highly conserved between different EPEC and EHEC strains, is inserted into the bacterial outer membrane, forming a β-barrel-like structure, and mediates dimerization (167). The C-terminal 280-amino-acid sequence of intimin (Int280) is variable, defines several intimin types (2, 65), extends from the bacterium, and interacts with receptors in the host cell plasma membrane (62). Int280 comprises three globular domains: two immunoglobulin-like domains and a C-type lectin-like domain; the latter, together with the last immunoglobulin-like domain, contains the receptor-binding site (7, 92, 111, 115). Compelling evidence has recently been accumulated showing that intimin binds, in addition to Tir, a receptor encoded by the host cell (65, 111). Two potential host cell-carried intimin receptors (Hir) have so far been identified, β1 integrin (63) and nucleolin (156, 157), although the physiological relevance of these interactions has yet to be demonstrated. Interaction of intimin with host cells stimulates production of microvilli-like processes (142), intimin types influence gut tissue tropism (58, 141, 145), and intimin-Hir interaction has been recently shown to be essential for EPEC-induced modulation of the tight junctions (41).
LEE-ENCODED EFFECTORS
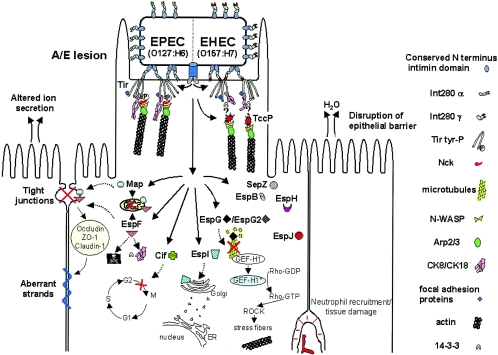
Tir, Map, EspF, EspG, EspH, SepZ, and EspB are known LEE-encoded effector proteins. Below we summarize the current knowledge about their functions (Fig. 1 and 3; Table 1).
FIG. 3.
EPEC and EHEC translocate LEE-encoded and other effector proteins into the host cell cytosol. These effectors trigger cytoskeleton rearrangements (Tir, EspH, EspF, and EspG), disruption of the epithelial barrier (EspF and Map), cytotoxicity (EspF and Cif), and host cell responses that ultimately generate watery diarrhea. Different from EPEC Tir, EHEC Tir is not tyrosine phosphorylated and does not interact with Nck in order to trigger actin polymerization at the site of bacterial adhesion; instead, EHEC translocates an effector protein, TccP/EspFu, which has an Nck-like activity and is essential to promote the reorganization of the actin cytoskeleton underneath adherent EHEC bacteria. ER, endoplasmic reticulum.
TABLE 1.
List of effector proteins of EHEC, EPEC, and C. rodentium
| Effector protein | Estimated molecular massb (kDa) | Localization in the genomec | Localization in host cells | Function(s) | Reference(s) |
|---|---|---|---|---|---|
| EPEC/EHEC/ C. rodentium | |||||
| Tir | 56-68* | LEE | Tip of pedestal | Intimate attachment; A/E lesion formation; actin polymerization | 97 |
| Map | 20 | LEE | Mitochondria | Disruption of TER and mitochondrial membrane potential; filopodium formation | 98, 99 |
| EspF | 20-31* | LEE | Mitochondria | Disruption of TER and mitochondrial membrane potential; induction at cell death | 30, 41, 126, 134, 137, 170, 171 |
| EspG | 44 | LEE | Pedestal/colocalization with tubulin | Destruction of microtubule network | 124 |
| EspH | 21 | LEE | Underneath microcolony | Cytoskeleton modulation | 168 |
| SepZ | 9.5 | LEE | Unknown | Unknown | 89 |
| EspB | 32 | LEE | Bacterial attachment site | Cytoskeleton modulation | 165 |
| EspI/NleA | 54 | CP-933P | Golgi | Unknown | 73, 130 |
| EspJ | 25 | CP-933U | Unknown | Regulation of clearance dynamics in vivo | 37 |
| NleBa | 39 | O island 122/CP-933K | Unknown | Unknown | 47 |
| NleC | 40 | CP-933K | Unknown | Unknown | 47 |
| NleD | 28 | CP-933K | Unknown | Colonization in calves | 47, 50 |
| NleE | 27 | O island 122 | Unknown | Unknown | 47 |
| NleF | 24 | O island 71 | Unknown | Unknown | 47 |
| NleHa | 33 | nt 2281573-2282484d/CP-933K | Unknown | Unknown | 47 |
| EPEC/EHEC | |||||
| Cif | 32 | λ Prophage | Unknown | Cyclomodulin activity | 121 |
| EHEC | |||||
| TccP/EspFu | 42.4 | CP-933U | Tip of pedestal | A/E lesion formation; actin polymerization | 15, 67 |
| EPEC | |||||
| EspG2 | 42 | EspC PAI | Pedestal/colocalization with tubulin | Destruction of microtubule network | 124 |
| C. rodentium | |||||
| NleG | 26 | Unknown | Unknown | 47 |
Several homologous genes found.
Asterisks indicate variations in molecular masses among, proteins of different A/E lesion-forming pathogens.
Localization in the EHEC O157:H7 genome.
Annotated position in EHEC O157:H7 genome. nt, nucleotides.
Map.
Map, or mitochondrion-associated protein, was given this name because it targets mitochondria via an N-terminal targeting sequence (94, 99). Map displays three distinct and independent functions: (i) it interferes with the cellular ability to maintain mitochondrial membrane potential, triggering the formation of misshapen mitochondria, mitochondrial swelling, and damage (94, 99); (ii) in initial stages of EPEC/EHEC infection, Map is responsible for the transient formation of filopodium-like structures at the sites of bacterial infection, a process that is dependent on the small G protein Cdc42 (98); and (iii) Map is essential for disruption of intestinal barrier function and alteration of tight junctions, and this activity is independent of mitochondrial targeting (41). The Citrobacter rodentium mouse model of EHEC and EPEC infection (116, 179) has shown that although Map is not essential for colonization and disease (47, 130), a map mutant is recovered in lower numbers than the wild-type strain and persists longer in the mouse gut (130). In a mixed infection with 50% wild-type and 50% map mutant bacteria, the latter strain was extensively outcompeted (130). Consistent with this finding, a map mutant was found among the attenuated strains in C. rodentium (132) and EHEC signature-tagged mutagenesis screens (50), suggesting that map expression is advantageous in a competitive environment.
EspF.
EspF is a proline-rich effector protein; it contains three proline-rich repeats in EPEC (126), four in EHEC (170), and five in C. rodentium (47). EspF has been shown to play a role in the disruption of the intestinal barrier function, being required for the loss of TER, for increased monolayer permeability, and for redistribution of the tight junction-associated protein occludin (127). Map and EspF are involved in disruption of the intestinal barrier function independently; however, they both require the presence of surface-located intimin to display this activity (41). A recent study has demonstrated another level of functional similarities between EspF and Map. Like Map, EspF is targeted to the host mitochondria via its N-terminal region and is involved in mitochondrion membrane permeabilization (134, 137). Moreover, it induces release of the toxic protein cytochrome c into the cytosol and cleavage of caspases 9 and 3, indicating that EspF plays a role at the beginning of the mitochondrial death pathway (134, 137). Additionally, EspF seems to play a direct role in EPEC-induced cell death, apparently via pure apoptosis (30), and at early time points postinfection it forms a complex with cytokeratin 18 and the adaptor protein 14-3-3 (zeta isoform), a complex that is dismantled at later stages (171). Consistent with this binding activity, EspF was shown to be involved in modulating the architecture of the IF network within infected cells (171). Recent studies using human intestinal in vitro organ cultures (IVOC) have shown that EspF plays a direct role in remodeling of the brush border microvilli (152). Using the C. rodentium model, two independent studies (47, 130) showed moderate attenuation in the level of colonization by an espF mutant strain, suggesting that EspF does not have a significant role in colonization. However, a recent study has shown that an espF C. rodentium mutant is avirulent (134). The reason behind the different phenotypes is, at this stage, not known.
EspG.
Recent studies have shown that EspG triggers the formation of actin stress fibers and destruction of microtubule networks underneath adherent bacteria in fibroblasts (124, 153a). EspG interacts with tubulins and stimulates microtubule destabilization in vitro (124) and colocalizes with tubulin during infection of polarized Caco-2 cells (153a). This destabilization triggers the activation of the RhoA-ROCK signaling pathway via guanine nucleotide exchange factor (GEF-H1) activity (124). EspG displays 21% identity at the amino acid level with the Shigella flexneri effector VirA, which has been shown to trigger host microtubule destabilization, leading to Rac1 stimulation and efficient bacterial internalization (182). Indeed, espG complemented a Shigella virA mutant (53). An espG mutant strain displays only slight attenuation in animals in the rabbit EPEC infection model and the C. rodentium mouse model (47, 53, 130).
EspH.
EspH localizes to the host cell membrane and modulates the host actin cytoskeleton structure, affecting filopodium and pedestal formation (168). EspH does not play a critical role in vivo, as mutant strains show only slight attenuation in the C. rodentium mouse model (47, 130). The precise role of EspH in infection is currently not known.
SepZ.
SepZ is the most recent LEE-encoded effector to be identified; its translocation has not yet been attributed to a specific phenotype or function (89).
EspB.
In addition to its role in translocation (181), EspB was reported to have an effector activity (166). Cytosolic EspB localizes to the region of bacterial attachment (166), and cells transfected with EspB display altered morphology with a reduced number of stress fibers (165). Additionaly, EHEC EspB has been shown to bind α-catenin, a cytoskeleton-associated molecule, consistent with a role in modulating the host cell cytoskeleton (105); EPEC EspB has been shown to bind alpha(1)-antitrypsin (AAT) (101). Indeed, EPEC-mediated hemolysis of RBC and actin polymerization were strongly reduced by AAT, suggesting that AAT could interfere with type III secretion by inhibiting the correct formation of the translocation pore (101).
Tir.
Tir (transmembrane intimin receptor) localizes to the host cell plasma membrane (43, 97). Containing two membrane-spanning transmembrane domains, Tir forms a hairpin-like structure with both its C and N termini located within the host cell and the region between the two transmembrane domains forming an extracellular loop, exposed on the surface of the cell, which interacts with intimin (42, 79, 95). Like intimin in the bacterial outer membrane (167), plasma membrane-bound Tir is a dimer (115). The conformation of the intimin dimer is such that each of the two Tir-binding domains interacts with Tir molecules belonging to different Tir dimers. This binding pattern generates a reticular array-like conformation that clusters Tir under adherent bacteria (167). Tir intracellular amino and carboxy termini interact with a number of focal adhesion and cytoskeletal proteins, linking the extracellular bacterium to the host cell cytoskeleton (17, 70). These interactions lead to the formation of actin-rich pedestals beneath adherent bacteria. After delivery into the host cells, EPEC Tir is phosphorylated on two serine residues (S434 and S463) by host kinases, resulting in a shift in molecular mass (175). It has been suggested that the sequential addition of two phosphate groups triggers conformational changes in Tir structure that supply the necessary energy for insertion of Tir into the plasma membrane (175), although Tir was shown to be targeted to the plasma membranes of RBCs in the absence of detectable protein phosphorylation (153). EPEC Tir is also phosphorylated on tyrosine 474 (Y474P); this modification is crucial for its ability to promote actin polymerization following infection of epithelial cells in vitro (95). However, recent studies have shown the existence of an alternative, Y474P-independent mechanism of EPEC Tir-induced actin polymerization in infected human intestinal organ cultures ex vivo (Y. Chong et al., unpublished results). Significantly, EHEC Tir lacks Y474 and promotes actin polymerization through another mechanism that will be discussed further in this review (16, 48, 49, 93).
Tir and Map, which share the chaperone CesMT, show antagonistic actions in regulating filopodium and pedestal formation and synergistic mechanisms to stimulate invasion, indicating that the activities of these two effectors are coordinated during infection (98).
PROPHAGE-CARRIED EFFECTORS
In addition to the above-described LEE-encoded effectors, it is now evident that a number of effector proteins carried on prophages or other PAIs are translocated into the host cell via the LEE-encoded FTTSS. However, so far, only four of the newly identified effectors have been characterized; we summarize below our current understanding of their functions (Fig. 1 and 3; Table 1).
Cif.
Cif (cycle inhibiting factor) was the first prophage-carried effector protein to be identified. It is carried on a λ prophage, which is integrated near the Bio operon and has been found in a subset of EPEC strains isolated from human and animal clinical specimens (not being present in EPEC E2348/69, EHEC O157:H7 strains, or C. rodentium). cif encodes an effector protein that acts as a bacterial cyclomodulin. It is required for the induction of an irreversible cytopathic effect, characterized by progressive recruitment of focal adhesion plaques, assembly of stress fibers, and inhibition of the cell cycle G2/M phase transition, leading to the accumulation of inactive phosphorylated Cdk1 (121).
EspI (NleA).
EspI (also called NleA for non-LEE-encoded effector A) is carried on the prophage CP-933P (21). EspI/NleA is not required for A/E lesion formation (73, 130), and although it does not display classical Golgi apparatus-targeting motifs, once translocated it colocalizes with Golgi markers (73). A large-scale screening of clinical isolates showed that espI was present in 53% of all LEE-positive EPEC strains tested. In contrast, espI was detected in 86% of the LEE-positive EHEC strains; interestingly, there is a statistically significant association between the presence of espI and the isolation of EHEC strains from patients suffering from symptomatic infections (131). Moreover, EspI/NleA was reported to play a critical but unknown role in virulence in the C. rodentium mouse model (73, 130).
EspJ.
espJ is located in prophage CP-933U, upstream of the TccP gene (Fig. 1). It encodes a translocated effector not required for A/E lesion formation. However, mutation in espJ influenced the dynamics of clearance of the pathogen from the host's intestinal tract in both the C. rodentium and the EHEC lamb models, suggesting a role in host survival and pathogen transmission (37).
TccP/EspFu, the EHEC lost link.
TccP (Tir-cytoskeleton coupling protein) (67)/EspFu (named because it displays 24% identity at the amino acid level with EspF [15]/U-EspF [170]) is a proline-rich effector protein carried within the EHEC prophage CP-933U and translocated through the LEE-encoded FTTSS.
EPEC and EHEC translocate Tir, which links the extracellular bacterium to the cell cytoskeleton. Although both converge on neuronal Wiskott-Aldrich syndrome protein (N-WASP), the processes of Tir-mediated actin accretion by EPEC and EHEC in cultured cells differ in that EPEC Tir requires both tyrosine phosphorylation (Y474) and the presence of the host adaptor protein Nck whereas EHEC Tir lacks a Y474 equivalent and utilizes TccP/EspFu as a linker instead. Indeed, following translocation, TccP/EspFu plays an essential role in actin accretion underneath adherent EHEC, displaying an Nck-like coupling activity. TccP/EspFu associates indirectly with Tir, binds N-WASP, and stimulates Nck-independent actin polymerization (15, 67). When expressed in EPEC, TccP/EspFu restores actin polymerization activity following infection of an Nck-deficient cell line (15, 67). Purified TccP/EspFu activates N-WASP, stimulating, in the presence of Arp2/3, actin polymerization in vitro (67). Moreover, TccP/EspFu displays similar biological activity on infected human intestinal explants ex vivo (67). In addition, TccP/EspFu seems to be involved in alteration of polarized epithelial barrier function as it complements an EPEC espF mutant (170).
ANOTHER EFFECTOR: EspG2
The third open reading frame (ORF3) within the espC PAI (Table 1) encodes a protein which shares 43.5% identity with LEE-encoded EspG and 19% identity with Shigella VirA (53). Present in EPEC (129), the ORF3 protein has been shown to be translocated (124, 153a) and to play a role similar to that of EspG; we therefore renamed the protein EspG2 (153a). EspG2 presents a clear example of effector functional redundancy (124).
INTEGRATION OF EFFECTOR PROTEIN FUNCTIONS
(i) Disruption of the normal host-commensal strain equilibrium: diarrhea.
Several factors contribute to EPEC-/EHEC-induced watery persistent diarrhea, such as loss of microvilli and malabsorption due to the brush border deficiency. Moreover, EPEC infection disrupts the barrier function through the loss of epithelial resistance and the increased monolayer permeability that occurs through disruption of the tight and adherent junctions. EPEC activates several pathways contributing to the disruption of barrier function. (i) One of these is the redistribution and activation of ezrin. Ezrin, a member of the ezrin-radixin-moesin family, is usually located in the microvilli of epithelial cells linking membrane and cytoskeleton (8, 148). During EPEC infection, ezrin is redistributed, accumulating under adherent bacteria (57, 70), and is activated by phosphorylation on threonine and tyrosine residues, leading to the disruption of intestinal epithelial tight junctions (154). (ii) Another activated pathway is the redistribution of the tight-junction proteins. During EPEC infection, occludin is dephosphorylated by host serine/threonine phosphatases and shifts from tight junctions to the cytoplasm (127, 155). Moreover, at the same time that tight-junction proteins lose their apical localization, aberrant strands appear at the lateral membrane containing claudin-1 and occludin (133). (iii) A third pathway is phosphorylation of the myosin light chain (MLC). EPEC infection enhances the association of the MLC with the cytoskeleton, and the cytoskeleton-associated MLC is phosphorylated by MLC kinase (120). MLC phosphorylation results in hydrolysis of ATP and movement of filaments past one another. This movement is associated with contraction of the ring of cytoskeletal proteins that underlies the intercellular tight junction (12, 91). Contraction of this perijunctional ring is involved in regulating tight-junction permeability (118). EPEC-induced phosphorylation of the MLC has been shown to contribute to the increase in paracellular permeability by driving contraction of the cytoskeleton (183). (iv) Finally, EPEC activates disruption of the adherent junctions. EPEC induces phosphorylation of protein kinase C, which associates with cadherins, leading to the dissociation of the cadherin/β-catenin complex, which constitutes the adherent junctions (119). EPEC/EHEC factors implicated in induction of these effects include EspF, outer membrane proteins (119), TccP/EspFu, and Map. EspF and Map play a large role in the EPEC-induced drop in TER and contribute to EPEC-driven disruption of tight junctions, as demonstrated by the redistribution of occludin during EPEC infection (41, 127). TccP/EspFu complements an EPEC espF mutant for intestinal barrier function alteration (170).
In addition to the disruption of the epithelial barrier function, EPEC/EHEC induces changes in the host cell electrolyte transport that contribute to secretory diarrhea. A decrease in cell resting membrane potential (158) and an increase in the short-circuit current (24) are observed in EPEC-infected cells, together with changes in the bicarbonate-dependent transport of chloride and stimulation of chloride secretion (80). These events are dependent on a functional TTSS. Mechanistically, EPEC induces tyrosine phosphorylation of phospholipase C-γ1, which, once activated, interacts with and cleaves phosphatidylinositol 4,5-biphosphate, releasing inositol 1,4,5-triphosphate and diacylglycerol, secondary messengers implicated in the activation of protein kinase C, which triggers a brisk secretion of ions and fluids (31, 96). Additionally, the study of the response of polarized intestinal epithelial cells to EPEC infection has recently revealed that several proteins involved in ion transport and ion channel function, such as calcium-activated chloride in the case of channel 4, are up-regulated in response to infection with a TTSS-competent EPEC strain (78).
(ii) Host cell cytoskeleton rearrangements.
In initial stages of EPEC infection (around 5 min postinfection), filopodium-like extensions are formed at the site of bacterial adhesion. These structures extend, retract, and sway from side to side for around 20 min and then are retracted into the host cell and disappear (98). This process is dependent on Map and the host GTP-binding protein, Cdc42. Down-regulation of filopodium formation is dependent on the putative GTPase-activating protein-like arginine finger motif at the C-terminal domain of Tir and is enhanced by EspH (98, 168).
Recent studies have shown a TTSS-dependent dramatic alteration in the architecture of the IF network in EPEC- and EHEC-infected epithelial cells. The IF proteins cytokeratin 18 (CK18) and cytokeratin 8 (CK8) have been shown to be recruited to the EPEC-/EHEC-induced pedestals (6); depletion of cells from CK18 diminished formation of actin-rich pedestals at the site of EPEC adhesion (6). Moreover, EPEC Tir was shown to form a complex with CK18 and the tau isoform of the adaptor protein 14-3-3 (I. F. Connerton, personal communication).
EspF was also recently implicated in subversion of the IF network (171). EspF forms, during early stages of infection, a complex with CK18 and the zeta isoform of 14-3-3; the complex disappears at a later time (171). Additionally, EspG and EspG2 are responsible for the destruction of the microtubule network located underneath adherent bacteria, which leads to the assembly of actin stress fibers by activation of the RhoA-ROCK signaling pathway via GEF-H1 (124).
The most striking effect of EPEC/EHEC infection is the massive rearrangement of the host cell actin microfilaments, triggering generation of A/E lesions. These lesions are characterized by the intimate attachment of the bacterium to the epithelial cell membrane and by the localized effacement of brush border microvilli (102). The epithelial cell beneath adherent bacteria is raised in a characteristic pedestal formation, which may extend up to 10 μm outwards from the cell to form pseudopod-like structures. Moreover, pedestals are not static: EPEC/EHEC can move across the surface of infected cells at speeds of up to 0.1 μm/s (147). EPEC-/EHEC-induced pedestals are composed of polymerized actin, IF, and other proteins normally associated with the cytoskeleton, such as focal adhesion proteins. Tir is the only known type III effector essential for A/E lesion formation by EPEC. Tir interacts via its N-terminal domain with several focal adhesion proteins including α-actinin, talin, and vinculin (19, 66, 71, 81). Focal adhesion proteins usually connect actin cytoskeleton to the membrane, either directly through interactions with the membrane phospholipids or indirectly via interaction with membrane-associated proteins. Recruitment of these proteins is not essential for pedestal formation, as deletion of the N-terminal Tir domain does not prevent A/E lesion formation (18). The C-terminal region of EPEC Tir is essential for pedestal formation. This domain includes Y474, whose phosphorylation by host kinases (95, 143, 161) is required for pedestal formation (17, 95). The C terminus of Tir recruits several host proteins to the site of bacterial attachment. However, the key event in EPEC pedestal formation is the recruitment of Nck by a 12-residue region encompassing Y474 (17, 18, 72). Nck is an adaptor protein, which in turn recruits and activates N-WASP (72). N-WASP activates the actin-nucleating Arp2/3 complex, triggering actin polymerization and pedestal formation (88, 112, 113). Additional activator proteins cortactin (20) and Gbr2 (70) are also recruited to pedestals, possibly amplifying the signal provided by Nck and N-WASP; indeed, cortactin has been shown to be required for pedestal formation (20). Other signaling and cytoskeletal host proteins that are recruited to the site of bacterial attachment include CD44 and calpactin, which are recruited independently of Tir delivery, and gelsolin, tropomyosin, and ezrin, which are recruited independently of Tir tyrosine phosphorylation. The role of these additional proteins in the pedestal is unknown (70).
EPEC and EHEC share similarities between their respective Tir molecules, the host components associated with their pedestals, and the triggered actin polymerization pathways converging on the N-WASP-Arp2/3 cascade. However, Tir-mediated actin accretion by EHEC differs in that EHEC Tir is not tyrosine phosphorylated and utilizes, instead of Nck, the bacterial effector protein TccP/EspFu (15, 17, 67, 72, 84, 95); TccP/EspFu binds N-WASP, stimulating actin polymerization and pedestal formation. The recent finding of TccP/EspFu as the second type III effector protein required for pedestal formation during EHEC infection has been an important advance in the study of bacterially driven mammalian pathways of actin signaling. The absence of a direct interaction between Tir and TccP/EspFu indicates the requirement of an additional bacterial or cellular factor(s) that will be the object of future investigation (15, 67).
CONCLUDING REMARKS
This review has been written at a time when the field of EPEC and EHEC studies is undergoing a minor revolution. Until April 2004, only five effectors were known. In December 2004, when we wrote this review, the number of known effectors (proven or deduced from the different genome projects) had risen to over 20. The discovery of TccP/EspFu has finally resolved the mystery of how EHEC induces actin polymerization in a tyrosine phosphorylation-/Nck-independent manner. At the same time, the use of human IVOC revealed the existence of a novel Tir Y474P-independent mechanism of EPEC-induced actin polymerization (Chong et al., unpublished results). Moreover, using human IVOC highlighted, for the first time, a role for an effector protein, EspF, in remodeling of the brush border microvilli, a poorly characterized aspect of EPEC and EHEC infection (152). However, despite the recent advances, there is still a long way to go until we fully discover and understand how EPEC and EHEC use their repertoire of effectors to inflict damage on host epithelial cells and to cause diarrhea.
Acknowledgments
This work was supported by the Wellcome Trust.
Editor: J. B. Kaper
REFERENCES
- 1.Abe, A., M. de Grado, R. A. Pfuetzner, C. Sanchez-Sanmartin, R. Devinney, J. L. Puente, N. C. Strynadka, and B. B. Finlay. 1999. Enteropathogenic Escherichia coli translocated intimin receptor, Tir, requires a specific chaperone for stable secretion. Mol. Microbiol. 33:1162-1175. [DOI] [PubMed] [Google Scholar]
- 2.Adu-Bobie, J., L. R. Trabulsi, M. M. Carneiro-Sampaio, G. Dougan, and G. Frankel. 1998. Identification of immunodominant regions within the C-terminal cell binding domain of intimin alpha and intimin beta from enteropathogenic Escherichia coli. Infect. Immun. 66:5643-5649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aizawa, S. I. 2001. Bacterial flagella and type III secretion systems. FEMS Microbiol. Lett. 202:157-164. [DOI] [PubMed] [Google Scholar]
- 4.Akeda, Y., and J. Galan. 2004. Genetic analysis of the Salmonella enterica type III secretion-associated ATPase InvC defines discrete functional domains. J. Bacteriol. 186:2402-2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allaoui, A., P. Sansonetti, and C. Parsot. 1992. MxiJ, a lipoprotein involved in secretion of Shigella Ipa invasins, is homologous to YscJ, a secretion factor of the Yersinia Yop proteins. J. Bacteriol. 174:7661-7669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Batchelor, M., J. Guignot, A. Patel, N. Cummings, J. Cleary, S. Knutton, D. W. Holden, I. Connerton, and G. Frankel. 2004. Involvement of the intermediate filament protein cytokeratin-18 in actin pedestal formation during EPEC infection. EMBO Rep. 5:104-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Batchelor, M., S. Prasannan, S. Daniell, S. Reece, I. Connerton, G. Bloomberg, G. Dougan, G. Frankel, and S. Matthews. 2000. Structural basis for recognition of the translocated intimin receptor (Tir) by intimin from enteropathogenic Escherichia coli. EMBO J. 19:2452-2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berryman, M., Z. Franck, and A. Bretscher. 1993. Ezrin is concentrated in the apical microvilli of a wide variety of epithelial cells whereas moesin is found primarily in endothelial cells. J. Cell Sci. 105:1025-1043. [DOI] [PubMed] [Google Scholar]
- 9.Blocker, A., P. Gounon, E. Larquet, K. Niebuhr, V. Cabiaux, C. Parsot, and P. Sansonetti. 1999. The tripartite type III secreton of Shigella flexneri inserts IpaB and IpaC into host membranes. J. Cell Biol. 147:683-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blocker, A., D. Holden, and G. Cornelis. 2000. Type III secretion systems: what is the translocator and what is translocated? Cell. Microbiol. 2:387-390. [DOI] [PubMed] [Google Scholar]
- 11.Blocker, A., N. Jouihri, E. Larquet, P. Gounon, F. Ebel, C. Parsot, P. Sansonetti, and A. Allaoui. 2001. Structure and composition of the Shigella flexneri “needle complex,” a part of its type III secreton. Mol. Microbiol. 39:652-663. [DOI] [PubMed] [Google Scholar]
- 12.Broschat, K. O., R. P. Stidwill, and D. R. Burgess. 1983. Phosphorylation controls brush border motility by regulating myosin structure and association with the cytoskeleton. Cell 35:561-571. [DOI] [PubMed] [Google Scholar]
- 13.Buttner, D., and U. Bonas. 2002. Port of entry—the type III secretion translocon. Trends Microbiol. 10:186-192. [DOI] [PubMed] [Google Scholar]
- 14.Cambronne, E. D., L. W. Cheng, and O. Schneewind. 2000. LcrQ/YscM1, regulators of the Yersinia yop virulon, are injected into host cells by a chaperone-dependent mechanism. Mol. Microbiol. 37:263-273. [DOI] [PubMed] [Google Scholar]
- 15.Campellone, K., D. Robbins, and J. Leong. 2004. EspF(U) is a translocated EHEC effector that interacts with Tir and N-WASP and promotes Nck-independent actin assembly. Dev. Cell 7:217-228. [DOI] [PubMed] [Google Scholar]
- 16.Campellone, K. G., A. Giese, D. J. Tipper, and J. M. Leong. 2002. A tyrosine-phosphorylated 12-amino-acid sequence of enteropathogenic Escherichia coli Tir binds the host adaptor protein Nck and is required for Nck localization to actin pedestals. Mol. Microbiol. 43:1227-1241. [DOI] [PubMed] [Google Scholar]
- 17.Campellone, K. G., and J. M. Leong. 2003. Tails of two Tirs: actin pedestal formation by enteropathogenic E. coli and enterohaemorrhagic E. coli O157:H7. Curr. Opin. Microbiol. 6:82-90. [DOI] [PubMed] [Google Scholar]
- 18.Campellone, K. G., S. Rankin, T. Pawson, M. W. Kirschner, D. J. Tipper, and J. M. Leong. 2004. Clustering of Nck by a 12-residue Tir phosphopeptide is sufficient to trigger localized actin assembly. J. Cell Biol. 164:406-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cantarelli, V. V., A. Takahashi, I. Yanagihara, Y. Akeda, K. Imura, T. Kodama, G. Kono, Y. Sato, and T. Honda. 2001. Talin, a host cell protein, interacts directly with the translocated intimin receptor, Tir, of enteropathogenic Escherichia coli, and is essential for pedestal formation. Cell. Microbiol. 3:745-751. [DOI] [PubMed] [Google Scholar]
- 20.Cantarelli, V. V., A. Takahashi, I. Yanagihara, Y. Akeda, K. Imura, T. Kodama, G. Kono, Y. Sato, T. Iida, and T. Honda. 2002. Cortactin is necessary for F-actin accumulation in pedestal structures induced by enteropathogenic Escherichia coli infection. Infect. Immun. 70:2206-2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chaudhuri, R. R., A. M. Khan, and M. J. Pallen. 2004. coliBASE: an online database for Escherichia coli, Shigella and Salmonella comparative genomics. Nucleic Acids Res. 32:D296-D299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen, H. D., and G. Frankel. 2005. Enteropathogenic Escherichia coli: unravelling pathogenesis. FEMS Microbiol. Rev. 29:83-98. [DOI] [PubMed]
- 23.Cleary, J., L. C. Lai, R. K. Shaw, A. Straatman-Iwanowska, M. S. Donnenberg, G. Frankel, and S. Knutton. 2004. Enteropathogenic Escherichia coli (EPEC) adhesion to intestinal epithelial cells: role of bundle-forming pili (BFP), EspA filaments and intimin. Microbiology 150:527-538. [DOI] [PubMed] [Google Scholar]
- 24.Collington, G. K., I. W. Booth, M. S. Donnenberg, J. B. Kaper, and S. Knutton. 1998. Enteropathogenic Escherichia coli virulence genes encoding secreted signalling proteins are essential for modulation of Caco-2 cell electrolyte transport. Infect. Immun. 66:6049-6053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coombes, B. K., N. F. Brown, Y. Valdez, J. H. Brumell, and B. B. Finlay. 2004. Expression and secretion of Salmonella pathogenicity island-2 virulence genes in response to acidification exhibit differential requirements of a functional type III secretion apparatus and SsaL. J. Biol. Chem. 279:49804-49815. [DOI] [PubMed] [Google Scholar]
- 26.Cornelis, G. 2002. The Yersinia Ysc-Yop virulence apparatus. Int. J. Med. Microbiol. 291:455-462. [DOI] [PubMed] [Google Scholar]
- 27.Cornelis, G. R., A. Boland, A. P. Boyd, C. Geuijen, M. Iriarte, C. Neyt, M. P. Sory, and I. Stainier. 1998. The virulence plasmid of Yersinia, an antihost genome. Microbiol. Mol. Biol. Rev. 62:1315-1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crago, A. M., and V. Koronakis. 1998. Salmonella InvG forms a ring-like multimer that requires the InvH lipoprotein for outer membrane localization. Mol. Microbiol. 30:47-56. [DOI] [PubMed] [Google Scholar]
- 29.Crane, J. K., S. Majumdar, and D. F. R. Pickhardt. 1999. Host cell death due to enteropathogenic Escherichia coli has features of apoptosis. Infect. Immun. 67:2575-2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crane, J. K., B. P. McNamara, and M. S. Donnenberg. 2001. Role of EspF in host cell death induced by enteropathogenic Escherichia coli. Cell. Microbiol. 3:197-211. [DOI] [PubMed] [Google Scholar]
- 31.Crane, J. K., and J. S. Ohm. 1997. Activation of host cell protein kinase C by enteropathogenic Escherichia coli. Infect. Immun. 65:3277-3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Creasey, E. A., R. M. Delahay, A. A. Bishop, R. K. Shaw, B. Kenny, S. Knutton, and G. Frankel. 2003. CesT is a bivalent enteropathogenic Escherichia coli chaperone required for translocation of both Tir and Map. Mol. Microbiol. 47:209-221. [DOI] [PubMed] [Google Scholar]
- 33.Creasey, E. A., R. M. Delahay, S. J. Daniell, and G. Frankel. 2003. Yeast two-hybrid system survey of interactions between LEE-encoded proteins of enteropathogenic Escherichia coli. Microbiology 149:2093-2106. [DOI] [PubMed] [Google Scholar]
- 34.Creasey, E. A., D. Friedberg, R. K. Shaw, T. Umanski, S. Knutton, I. Rosenshine, and G. Frankel. 2003. CesAB is an enteropathogenic Escherichia coli chaperone for the type-III translocator proteins EspA and EspB. Microbiology 149:3639-3647. [DOI] [PubMed] [Google Scholar]
- 35.Crepin, V. F., S. Prasannan, R. K. Shaw, R. K. Wilson, E. Creasey, C. M. Abe, S. Knutton, G. Frankel, and S. Matthews. 2005. Structural and functional studies of the enteropathogenic Escherichia coli type III needle complex protein EscJ. Mol. Microbiol. 55:1658-1670. [DOI] [PubMed]
- 35a.Crepin, V. F., R. Shaw, C. M. Abe, S. Knutton, and G. Frankel. 2005. Polarity of enteropathogenic Escherichia coli EspA filament assembly and protein secretion. J. Bacteriol., in press. [DOI] [PMC free article] [PubMed]
- 36.Dahan, S., S. Knutton, R. K. Shaw, V. F. Crepin, G. Dougan, and G. Frankel. 2004. The transcriptome of enterohemorrhagic Escherichia coli O157 adhering to eukaryotic plasma membranes. Infect. Immun. 72:5452-5459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dahan, S., S. Wiles, R. M. La Ragione, A. Best, M. J. Woodward, M. P. Stevens, R. K. Shaw, Y. Chong, S. Knutton, A. Phillips, and G. Frankel. 2005. EspJ is a prophage-carried type III effector protein of attaching and effacing pathogens that modulates infection dynamics. Infect. Immun. 73:679-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Daniell, S. J., R. M. Delahay, R. K. Shaw, E. L. Hartland, M. J. Pallen, F. Booy, F. Ebel, S. Knutton, and G. Frankel. 2001. Coiled-coil domain of enteropathogenic Escherichia coli type III secreted protein EspD is involved in EspA filament-mediated cell attachment and hemolysis. Infect. Immun. 69:4055-4064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Daniell, S. J., E. Kocsis, E. Morris, S. Knutton, F. P. Booy, and G. Frankel. 2003. 3D structure of EspA filaments from enteropathogenic Escherichia coli. Mol. Microbiol. 49:301-308. [DOI] [PubMed] [Google Scholar]
- 40.Daniell, S. J., N. Takahashi, R. Wilson, D. Friedberg, I. Rosenshine, F. P. Booy, R. K. Shaw, S. Knutton, G. Frankel, and S. Aizawa. 2001. The filamentous type III secretion translocon of enteropathogenic Escherichia coli. Cell. Microbiol. 3:865-871. [DOI] [PubMed] [Google Scholar]
- 41.Dean, P., and B. Kenny. 2004. Intestinal barrier dysfunction by enteropathogenic Escherichia coli is mediated by two effector molecules and a bacterial surface protein. Mol. Microbiol. 54:665-675. [DOI] [PubMed] [Google Scholar]
- 42.de Grado, M., A. Abe, A. Gauthier, O. Steele-Mortimer, R. DeVinney, and B. B. Finlay. 1999. Identification of the intimin binding domain of Tir of enteropathogenic Escherichia coli. Cell. Microbiol. 1:7-18. [DOI] [PubMed] [Google Scholar]
- 43.Deibel, C., S. Kramer, T. Chakraborty, and F. Ebel. 1998. EspE, a novel secreted protein of attaching and effacing bacteria, is directly translocated into infected host cells, where it appears as a tyrosine-phosphorylated 90 kDa protein. Mol. Microbiol. 28:463-474. [DOI] [PubMed] [Google Scholar]
- 44.Delahay, R. M., and G. Frankel. 2002. Coiled-coil proteins associated with type III secretion systems: a versatile domain revisited. Mol. Microbiol. 45:905-916. [DOI] [PubMed] [Google Scholar]
- 45.Delahay, R. M., S. Knutton, R. K. Shaw, E. L. Hartland, M. J. Pallen, and G. Frankel. 1999. The coiled-coil domain of EspA is essential for the assembly of the type III secretion translocon on the surface of enteropathogenic E. coli. J. Biol. Chem. 274:35969-35974. [DOI] [PubMed] [Google Scholar]
- 46.Deng, W., and H. Huang. 1999. Cellular locations of Pseudomonas syringae pv. syringae HrcC and HrcJ proteins, required for harpin secretion via the type III pathway. J. Bacteriol. 181:2298-2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Deng, W., J. L. Puente, S. Gruenheid, Y. Li, B. A. Vallance, A. Vazquez, J. Barba, J. A. Ibarra, P. O'Donnell, P. Metalnikov, K. Ashman, S. Lee, D. Goode, T. Pawson, and B. B. Finlay. 2004. Dissecting virulence: systematic and functional analyses of a pathogenicity island. Proc. Natl. Acad. Sci. USA 101:3597-3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.DeVinney, R., J. L. Puente, A. Gauthier, D. Goosney, and B. B. Finlay. 2001. Enterohaemorrhagic and enteropathogenic Escherichia coli use a different Tir-based mechanism for pedestal formation. Mol. Microbiol. 41:1445-1458. [DOI] [PubMed] [Google Scholar]
- 49.DeVinney, R., M. Stein, D. Reinscheid, A. Abe, S. Ruschkowski, and B. B. Finlay. 1999. Enterohemorrhagic Escherichia coli O157:H7 produces Tir, which is translocated to the host cell membrane but is not tyrosine phosphorylated. Infect. Immun. 67:2389-2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dziva, F., P. M. van Diemen, M. P. Stevens, A. J. Smith, and T. S. Wallis. 2004. Identification of Escherichia coli O157: H7 genes influencing colonization of the bovine gastrointestinal tract using signature-tagged mutagenesis. Microbiology 150:3631-3645. [DOI] [PubMed] [Google Scholar]
- 51.Ebel, F., T. Podzadel, M. Rohde, A. U. Kresse, S. Kramer, C. Deibel, C. A. Guzman, and T. Chakraborty. 1998. Initial binding of Shiga toxin-producing Escherichia coli to host cells and subsequent induction of actin rearrangements depend on filamentous EspA-containing surface appendages. Mol. Microbiol. 30:147-161. [DOI] [PubMed] [Google Scholar]
- 52.Elliott, S. J., M. S. Dubois, S. W. Hutcheson, L. A. Wainwright, M. Batchelor, G. Frankel, S. Knutton, and J. B. Kaper. 1999. Identification of CesT, a chaperone for the type III secretion of Tir in enteropathogenic Escherichia coli. Mol. Microbiol. 33:1176-1189. [DOI] [PubMed] [Google Scholar]
- 53.Elliott, S. J., E. O. Krejany, J. L. Mellies, R. M. Robins-Browne, C. Sasakawa, and J. B. Kaper. 2001. EspG, a novel type III system-secreted protein from enteropathogenic Escherichia coli with similarities to VirA of Shigella flexneri. Infect. Immun. 69:4027-4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Elliott, S. J., C. B. O'Connell, A. Koutsouris, C. Brinkley, M. S. Donnenberg, G. Hecht, and J. B. Kaper. 2002. A gene from the locus of enterocyte effacement that is required for enteropathogenic Escherichia coli to increase tight-junction permeability encodes a chaperone for EspF. Infect. Immun. 70:2271-2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Elliott, S. J., L. A. Wainwright, T. K. McDaniel, K. G. Jarvis, Y. K. Deng, L. C. Lai, B. P. McNamara, M. S. Donnenberg, and J. B. Kaper. 1998. The complete sequence of the locus of enterocyte effacement (LEE) from enteropathogenic Escherichia coli E2348/69. Mol. Microbiol. 28:1-4. [DOI] [PubMed] [Google Scholar]
- 56.Emerson, S. U., K. Tokuyasu, and M. I. Simon. 1970. Bacterial flagella: polarity of elongation. Science 169:190-192. [DOI] [PubMed] [Google Scholar]
- 57.Finlay, B. B., I. Rosenshine, M. S. Donnenberg, and J. B. Kaper. 1992. Cytoskeletal composition of attaching and effacing lesions associated with enteropathogenic Escherichia coli adherence to HeLa cells. Infect. Immun. 60:2541-2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fitzhenry, R. J., D. J. Pickard, E. L. Hartland, S. Reece, G. Dougan, A. D. Phillips, and G. Frankel. 2002. Intimin type influences the site of human intestinal mucosal colonisation by enterohaemorrhagic Escherichia coli O157:H7. Gut 50:180-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fivaz, M., and F. G. van der Goot. 1999. The tip of a molecular syringe. Trends Microbiol. 7:341-343. [DOI] [PubMed] [Google Scholar]
- 60.Foster, D. B., M. Abul-Milh, M. Huesca, and C. A. Lingwood. 2000. Enterohemorrhagic Escherichia coli induces apoptosis which augments bacterial binding and phosphatidylethanolamine exposure on the plasma membrane outer leaflet. Infect. Immun. 68:3108-3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Francis, M. S., and H. Wolf-Watz. 1998. YopD of Yersinia pseudotuberculosis is translocated into the cytosol of HeLa epithelial cells: evidence of a structural domain necessary for translocation. Mol. Microbiol. 29:799-813. [DOI] [PubMed] [Google Scholar]
- 62.Frankel, G., D. C. Candy, P. Everest, and G. Dougan. 1994. Characterization of the C-terminal domains of intimin-like proteins of enteropathogenic and enterohemorrhagic Escherichia coli, Citrobacter freundii, and Hafnia alvei. Infect. Immun. 62:1835-1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Frankel, G., O. Lider, R. Hershkoviz, A. P. Mould, S. G. Kachalsky, D. C. A. Candy, L. Cahalon, M. J. Humphries, and G. Dougan. 1996. The cell-binding domain of intimin from enteropathogenic Escherichia coli binds to beta1 integrins. J. Biol. Chem. 271:20359-20364. [DOI] [PubMed] [Google Scholar]
- 64.Frankel, G., A. D. Phillips, I. Rosenshine, G. Dougan, J. B. Kaper, and S. Knutton. 1998. Enteropathogenic and enterohaemorrhagic Escherichia coli: more subversive elements. Mol. Microbiol. 30:911-921. [DOI] [PubMed] [Google Scholar]
- 65.Frankel, G., A. D. Phillips, L. R. Trabulsi, S. Knutton, G. Dougan, and S. Matthews. 2001. Intimin and the host cell—is it bound to end in Tir(s)? Trends Microbiol. 9:214-218. [DOI] [PubMed] [Google Scholar]
- 66.Freeman, N. L., D. V. Zurawski, P. Chowrashi, J. C. Ayoob, L. Huang, B. Mittal, J. M. Sanger, and J. W. Sanger. 2000. Interaction of the enteropathogenic Escherichia coli protein, translocated intimin receptor (Tir), with focal adhesion proteins. Cell Motil. Cytoskelet. 47:307-318. [DOI] [PubMed] [Google Scholar]
- 67.Garmendia, J., A. D. Phillips, M. F. Carlier, Y. Chong, S. Schuller, O. Marches, S. Dahan, E. Oswald, R. K. Shaw, S. Knutton, and G. Frankel. 2004. TccP is an enterohaemorrhagic Escherichia coli O157:H7 type III effector protein that couples Tir to the actin-cytoskeleton. Cell. Microbiol. 6:1167-1183. [DOI] [PubMed] [Google Scholar]
- 68.Gauthier, A., and B. B. Finlay. 2003. Translocated intimin receptor and its chaperone interact with ATPase of the type III secretion apparatus of enteropathogenic Escherichia coli. J. Bacteriol. 185:6747-6755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gauthier, A., J. Puente, and B. Finlay. 2003. Secretin of the enteropathogenic Escherichia coli type III secretion system requires components of the type III apparatus for assembly and localization. Infect. Immun. 71:3310-3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Goosney, D. L., R. DeVinney, and B. B. Finlay. 2001. Recruitment of cytoskeletal and signaling proteins to enteropathogenic and enterohaemorrhagic Escherichia coli pedestals. Infect. Immun. 69:3315-3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Goosney, D. L., R. DeVinney, R. A. Pfuetzner, E. A. Frey, N. C. Strynadka, and B. B. Finlay. 2000. Enteropathogenic E. coli translocated intimin receptor, Tir, interacts directly with alpha-actinin. Curr. Biol. 10:735-738. [DOI] [PubMed] [Google Scholar]
- 72.Gruenheid, S., R. DeVinney, F. Bladt, D. Goosney, S. Gelkop, G. D. Gish, T. Pawson, and B. B. Finlay. 2001. Enteropathogenic E. coli Tir binds Nck to initiate actin pedestal formation in host cells. Nat. Cell Biol. 3:856-859. [DOI] [PubMed] [Google Scholar]
- 73.Gruenheid, S., I. Sekirov, N. A. Thomas, W. Deng, P. O'Donnell, D. Goode, Y. Li, E. A. Frey, N. F. Brown, P. Metalnikov, T. Pawson, K. Ashman, and B. B. Finlay. 2004. Identification and characterization of NleA, a non-LEE encoded type III translocated virulence factor of enterohaemorrhagic Escherichia coli O157:H7. Mol. Microbiol. 51:1233-1249. [DOI] [PubMed] [Google Scholar]
- 74.Hacker, J., L. Bender, M. Ott, J. Wingender, B. Lund, R. Marre, and W. Goebel. 1990. Deletions of chromosomal regions coding for fimbriae and hemolysins occur in vitro and in vivo in various extraintestinal Escherichia coli isolates. Microb. Pathog. 8:213-225. [DOI] [PubMed] [Google Scholar]
- 75.Hacker, J., G. Blum-Oehler, I. Muhldorfer, and H. Tschape. 1997. Pathogenicity islands of virulent bacteria: structure, function and impact on microbial evolution. Mol. Microbiol. 23:1089-1097. [DOI] [PubMed] [Google Scholar]
- 76.Hacker, J., and J. B. Kaper. 2000. Pathogenicity islands and the evolution of microbes. Annu. Rev. Microbiol. 54:641-679. [DOI] [PubMed] [Google Scholar]
- 77.Hakansson, S., K. Schesser, C. Persson, E. E. Galyov, R. Rosqvist, F. Homble, and H. Wolf-Watz. 1996. The YopB protein of Yersinia pseudotuberculosis is essential for the translocation of Yop effector proteins across the target cell plasma membrane and displays a contact-dependent membrane disrupting activity. EMBO J. 15:5812-5823. [PMC free article] [PubMed] [Google Scholar]
- 78.Hardwidge, P. R., I. Rodriguez-Escudero, D. Goode, S. Donohoe, J. Eng, D. R. Goodlett, R. Aebersold, and B. B. Finlay. 2004. Proteomic analysis of the intestinal epithelial cell response to enteropathogenic Escherichia coli. J. Biol. Chem. 279:20127-20136. [DOI] [PubMed] [Google Scholar]
- 79.Hartland, E. L., M. Batchelor, R. M. Delahay, C. Hale, S. Matthews, G. Dougan, S. Knutton, I. Connerton, and G. Frankel. 1999. Binding of intimin from enteropathogenic Escherichia coli to Tir and to host cells. Mol. Microbiol. 32:151-158. [DOI] [PubMed] [Google Scholar]
- 80.Hecht, G., and A. Koutsouris. 1999. Enteropathogenic E. coli attenuates secretagogue-induced net intestinal ion transport but not Cl− secretion. Am. J. Physiol. 276:G781-G788. [DOI] [PubMed] [Google Scholar]
- 81.Huang, L., B. Mittal, J. W. Sanger, and J. M. Sanger. 2002. Host focal adhesion protein domains that bind to the translocated intimin receptor (Tir) of enteropathogenic Escherichia coli (EPEC). Cell Motil. Cytoskelet. 54:255-265. [DOI] [PubMed] [Google Scholar]
- 82.Hueck, C. J. 1998. Type III protein secretion systems in bacterial pathogens of animals and plants. Microbiol. Mol. Biol. Rev. 62:379-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ide, T., S. Laarmann, L. Greune, H. Schillers, H. Oberleithner, and M. A. Schmidt. 2001. Characterization of translocation pores inserted into plasma membranes by type III-secreted Esp proteins of enteropathogenic Escherichia coli. Cell. Microbiol. 3:669-679. [DOI] [PubMed] [Google Scholar]
- 84.Ismaili, A., D. J. Philpott, M. T. Dytoc, R. Soni, S. Ratnam, and P. M. Sherman. 1995. Alpha-actinin accumulation in epithelial cells infected with attaching and effacing gastrointestinal pathogens. J. Infect. Dis. 172:1393-1396. [DOI] [PubMed] [Google Scholar]
- 85.Jerse, A. E., J. Yu, B. D. Tall, and J. B. Kaper. 1990. A genetic locus of enteropathogenic Escherichia coli necessary for the production of attaching and effacing lesions on tissue culture cells. Proc. Natl. Acad. Sci. USA 87:7839-7843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jouihri, N., M. Sory, A. Page, P. Gounon, C. Parsot, and A. Allaoui. 2003. MxiK and MxiN interact with the Spa47 ATPase and are required for transit of the needle components MxiH and MxiI, but not of Ipa proteins, through the type III secretion apparatus of Shigella flexneri. Mol. Microbiol. 49:755-767. [DOI] [PubMed] [Google Scholar]
- 87.Journet, L., C. Agrain, P. Broz, and G. R. Cornelis. 2003. The needle length of bacterial injectisomes is determined by a molecular ruler. Science 302:1757-1760. [DOI] [PubMed] [Google Scholar]
- 88.Kalman, D., O. D. Weiner, D. L. Goosney, J. W. Sedat, B. B. Finlay, A. Abo, and J. M. Bishop. 1999. Enteropathogenic E. coli acts through WASP and Arp2/3 complex to form actin pedestals. Nat. Cell Biol. 1:389-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kanack, K. J., J. A. Crawford, M. A. Karmali, and J. B. Kaper. 2004. Abstr. 104th Gen. Meet. Am. Soc. Microbiol., abstr. B-085. p. 47. American Society for Microbiology, Washington, D.C.
- 90.Kaper, J. B., J. P. Nataro, and H. L. Mobley. 2004. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2:123-140. [DOI] [PubMed] [Google Scholar]
- 91.Keller, T. C. R., and M. S. Mooseker. 1982. Ca++-calmodulin-dependent phosphorylation of myosin, and its role in brush border contraction in vitro. J. Cell Biol. 95:943-959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kelly, G., S. Prasannan, S. Daniel, G. Frankel, G. Dougan, I. Connerton, and S. Mathews. 1998. Sequential assignment of the triple labelled 30.1 kDa cell adhesion domain of intimin from enteropathogenic E. coli. J. Biomol. NMR 12:189-191. [DOI] [PubMed] [Google Scholar]
- 93.Kenny, B. 2001. The enterohaemorrhagic Escherichia coli (serotype O157:H7) Tir molecule is not functionally interchangeable for its enteropathogenic E. coli (serotype O127:H6) homologue. Cell. Microbiol. 3:499-510. [DOI] [PubMed] [Google Scholar]
- 94.Kenny, B. 2002. Mechanism of action of EPEC type III effector molecules. Int. J. Med. Microbiol. 291:469-477. [DOI] [PubMed] [Google Scholar]
- 95.Kenny, B. 1999. Phosphorylation of tyrosine 474 of the enteropathogenic Escherichia coli (EPEC) Tir receptor molecule is essential for actin nucleating activity and is preceded by additional host modifications. Mol. Microbiol. 31:1229-1241. [DOI] [PubMed] [Google Scholar]
- 96.Kenny, B., A. Abe, M. Stein, and B. B. Finlay. 1997. Enteropathogenic Escherichia coli protein secretion is induced in response to conditions similar to those in the gastrointestinal tract. Infect. Immun. 65:2606-2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kenny, B., R. DeVinney, M. Stein, D. J. Reinscheid, E. A. Frey, and B. B. Finlay. 1997. Enteropathogenic E. coli (EPEC) transfers its receptor for intimate adherence into mammalian cells. Cell 91:511-520. [DOI] [PubMed] [Google Scholar]
- 98.Kenny, B., S. Ellis, A. D. Leard, J. Warawa, H. Mellor, and M. A. Jepson. 2002. Co-ordinate regulation of distinct host cell signalling pathways by multifunctional enteropathogenic Escherichia coli effector molecules. Mol. Microbiol. 44:1095-1107. [DOI] [PubMed] [Google Scholar]
- 99.Kenny, B., and M. Jepson. 2000. Targeting of an enteropathogenic Escherichia coli (EPEC) effector protein to host mitochondria. Cell. Microbiol. 2:579-590. [DOI] [PubMed] [Google Scholar]
- 100.Kimbrough, T. G., and S. I. Miller. 2000. Contribution of Salmonella typhimurium type III secretion components to needle complex formation. Proc. Natl. Acad. Sci. USA 97:11008-11013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Knappstein, S., T. Ide, M. A. Schmidt, and G. Heusipp. 2004. Alpha 1-antitrypsin binds to and interferes with functionality of EspB from atypical and typical enteropathogenic Escherichia coli strains. Infect. Immun. 72:4344-4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Knutton, S., D. R. Lloyd, and A. S. McNeish. 1987. Adhesion of enteropathogenic Escherichia coli to human intestinal enterocytes and cultured human intestinal mucosa. Infect. Immun. 55:69-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Knutton, S., I. Rosenshine, M. J. Pallen, I. Nisan, B. C. Neves, C. Bain, C. Wolff, G. Dougan, and G. Frankel. 1998. A novel EspA-associated surface organelle of enteropathogenic Escherichia coli involved in protein translocation into epithelial cells. EMBO J. 17:2166-2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Knutton, S., R. Shaw, and G. Frankel. 2002. Interaction of enteropathogenic Escherichia coli with red blood cell monolayers. Methods Enzymol. 358:350-355. [DOI] [PubMed] [Google Scholar]
- 105.Kodama, T., Y. Akeda, G. Kono, A. Takahashi, K. Imura, T. Iida, and T. Honda. 2002. The EspB protein of enterohaemorrhagic Escherichia coli interacts directly with alpha-catenin. Cell. Microbiol. 4:213-222. [DOI] [PubMed] [Google Scholar]
- 106.Koster, M., W. Bitter, H. de Cock, A. Allaoui, G. R. Cornelis, and J. Tommassen. 1997. The outer membrane component, YscC, of the Yop secretion machinery of Yersinia enterocolitica forms a ring-shaped multimeric complex. Mol. Microbiol. 26:789-797. [DOI] [PubMed] [Google Scholar]
- 107.Kresse, A. U., M. Rohde, and C. A. Guzman. 1999. The EspD protein of enterohemorrhagic Escherichia coli is required for the formation of bacterial surface appendages and is incorporated in the cytoplasmic membranes of target cells. Infect. Immun. 67:4834-4842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kubori, T., Y. Matsushima, D. Nakamura, J. Uralil, M. Lara-Tejero, A. Sukhan, J. E. Galan, and S. I. Aizawa. 1998. Supramolecular structure of the Salmonella typhimurium type III protein secretion system. Science 280:602-605. [DOI] [PubMed] [Google Scholar]
- 109.Kubori, T., A. Sukhan, S. I. Aizawa, and J. E. Galan. 2000. Molecular characterization and assembly of the needle complex of the Salmonella typhimurium type III protein secretion system. Proc. Natl. Acad. Sci. USA 97:10225-10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lai, L. C., L. A. Wainwright, K. D. Stone, and M. S. Donnenberg. 1997. A third secreted protein that is encoded by the enteropathogenic Escherichia coli pathogenicity island is required for transduction of signals and for attaching and effacing activities in host cells. Infect. Immun. 65:2211-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Liu, H., L. Magoun, S. Luperchio, D. B. Schauer, and J. M. Leong. 1999. The Tir-binding region of enterohaemorrhagic Escherichia coli intimin is sufficient to trigger actin condensation after bacterial-induced host cell signalling. Mol. Microbiol. 34:67-81. [DOI] [PubMed] [Google Scholar]
- 112.Lommel, S., S. Benesch, M. Rohde, J. Wehland, and K. Rottner. 2004. Enterohaemorrhagic and enteropathogenic Escherichia coli use different mechanisms for actin pedestal formation that converge on N-WASP. Cell. Microbiol. 6:243-254. [DOI] [PubMed] [Google Scholar]
- 113.Lommel, S., S. Benesch, K. Rottner, T. Franz, J. Wehland, and R. Kuhn. 2001. Actin pedestal formation by enteropathogenic Escherichia coli and intracellular motility of Shigella flexneri are abolished in N-WASP-defective cells. EMBO Rep. 2:850-857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Luo, Y., M. G. Bertero, E. A. Frey, R. A. Pfuetzner, M. R. Wenk, L. Creagh, S. L. Marcus, D. Lim, F. Sicheri, C. Kay, C. Haynes, B. B. Finlay, and N. C. Strynadka. 2001. Structural and biochemical characterization of the type III secretion chaperones CesT and SigE. Nat. Struct. Biol. 8:1031-1036. [DOI] [PubMed] [Google Scholar]
- 115.Luo, Y., E. A. Frey, R. A. Pfuetzner, A. L. Creagh, D. G. Knoechel, C. A. Haynes, B. B. Finlay, and N. C. Strynadka. 2000. Crystal structure of enteropathogenic Escherichia coli intimin-receptor complex. Nature 405:1073-1077. [DOI] [PubMed] [Google Scholar]
- 116.Luperchio, S. A., and D. B. Schauer. 2001. Molecular pathogenesis of Citrobacter rodentium and transmissible murine colonic hyperplasia. Microbes Infect. 3:333-340. [DOI] [PubMed] [Google Scholar]
- 117.Macnab, R. M. 2003. How bacteria assemble flagella. Annu. Rev. Microbiol. 57:77-100. [DOI] [PubMed] [Google Scholar]
- 118.Madara, J. L., C. Parkos, S. Colgan, A. Nusrat, K. Atisook, and P. Kaoutzani. 1992. The movement of solutes and cells across tight junctions. Ann. N. Y. Acad. Sci. 664:47-60. [DOI] [PubMed] [Google Scholar]
- 119.Malladi, V., B. Shankar, P. H. Williams, and A. Balakrishnan. 2004. Enteropathogenic Escherichia coli outer membrane proteins induce changes in cadherin junctions of Caco-2 cells through activation of PKCalpha. Microbes Infect. 6:38-50. [DOI] [PubMed] [Google Scholar]
- 120.Manjarrez-Hernandez, H. A., T. J. Baldwin, P. H. Williams, R. Haigh, S. Knutton, and A. Aitken. 1996. Phosphorylation of myosin light chain at distinct sites and its association with the cytoskeleton during enteropathogenic Escherichia coli infection. Infect. Immun. 64:2368-2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Marches, O., T. N. Ledger, M. Boury, M. Ohara, X. Tu, F. Goffaux, J. Mainil, I. Rosenshine, M. Sugai, J. De Rycke, and E. Oswald. 2003. Enteropathogenic and enterohaemorrhagic Escherichia coli deliver a novel effector called Cif, which blocks cell cycle G2/M transition. Mol. Microbiol. 50:1553-1567. [DOI] [PubMed] [Google Scholar]
- 122.Marenne, M., L. Journet, L. Mota, and G. Cornelis. 2003. Genetic analysis of the formation of the Ysc-Yop translocation pore in macrophages by Yersinia enterocolitica: role of LcrV, YscF and YopN. Microb. Pathog. 35:243-258. [DOI] [PubMed] [Google Scholar]
- 123.Marlovits, T. C., T. Kubori, A. Sukhan, D. R. Thomas, J. E. Galan, and V. M. Unger. 2004. Structural insights into the assembly of the type III secretion needle complex. Science 306:1040-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Matsuzawa, T., A. Kuwae, S. Yoshida, C. Sasakawa, and A. Abe. 2004. Enteropathogenic Escherichia coli activates the RhoA signaling pathway via the stimulation of GEF-H1. EMBO J. 23:3570-3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.McDaniel, T. K., K. G. Jarvis, M. S. Donnenberg, and J. B. Kaper. 1995. A genetic locus of enterocyte effacement conserved among diverse enterobacterial pathogens. Proc. Natl. Acad. Sci. USA 92:1664-1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.McNamara, B. P., and M. S. Donnenberg. 1998. A novel proline-rich protein, EspF, is secreted from enteropathogenic Escherichia coli via the type III export pathway. FEMS Microbiol. Lett. 166:71-78. [DOI] [PubMed] [Google Scholar]
- 127.McNamara, B. P., A. Koutsouris, C. B. O'Connell, J. P. Nougayrede, M. S. Donnenberg, and G. Hecht. 2001. Translocated EspF protein from enteropathogenic Escherichia coli disrupts host intestinal barrier function. J. Clin. Investig. 107:621-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mellies, J. L., S. J. Elliott, V. Sperandio, M. S. Donnenberg, and J. B. Kaper. 1999. The Per regulon of enteropathogenic Escherichia coli: identification of a regulatory cascade and a novel transcriptional activator, the locus of enterocyte effacement (LEE)-encoded regulator (Ler). Mol. Microbiol. 33:296-306. [DOI] [PubMed] [Google Scholar]
- 129.Mellies, J. L., F. Navarro-Garcia, I. Okeke, J. Frederickson, J. P. Nataro, and J. B. Kaper. 2001. espC pathogenicity island of enteropathogenic Escherichia coli encodes an enterotoxin. Infect. Immun. 69:315-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mundy, M., L. Petrovska, K. Smollett, N. Simpson, R. K. Wilson, J. Yu, X. Tu, I. Rosenshine, S. Clare, G. Dougan, and G. Frankel. 2004. Identification of a novel Citrobacter rodentium type III secreted protein, EspI, and the roles of this and other secreted proteins in infection. Infect. Immun. 72:2288-2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mundy, R., C. Jenkins, J. Yu, H. Smith, and G. Frankel. 2004. Distribution of espI among clinical enterohaemorrhagic and enteropathogenic Escherichia coli isolates. J. Med. Microbiol. 53:1145-1149. [DOI] [PubMed] [Google Scholar]
- 132.Mundy, R., D. Pickard, R. K. Wilson, C. P. Simmons, G. Dougan, and G. Frankel. 2003. Identification of a novel type IV pilus gene cluster required for gastrointestinal colonization of Citrobacter rodentium. Mol. Microbiol. 48:795-809. [DOI] [PubMed] [Google Scholar]
- 133.Muza-Moons, M. M., E. E. Schneeberger, and G. A. Hecht. 2004. Enteropathogenic Escherichia coli infection leads to appearance of aberrant tight junctions strands in the lateral membrane of intestinal epithelial cells. Cell. Microbiol. 6:783-793. [DOI] [PubMed] [Google Scholar]
- 134.Nagai, T., A. Abe, and C. Sasakawa. 2005. Targeting of enteropathogenic Escherichia coli EspF to host mitochondria is essential for the bacterial pathogenesis: critical role of the 16th leucine residue in EspF. J. Biol. Chem. 280:2998-3011. [DOI] [PubMed]
- 135.Nataro, J. P., and J. B. Kaper. 1998. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 11:142-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Neves, B. C., R. Mundy, L. Petrovska, G. Dougan, S. Knutton, and G. Frankel. 2003. CesD2 of enteropathogenic Escherichia coli is a second chaperone for the type III secretion translocator protein EspD. Infect. Immun. 71:2130-2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Nougayrède, J.-P., and M. S. Donnenberg. 2004. Enteropathogenic Escherichia coli EspF is targeted to mitochondria and is required to initiate the mitochondrial death pathway. Cell. Microbiol. 11:1097-1111. [DOI] [PubMed] [Google Scholar]
- 138.O'Connell, C. B., E. A. Creasey, S. Knutton, S. Elliott, L. J. Crowther, W. Luo, M. J. Albert, J. B. Kaper, G. Frankel, and M. S. Donnenberg. 2004. SepL, a protein required for enteropathogenic Escherichia coli type III translocation, interacts with secretion component SepD. Mol. Microbiol. 52:1613-1625. [DOI] [PubMed] [Google Scholar]
- 139.Page, A. L., M. Fromont-Racine, P. Sansonetti, P. Legrain, and C. Parsot. 2001. Characterization of the interaction partners of secreted proteins and chaperones of Shigella flexneri. Mol. Microbiol. 42:1133-1145. [DOI] [PubMed] [Google Scholar]
- 140.Parsot, C., C. Hamiaux, and A. L. Page. 2003. The various and varying roles of specific chaperones in type III secretion systems. Curr. Opin. Microbiol. 6:7-14. [DOI] [PubMed] [Google Scholar]
- 141.Phillips, A. D., and G. Frankel. 2000. Intimin-mediated tissue specificity in enteropathogenic Escherichia coli interaction with human intestinal organ cultures. J. Infect. Dis. 181:1496-1500. [DOI] [PubMed] [Google Scholar]
- 142.Phillips, A. D., J. Giron, S. Hicks, G. Dougan, and G. Frankel. 2000. Intimin from enteropathogenic Escherichia coli mediates remodelling of the eukaryotic cell surface. Microbiology 146:1333-1344. [DOI] [PubMed] [Google Scholar]
- 143.Phillips, N., R. D. Hayward, and V. Koronakis. 2001. Phosphorylation of the enteropathogenic E. coli receptor by the Src-family kinase c-Fyn triggers actin pedestal formation. Nat. Cell Biol. 6:618-625. [DOI] [PubMed] [Google Scholar]
- 144.Plano, G. V., and S. C. Straley. 1995. Mutations in yscC, yscD, and yscG prevent high-level expression and secretion of V antigen and Yops in Yersinia pestis. J. Bacteriol. 177:3843-3854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Reece, S., C. P. Simmons, R. J. Fitzhenry, S. Matthews, A. D. Phillips, G. Dougan, and G. Frankel. 2001. Site-directed mutagenesis of intimin alpha modulates intimin-mediated tissue tropism and host specificity. Mol. Microbiol. 40:86-98. [DOI] [PubMed] [Google Scholar]
- 146.Russmann, H., T. Kubori, J. Sauer, and J. E. Galan. 2002. Molecular and functional analysis of the type III secretion signal of the Salmonella enterica InvJ protein. Mol. Microbiol. 46:769-779. [DOI] [PubMed] [Google Scholar]
- 147.Sanger, J. M., R. Chang, F. Ashton, J. B. Kaper, and J. W. Sanger. 1996. Novel form of actin-based motility transports bacteria on the surfaces of infected cells. Cell Motil. Cytoskelet. 34:279-287. [DOI] [PubMed] [Google Scholar]
- 148.Sato, N., N. Funayama, A. Nagafuchi, S. Yonemura, S. Tsukita, and S. Tsukita. 1992. A gene family consisting of ezrin, radixin and moesin. Its specific localization at actin filament/plasma membrane association sites. J. Cell Sci. 103:131-143. [DOI] [PubMed] [Google Scholar]
- 149.Savkovic, S. D., A. Koutsouris, and G. Hecht. 1997. Activation of NF-kappaB in intestinal epithelial cells by enteropathogenic Escherichia coli. Am. J. Physiol. 273:C1160-C1167. [DOI] [PubMed] [Google Scholar]
- 150.Schuch, R., and A. T. Maurelli. 2001. MxiM and MxiJ, base elements of the Mxi-Spa type III secretion system of Shigella, interact with and stabilize the MxiD secretin in the cell envelope. J. Bacteriol. 183:6991-6998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sekiya, K., M. Ohishi, T. Ogino, K. Tamano, C. Sasakawa, and A. Abe. 2001. Supermolecular structure of the enteropathogenic Escherichia coli type III secretion system and its direct interaction with the EspA-sheath-like structure. Proc. Natl. Acad. Sci. USA 98:11638-11643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Shaw, R. K., J. Cleary, M. S. Murphy, G. Frankel, and S. Knutton. 2005. Interaction of enteropathogenic Escherichia coli with human intestinal mucosa: role of effector proteins in brush border remodelling and formation of attaching and effacing lesions. Infect. Immun. 73:1243-1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Shaw, R. K., S. Daniell, F. Ebel, G. Frankel, and S. Knutton. 2001. EspA filament-mediated protein translocation into red blood cells. Cell. Microbiol. 3:213-222. [DOI] [PubMed] [Google Scholar]
- 153a.Shaw, R. K., K. Smollett, J. Cleary, J. Carmendia, A. Straatman-Iwanowska, G. Frankel, and S. Knutton. Enteropathogenic Escherichia coli type III effectors EspG and EspG2 disrupt the microtubule network of intestinal epithelial cells. Infect. Immun., in press. [DOI] [PMC free article] [PubMed]
- 154.Simonovic, I., M. Arpin, A. Koutsouris, H. J. Falk-Krzesinski, and G. Hecht. 2001. Enteropathogenic Escherichia coli activates ezrin, which participates in disruption of tight junction barrier function. Infect. Immun. 69:5679-5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Simonovic, I., J. Rosenberg, A. Koutsouris, and G. Hecht. 2000. Enteropathogenic Escherichia coli dephosphorylates and dissociates occludin from intestinal epithelial tight junctions. Cell. Microbiol. 2:305-315. [DOI] [PubMed] [Google Scholar]
- 156.Sinclair, J. F., and A. D. O'Brien. 2002. Cell surface-localized nucleolin is a eukaryotic receptor for the adhesin intimin-gamma of enterohemorrhagic Escherichia coli O157:H7. J. Biol. Chem. 277:2876-2885. [DOI] [PubMed] [Google Scholar]
- 157.Sinclair, J. F., and A. D. O'Brien. 2004. Intimin types alpha, beta, and gamma bind to nucleolin with equivalent affinity but lower avidity than to the translocated intimin receptor. J. Biol. Chem. 279:33751-33758. [DOI] [PubMed] [Google Scholar]
- 158.Stein, M. A., D. A. Mathers, H. Yan, K. G. Baimbridge, and B. B. Finlay. 1996. Enteropathogenic Escherichia coli markedly decreases the resting membrane potential of Caco-2 and HeLa human epithelial cells. Infect. Immun. 64:4820-4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Sukhan, A., T. Kubori, and J. Galan. 2003. Synthesis and localization of the Salmonella SPI-1 type III secretion needle complex proteins PrgI and PrgJ. J. Bacteriol. 185:3480-3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Sukhan, A., T. Kubori, J. Wilson, and J. E. Galan. 2001. Genetic analysis of assembly of the Salmonella enterica serovar Typhimurium type III secretion-associated needle complex. J. Bacteriol. 183:1159-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Swimm, A., B. Bommarius, Y. Li, D. Cheng, P. Reeves, M. Sherman, D. Veach, W. Bornmann, and D. Kalman. 2004. Enteropathogenic Escherichia coli use redundant tyrosine kinases to form actin pedestals. Mol. Biol. Cell 15:3520-3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Tamano, K., S. Aizawa, E. Katayama, T. Nonaka, S. Imajoh-Ohmi, A. Kuwae, S. Nagai, and C. Sasakawa. 2000. Supramolecular structure of the Shigella type III secretion machinery: the needle part is changeable in length and essential for delivery of effectors. EMBO J. 19:3876-3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Tamano, K., E. Katayama, T. Toyotome, and C. Sasakawa. 2002. Shigella Spa32 is an essential secretory protein for functional type III secretion machinery and uniformity of its needle length. J. Bacteriol. 184:1244-1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Tampakaki, A., V. Fadouloglou, A. Gazi, N. Panopoulos, and M. Kokkinidis. 2004. Conserved features of type III secretion. Cell. Microbiol. 6:805-816. [DOI] [PubMed] [Google Scholar]
- 165.Taylor, K. A., P. W. Luther, and M. S. Donnenberg. 1999. Expression of the EspB protein of enteropathogenic Escherichia coli within HeLa cells affects stress fibers and cellular morphology. Infect. Immun. 67:120-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Taylor, K. A., C. B. O'Connell, P. W. Luther, and M. S. Donnenberg. 1998. The EspB protein of enteropathogenic Escherichia coli is targeted to the cytoplasm of infected HeLa cells. Infect. Immun. 66:5501-5507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Touze, T., R. D. Hayward, J. Eswaran, J. M. Leong, and V. Koronakis. 2004. Self-association of EPEC intimin mediated by the beta-barrel-containing anchor domain: a role in clustering of the Tir receptor. Mol. Microbiol. 51:73-87. [DOI] [PubMed] [Google Scholar]
- 168.Tu, X., I. Nisan, C. Yona, E. Hanski, and I. Rosenshine. 2003. EspH, a new cytoskeleton-modulating effector of enterohaemorrhagic and enteropathogenic Escherichia coli. Mol. Microbiol. 47:595-606. [DOI] [PubMed] [Google Scholar]
- 169.Ueno, T., K. Oosawa, and S. Aizawa. 1994. Domain structures of the MS ring component protein (FliF) of the flagellar basal body of Salmonella typhimurium. J. Mol. Biol. 236:546-555. [DOI] [PubMed] [Google Scholar]
- 170.Viswanathan, V. K., A. Koutsouris, S. Lukic, M. Pilkinton, I. Simonovic, M. Simonovic, and G. Hecht. 2004. Comparative analysis of EspF from enteropathogenic and enterohemorrhagic Escherichia coli in alteration of epithelial barrier function. Infect. Immun. 72:3218-3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Viswanathan, V. K., S. Lukic, A. Koutsouris, R. Miao, M. M. Muza, and G. Hecht. 2004. Cytokeratin 18 interacts with the enteropathogenic Escherichia coli secreted protein F (EspF) and is redistributed after infection. Cell. Microbiol. 6:987-997. [DOI] [PubMed] [Google Scholar]
- 172.Wachter, C., C. Beinke, M. Mattes, and M. A. Schmidt. 1999. Insertion of EspD into epithelial target cell membranes by infecting enteropathogenic Escherichia coli. Mol. Microbiol. 31:1695-1707. [DOI] [PubMed] [Google Scholar]
- 173.Wainwright, L. A., and J. B. Kaper. 1998. EspB and EspD require a specific chaperone for proper secretion from enteropathogenic Escherichia coli. Mol. Microbiol. 27:1247-1260. [DOI] [PubMed] [Google Scholar]
- 174.Warawa, J., B. B. Finlay, and B. Kenny. 1999. Type III secretion-dependent hemolytic activity of enteropathogenic Escherichia coli. Infect. Immun. 67:5538-5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Warawa, J., and B. Kenny. 2001. Phosphoserine modification of the enteropathogenic Escherichia coli Tir molecule is required to trigger conformational changes in Tir and efficient pedestal elongation. Mol. Microbiol. 42:1269-1280. [DOI] [PubMed] [Google Scholar]
- 176.Wattiau, P., B. Bernier, P. Deslee, T. Michiels, and G. R. Cornelis. 1994. Individual chaperones required for Yop secretion by Yersinia. Proc. Natl. Acad. Sci. USA 91:10493-10497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Wattiau, P., S. Woestyn, and G. R. Cornelis. 1996. Customized secretion chaperones in pathogenic bacteria. Mol. Microbiol. 20:255-262. [DOI] [PubMed] [Google Scholar]
- 178.Wieler, L. H., T. K. McDaniel, T. S. Whittam, and J. B. Kaper. 1997. Insertion site of the locus of enterocyte effacement in enteropathogenic and enterohemorrhagic Escherichia coli differs in relation to the clonal phylogeny of the strains. FEMS Microbiol. Lett. 156:49-53. [DOI] [PubMed] [Google Scholar]
- 179.Wiles, S., S. Clare, J. Harker, A. Huett, D. Young, G. Dougan, and G. Frankel. 2004. Organ specificity, colonization and clearance dynamics in vivo following oral challenges with the murine pathogen Citrobacter rodentium. Cell. Microbiol. 6:963-972. [DOI] [PubMed] [Google Scholar]
- 180.Wilson, R. K., R. K. Shaw, S. Daniell, S. Knutton, and G. Frankel. 2001. Role of EscF, a putative needle complex protein, in the type III protein translocation system of enteropathogenic Escherichia coli. Cell. Microbiol. 3:753-762. [DOI] [PubMed] [Google Scholar]
- 181.Wolff, C., I. Nisan, E. Hanski, G. Frankel, and I. Rosenshine. 1998. Protein translocation into HeLa cells by infecting enteropathogenic Escherichia coli. Mol. Microbiol. 28:143-155. [DOI] [PubMed] [Google Scholar]
- 182.Yoshida, S., E. Katayama, A. Kuwae, H. Mimuro, T. Suzuki, and C. Sasakawa. 2002. Shigella deliver an effector protein to trigger host microtubule destabilization, which promotes Rac1 activity and efficient bacterial internalization. EMBO J. 21:2923-2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Yuhan, R., A. Koutsouris, S. D. Savkovic, and G. Hecht. 1997. Enteropathogenic Escherichia coli-induced myosin light chain phosphorylation alters intestinal epithelial permeability. Gastroenterology 113:1873-1882. [DOI] [PubMed] [Google Scholar]