The origin of the name malaria, meaning ‘bad air’, stems from the Roman period in Italy when the Romans first associated the disease with the stench of swamps. Indeed, malaria is one of humankind’s oldest enemies. Alexander the Great died at the age of 32 in Babylon, most probably weakened by malaria, which he acquired during his campaigns. Even earlier, the Greek physician Hippocrates of Cos described the symptoms while travelling through Egypt. His description is so accurate that medical doctors still used it to diagnose malaria until the last century.
Malaria has been a scourge of humanity since antiquity and it remains so today. It has been over 100 years since the discovery that ‘the ague’—now commonly known as malaria—is caused by infection with the protozoan Plasmodia, which is transmitted between humans by the bite of the female mosquito Anopheles. There are various Plasmodia species but the most severe form of the disease is caused by Plasmodium falciparum. With the discovery of the cheap and effective anti-malarial chloroquine in World War II, it looked like malaria could be kept at bay. But the last decades have seen the rise of drug-resistant strains of Plasmodium particularly in Southeast Asia and sub-Saharan Africa. It is therefore increasingly important to find new drugs or a vaccine. The sequencing of the Plasmodium genome will be completed soon and analysis of this information may result in new drug targets and ultimately new treatments for malaria. But to successfully control the disease in the meantime, new ways must be found to circumvent drug resistance to the existing treatments.
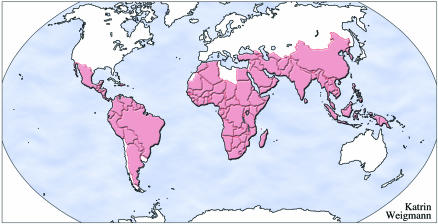
The World Health Organization estimates that malaria is endemic in more than 100 countries (Figure 1), which puts 2.4 billion people—over 40% of the world’s population–at risk. More than 300 million people develop clinical malaria and at least 1 million die each year. Depressingly, these statistics have changed little since the 1950s when the first initiatives to eliminate the mosquito vector were put into place. The geographical area affected by malaria has shrunk considerably, but control of the disease is becoming more difficult and gains achieved over the past 50 years are slowly being eroded due to the increasing drug resistance of the protozoan. There have been huge reductions in mortality and morbidity in areas such as South Asia, but malaria still remains the largest paediatric killer in many parts of sub-Saharan Africa, which bears the greatest global burden of disease.
Fig. 1. Countries where malaria is endemic according to the WHO.
In addition, malaria puts an economic burden on those countries that are already among the poorest in the world. WHO, Harvard University and the London School of Hygiene and Tropical Medicine estimate that the disease slows Africa’s economic growth by up to 1.3% each year by increasing costs for health care and preventing the improvement of living standards. Their report puts the short-term economic benefits of malaria control for African countries at between $3 and $12 billion each year.
Recently, there has been a strengthening of political will to combat malaria including the initiation of the Roll Back Malaria Global Partnership and the Multilateral Initiative for Malaria of the WHO. In March 2000, the Medicines for Malaria Venture, a WHO-organised public/private partnership for the discovery of new antimalarial drugs, received $25 million funding from the Bill and Melinda Gates Foundation. This has coincided with an improved ability to genetically analyse the parasite as well as the near completion of the P. falciparum genome-sequencing project. A major challenge for the coming years is therefore the appropriate use of this knowledge in the control of the disease.
The greatest and most pressing concern now is the growing drug resistance of Plasmodium, for it has led to a large increase in mortality across the world. In one area of West Africa, childhood mortality increased more than 5-fold over a period when resistance to chloroquine was taking hold (Trape et al., 1998). Indeed, a group of malaria experts have viewed the challenges of circumventing drug resistance as ‘averting a malaria disaster’ (White et al., 1999). Following its development in World War II, chloroquine became a popular replacement for the toxic drug quinine—being safe and relatively affordable. Unfortunately, resistance to chloroquine arose in the early 1960s in South America and Southeast Asia. Since then, chloroquine resistance has spread inexorably across the world and is now autochthonous with the presence of malaria.
The border areas of Southeast Asia provide a scenario for what may happen in Africa in the near future. By the early 1980s, chloroquine and the antifolate combination pyrimethamine/sulphadoxine, which is commonly used to treat chloroquine-resistant malaria, were virtually useless. Mefloquine, a drug with psychotropic side-effects, was found to be still effective throughout the 1980s, but by the early 1990s even its efficacy had dropped to less than 50% in some areas (Fontanet and Walker, 1993). The situation in Africa is following a similarly alarming evolution of drug resistance. The affordable drugs chloroquine and pyrimethamine/sulphadoxine—less than US$0.20 per treatment—are rapidly losing their efficacy throughout the continent. The next drug in line, mefloquine, is many times more expensive, which makes it unaffordable for most African patients as the per capita expenditure on drugs is much less than in Southeast Asia.
There is an urgent need for the rational understanding of drug resistance in order to maintain the efficacy of the existing antimalarial armamentarium and to develop new drugs. Our understanding of the molecular mechanisms of drug resistance in P. falciparum has increased greatly over the last decade. The antifolate drugs pyrimethamine and sulphadoxine respectively, target the enzymes dihydrofolate reductase and dihydropteroate synthase, two enzymes involved in the biosynthesis of folate cofactors. A specific set of mutations in these enzymes has been identified and shown to be responsible for resistance to these drugs in P. falciparum by reducing their ability to bind to the active site (Wu et al., 1996; Triglia et al., 1998). A complete understanding of how the parasite evades the lethal effect of chloroquine and other antimalarials such as mefloquine is still some way off. However, Fidock and co-workers recently identified the P. falciparum gene pfcrt that plays an important role in chloroquine resistance (Fidock et al., 2000). In addition, polymorphisms in the pfmdr1 gene have been shown to modulate resistance to chloroquine, mefloquine, quinine and the new antimalarial artemisinin (Reed et al., 2000).
This identification of alleles that are responsible for drug resistance will allow health workers to keep track of the rise and spread of resistant Plasmodium in malaria-endemic areas. The ability to identify drug resistance alleles by simple PCR-based techniques in the field has already been shown to be feasible. Such molecular surveys are logistically much easier and less expensive than in vivo assessments of drug susceptibility. Improved surveillance of these alleles can have direct implications for the development of judicious drug treatment and intervention policies by relevant governments. This may prolong the shelf-life of some antimalarials by reducing the selective pressure for drug resistance at an appropriate time. Furthermore, the analysis of drug resistance alleles with epidemiological and clinical data may also be helpful in devising new treatments
Clearly, P. falciparum has adapted extremely well to the challenge of our current armamentarium of antimalarial compounds. It will be necessary to develop new drugs that are not only effective against resistant Plasmodia but that may also prevent the parasite from developing resistance. The ability of drugs such as verapamil to modulate chloroquine resistance in P. falciparum has highlighted the possibility of developing reversal agents that could be used in combination to counteract the resistance of the parasite (Martin et al., 1987). A recent development has been the use of cheap and widely available antihistamines, such as promethazine, for the reversal of chloroquine resistance in West Africa (Oduola et al., 1998). This is particularly interesting when considering it appears that both of the proteins implicated in chloroquine resistance modulate ion fluxes within the parasite.
The use of drug combinations will be an important feature of future treatment regimens as they are hypothesised to protect existing antimalarials from the emergence of drug resistance or, at least, to slow the evolution of resistance. Combinations of the antimalarials artesunate and mefloquine in Thailand has halted the progression of mefloquine resistance in P. falciparum (White, 1998). Indeed, one strategy, which is being strongly promoted, is the use of artemisinin and its derivatives combined with one or more of the other antimalarials to minimise the emergence of resistant parasites (White et al., 1999). The molecular analytical tools to test the efficacy of this approach are now available for use in conjunction with field trials. At the same time, the development of new combinations such as atovaquone and proguanil (malarone) will provide important alternatives for the treatment and control of malaria.
The full genome sequence of P. falciparum is fast nearing completion and this immensely valuable resource will greatly enhance our understanding of the parasite and its interactions with the human host (Gardner et al., 1998). New technologies are emerging that will enable the analysis of gene function and genetic perturbations on a whole genome scale and rapidly yield a wealth of information. It is important that this new information is efficiently utilised to assist in the development of novel antimalarial compounds and vaccines.
However, an important point to remember while pushing for high-technology solutions, is that new drugs may not be affordable for those most at risk in poor countries. Malaria is a disease of the poor and cost is usually the major factor that determines the development and use of antimalarial drugs and new treatments. The most vulnerable and impoverished groups may not be reached with effective treatments and vaccines without substantial international co-operation. Moreover, with little prospect of adequate commercial returns, the pharmaceutical industry does not put the development of new antimalarials or vaccines high on the agenda. International co-operation of private and public enterprises therefore gains more importance in developing new treatments.
Malaria has been a companion of humans throughout history. The numerous attempts to control it have been defeated by a combination of the ability of the parasite and the mosquito to adapt to the challenges set by humans. But as the light microscope increased our understanding of the aetiology of malaria at the turn of the 19th century (Laveran, 1880), the sensitive new techniques of genetic analyses—when appropriately used in the field and the laboratory—might help us to expand our armamentarium against this ancient foe. It is hoped that the increased commitment to the eradication of malaria, together with the full exploitation of the scientific advances associated with our increased knowledge of the P. falciparum genome, will eventually bring this old enemy under control.


REFERENCES
- Fidock D.A. et al. (2000) Mutations in the P. falciparum digestive vacuole transmembrane protein PfCRT and evidence for their role in chloroquine resistance. Mol. Cell, 6, 861–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontanet A.L. and Walker, A.M. (1993) Predictors of treatment failure in multiple drug-resistant falciparum malaria: Results from a 42-day follow-up of 224 patients in Eastern Thailand. Am. J. Trop. Med. Hyg., 49, 465–472. [DOI] [PubMed] [Google Scholar]
- Gardner M.J. et al. (1998) Chromosome 2 sequence of the human malaria parasite Plasmodium falciparum. Science, 282, 1126–1132. [DOI] [PubMed] [Google Scholar]
- Laveran A. (1880) Un nouveau parasite trouvé dans le sang des malades atteints de fi èvre palustre origine parasitaire des accidents de l’impaludisme. Communication faite à la Société médicales des hôpitaux dans la séance du 24 décembre 1880. Bull. Mem. Soc. Med. Hop. Paris, 17, 158–164. [Google Scholar]
- Martin S.K., Oduola, A.M. and Milhous, W.K. (1987) Reversal of chloroquine resistance in Plasmodium falciparum by verapamil. Science, 235, 899–901. [DOI] [PubMed] [Google Scholar]
- Oduola A.M. et al (1998) In vitro and in vivo reversal of chloroquine resistance in Plasmodium falciparum with promethazine. Am. J. Trop. Med. Hyg., 58, 625–629. [DOI] [PubMed] [Google Scholar]
- Reed M.B., Saliba, K.J., Caruana, S.R., Kirk, K. and Cowman, A.F. (2000) Pgh1 modulates sensitivity and resistance to multiple antimalarials in Plasmodium falciparum. Nature, 403, 906–909. [DOI] [PubMed] [Google Scholar]
- Trape J.F. et al. (1998) Impact of chloroquine resistance on malaria mortality. C. R. Acad. Sci. III, 321, 689–97. [DOI] [PubMed] [Google Scholar]
- Triglia T., Wang, P., Sims, P.F.G., Hyde, J.E. and Cowman, A.F. (1998) Allelic exchange at the endogenous genomic locus in Plasmodium falciparum proves the role of dihydropteroate synthase in sulfadoxine-resistant malaria. EMBO J., 17, 3807–3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White N.J. (1998) Preventing antimalarial drug resistance through combinations. Drug Resis. Updates, 1, 3–9. [DOI] [PubMed] [Google Scholar]
- White N.J. et al. (1999) Averting a malaria disaster. Lancet, 353, 1965–1968. [DOI] [PubMed] [Google Scholar]
- Wu Y., Kirkman, L.A. and Wellems, T.E. (1996) Transformation of Plasmodium falciparum malaria parasites by homologous integration of plasmids that confer resistance to pyrimethamine. Proc. Natl Acad. Sci. USA, 93, 1130–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]