Abstract
Encapsulation of soluble protein antigens in liposomes was previously shown to result in processing of antigen via the major histocompatibility complex class I pathway, as evidenced by costaining of the trans-Golgi region of murine bone marrow-derived macrophages (BMs) by fluorophore-labeled liposomal antigen and by a trans-Golgi-specific fluorescent lipid. Evidence is presented here that free or liposome-encapsulated RTS,S, a particulate malaria antigen consisting of hepatitis B particles coexpressed with epitopes from the Plasmodium falciparum circumsporozoite protein, also was localized in the trans-Golgi after incubation with BMs, suggesting processing by the class I pathway. An in vivo cytotoxic T-lymphocyte (CTL) response was detected, however, only after immunization with RTS,S encapsulated in liposomes containing lipid A and not after immunization with free RTS,S or with RTS,S encapsulated in liposomes lacking lipid A. Therefore, intracellular delivery of antigen containing CTL epitopes to the Golgi of BMs does not necessarily result in a CTL response in vivo unless an additional adjuvant, such as liposomes containing lipid A, is utilized. Encapsulation of RTS,S in liposomes containing monophosphoryl lipid A (MPL) resulted in a dose-dependent enhancement of the NANP-specific immunoglobulin G (IgG) antibody response compared to that of free RTS,S. The IgG1 and IgG2a subclasses predominated after immunization with RTS,S encapsulated in liposomes containing MPL. These results demonstrate that encapsulation of a lipid-containing particulate antigen, such as RTS,S, in liposomes containing lipid A can enhance both humoral and cellular immune responses.
Induction of an immune response against a protein antigen is currently believed to involve the interaction of the protein with an antigen-presenting cell (APC) that partially degrades the antigen and channels the resulting peptides into either the major histocompatibility complex (MHC) class I or class II pathway (10, 14). Endogenous cellular proteins are thought to be presented via the class I pathway, while exogenous (extracellular) antigens are thought to be presented via the class II pathway. Exogenous antigens generally cannot be presented by class I molecules because of their inability to reach the cytosol. As a consequence most soluble antigens are poor at priming MHC class I-restricted cytotoxic T-lymphocyte (CTL) responses unless they are introduced into the cytoplasm by such artificial mechanisms as osmotic loading (27), covalent or noncovalent association with lipid carriers (12, 24, 36), conjugation to latex beads (18, 21), or encapsulation in liposomes (4, 6, 23, 26, 33, 44, 45).
In the classical MHC class I pathway for presentation of intracellular antigens on APCs, the endogenous proteins are hydrolyzed into peptides in the cytosol by proteasomes and then delivered to the endoplasmic reticulum by transporters associated with antigen processing (TAP) (8, 37). Studies utilizing fluorophore-labeled proteins encapsulated in liposomes demonstrated that after phagocytosis of the liposomes, the fluorescent liposomal protein, initially associated with the liposomal lipids in phagosomes, later entered the cytoplasm, and the processed protein was subsequently visualized in the trans-Golgi as fluorescent peptide (31). The translocation of the peptides into the trans-Golgi area also was shown to be dependent upon the TAP1 protein (31).
Class I presentation and induction of CTLs by liposomal antigens, both in vivo and in vitro, have been demonstrated for a variety of antigens (reviewed in references 6, 30, and 31). A liposome formulation developed in our laboratory, referred to as Walter Reed liposomes (42), has been shown to be an effective vehicle for delivery of proteins or peptides to APCs for presentation via the MHC class I pathway (4, 43–45). These liposomes contain either lipid A or monophosphoryl lipid A (MPL), its monophosphoryl derivative, as an intrinsic adjuvant in addition to dimyristoyl phosphatidylcholine, dimyristoyl phosphatidylglycerol, and cholesterol. Liposomes containing MPL and encapsulated RLF, a recombinant malaria antigen derived from the nonrepeat sequences of the Plasmodium falciparum circumsporozoite protein (CSP), have been shown to induce MHC class I-restricted CTLs specific for the CSP T-cell epitope (22), which includes amino acids 367 to 390 (19, 44). Immunization of mice with a myristoylated peptide consisting of the 367-to-390 sequence encapsulated in liposomes containing lipid A also was demonstrated to elicit CTLs specific for this T-cell epitope (4).
In this study we used a malaria sporozoite antigen (known as RTS,S) which consists of amino acids 210 to 398 of the carboxyl terminus of the P. falciparum CSP, including 19 NANP repeats, coexpressed in yeast with hepatitis B surface antigen (HBsAg) (16, 35). RTS,S exists in the form of lipid-containing particles with the CSP sequences exposed on the exterior, as evidenced by the induction in immunized animals of a high-titer antibody response against the CSP epitopes (35). In order to determine if RTS,S, since it is a lipid-containing particulate antigen, is processed by APCs by the class I pathway like liposomal antigens or is processed by the same pathway as soluble antigens, we have examined the intracellular trafficking of RTS,S. Our results indicate that, whether free or encapsulated in liposomes, RTS,S is localized in the trans-Golgi and/or cytoplasm of bone marrow-derived macrophages (BMs). The ability of free or liposome-encapsulated RTS,S to induce antibody and CTL responses in mice has also been investigated.
The role played by immunoglobulin G (IgG) subclasses in the immune response and in protection against disease is not yet fully understood. However, there is evidence that different IgG subclasses possess unique immunomodulatory characteristics (1). Murine antibody responses to soluble proteins are generally restricted to the IgG1 subclass despite the fact that IgG2a represents the major component of mouse serum IgG (28). Immunization with DNA encoding the S region of HBsAg encapsulated in cationic liposomes also resulted in a predominance of IgG1 (17). In contrast, encapsulation of a synthetic malaria antigen in liposomes containing lipid A resulted in a shift of the IgG subclass pattern from a predominance of IgG1 to a predominance of IgG2a (15). The results presented here suggested that the IgG subclass pattern, with IgG1 predominating, was similar whether RTS,S was free or encapsulated in liposomes.
MATERIALS AND METHODS
Liposome components.
Dimyristoyl phosphatidylcholine, dimyristoyl phosphatidylglycerol, and cholesterol used to prepare liposomes were obtained from Avanti Polar Lipids, Alabaster, Ala. MPL was from Ribi ImmunoChem, Hamilton, Mont.
Antigens.
RTS,S is a yeast-expressed HBsAg that has coexpressed epitopes from the CSP of P. falciparum 7G8 (16). The CSP epitopes contain amino acids 210 to 398 of the C terminus of the CSP, which includes 19 NANP repeats and amino acids 367 to 390, which have been identified as a CSP T-cell epitope (22). RTS,S exists as a lipid-containing particle, since it contains lipids (primarily phospholipid) in addition to protein. RTS,S was kindly provided by SmithKline Beecham Biologicals, Rixensart, Belgium. RTS,S was conjugated with Texas red (TR) (Molecular Probes, Inc., Eugene, Oreg.) according to the procedure of Titus et al. (39).
The peptide containing the 367-to-390 CTL epitope was synthesized by SynPep Corporation, Dublin, Calif.
Antibodies.
Hybridomas producing monoclonal antibodies against CD8 (8312.5) and CD4 (2RL) were kindly provided by R. Hodes, National Institutes of Health, Bethesda, Md. Hybridomas HB 20 (kkDk) and HB 6 (I-Ek) were obtained from the American Type Culture Collection, Rockville, Md.
Mice.
B10.Br (H-2k) mice were purchased from Jackson Laboratories, Bar Harbor, Maine. Each group of three or five mice was housed in an individual cage and given water and food ad libitum. Care and handling of the mice were conducted according to the principles set forth in the Animal Welfare Act of 1985 and reference 19a.
Preparation of liposomes.
Liposomes were prepared essentially as described previously (5, 43). The liposomes were composed of dimyristoyl phosphatidylcholine, dimyristoyl phosphatidylglycerol, and cholesterol in molar ratios of 0.9:0.1:0.75 and, where indicated, MPL. Lipids were dried from chloroform solution as a thin film under vacuum, suspended in water, aliquoted into vaccine vials, and lyophilized. For the immunizations, the lyophilized liposomes were reconstituted with RTS,S, diluted with buffer, and centrifuged to remove RTS,S not associated with the liposomes. Since unencapsulated RTS,S was recovered in the supernatant fraction after centrifugation, the centrifugation conditions used did not cause free RTS,S to pellet. The washed liposomes were aliquoted into vaccine vials and stored at 2 to 6°C until they were used for immunization. For the trafficking experiments, lyophilized liposomes either containing or lacking MPL were reconstituted with TR-labeled RTS,S (TR-RTS,S).
APCs.
Bone marrow stem cells that were cultured under conditions promoting only the growth of macrophages (BMs) were used as the APCs for the intracellular trafficking experiments. Marrows from the femurs of 8- to 10-week-old B10.Br mice were isolated as described previously (32, 41), and cells were seeded at a density of 2 × 105 on acid-washed circular glass coverslips (no. 1; VWR Scientific, West Chester, Pa.) in macrophage growth medium (RPMI 1640 containing 10% heat-inactivated fetal bovine serum, 10% L-929 cell-conditioned medium, 8 mM l-glutamine, 100 U of penicillin/ml, and 100 μg of streptomycin/ml [all from Gibco-BRL, Life Technologies, Grand Island, N.Y.]). On day 9, the macrophage cultures were supplemented with 10 U of murine gamma interferon (Gibco-BRL) per ml, and they were used as APCs the next day.
Immunofluorescence.
BMs grown on coverslips as described above were washed in Hanks balanced salt solution without phenol red (HBSS), pH 7.4, and then incubated in a total volume of 1 ml of HBSS containing either 200 μg of free TR-RTS,S, 30 μg of TR-RTS,S encapsulated in liposomes lacking lipid A, or 45 μg of TR-RTS,S encapsulated in liposomes containing lipid A for 90 min at 37°C. A greater amount of free TR-RTS,S than of liposomal TR-RTS,S was used, based on previous experiments with antigens not encapsulated in liposomes (31), to ensure that any low-level trafficking to the Golgi could be detected. The doses of liposomal TR-RTS,S were adjusted to give equal amounts of liposomal phospholipid and varied between the liposomes containing and lacking lipid A due to small differences in the TR-RTS,S encapsulation. At the end of the incubation period, the BMs were washed in HBSS and then incubated for an additional 2 h (chase) in HBSS or they were mounted immediately on a depression slide and the live cells were observed under a Leitz Orthoplan microscope (Leica) with an oil immersion 63× objective. Images were collected and processed as described previously (31).
Labeling of the trans-Golgi complex.
At the end of the incubation period, the trans-Golgi was visualized by staining the cells with a green-fluorescent analog of ceramide [N-(ɛ-nitrobenzooxadiazole-aminohexanoyl)-d-erythro-sphingosine (hereafter referred to as C6-NBD-ceramide); Molecular Probes, Inc.] as described previously (29, 31). Briefly, coverslips containing BMs from B10.Br mice were incubated on ice with 2 nmol of C6-NBD-ceramide/ml for 30 min. The cells were then washed twice with HBSS and incubated at 37°C for 15 min. After being washed twice more with HBSS, the cells were mounted and viewed as described above.
Immunizations.
B10.Br mice (five per group) were immunized intraperitoneally at 0 and 4 weeks with liposomal RTS,S (0.8, 1.4, 4.2, or 8.5 μg of RTS,S per 0.2-ml dose) or free RTS,S (10 μg of RTS,S per 0.1-ml dose) and bled at weeks 0, 2, 4, and 6 after the primary immunization. Each dose of the liposomal RTS,S formulations contained, in addition, 20 μmol (13.6 mg) of phospholipid, 15 μmol (5.8 mg) of cholesterol, and 40 μg of MPL.
For the experiment to study the effect of liposomal lipid A on CTL induction, B10.Br mice (three per group) were immunized as described above with RTS,S (4 μg per 0.1-ml dose) encapsulated in liposomes containing or lacking MPL. Each dose of these liposomal RTS,S formulations contained, in addition, 5.5 μmol (3.7 mg) of phospholipid, 4 μmol (1.5 mg) of cholesterol, and, where indicated, 18 μg of MPL.
Assay for antigen-specific CTLs.
Splenic lymphocytes were collected 2 weeks after the boosting immunization. The cells were cultured in vitro in complete medium (RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum, 8 mM l-glutamine, 100 U of penicillin/ml, 100 μg of streptomycin/ml, and 100 μM 2-mercaptoethanol) for 6 days, with the addition of 5% rat concanavalin A supernatant (as a source of interleukin 2), either without any antigen or with RTS,S (10 μg/ml) or the 367-to-390 CTL peptide (10 μg/ml) as an antigen. Target cells consisted of syngeneic (H-2k) L929 cells, L929 cells transfected with the P. falciparum gene (LPF), L929 cells pulsed with the 367-to-390 CTL peptide, or allogeneic (H-2b) EL-4 cells pulsed with the 367-to-390 CTL peptide. The target cells were labeled for 1 h with 51Cr (Na2CrO4; Amersham Life Science, Arlington Heights, Ill.) (100 μCi/106 cells), and then they were added to effector cells at ratios ranging from 3:1 to 100:1. The cell mixtures were incubated in 96-well round-bottom tissue culture plates (Costar, Cambridge, Mass.) in 0.2 ml of complete RPMI medium for 4 h at 37°C in a 5% CO2 humidified atmosphere. During the 4-h assay, a 1:5 final concentration of 8312.5, 2RL, HB 20, or HB 6 culture supernatant was added to the appropriate wells. At the end of the 4-h culture, the supernatants were absorbed by cotton wicks and processed for the determination of 51Cr release. Specific lysis was determined by the following formula: percent specific lysis = 100 × (experimental release − spontaneous release)/(maximal release − spontaneous release).
ELISA.
Solid-phase enzyme-linked immunosorbent assays (ELISAs) were performed to measure the levels of IgG antibody against the NANP repeat epitopes of the P. falciparum CSP by using R32LR, a recombinant protein with the sequence MDP(NANP)15NVDP(NANP)15NVDPLR (9), as the capture antigen, essentially as described previously (34, 41). Immulon-2 96-well U-bottom polystyrene plates (Dynatech Laboratories, Chantilly, Va.) were incubated with R32LR (2 μg/ml; 50 μl/well) in antigen diluent (4 μg/ml boiled casein in phosphate-buffered saline [PBS]) overnight at 4 to 6°C and then blocked with 0.5% casein in PBS containing 1% Tween 20 (PBS-casein-Tween). Individual mouse sera diluted in PBS-casein-Tween were added to the plates in triplicate wells and incubated overnight at 4 to 6°C. After being washed with 0.05% Tween 20 in PBS (PBS-Tween), the plates were incubated with phosphatase-labeled goat anti-mouse IgG (Kirkegaard & Perry Laboratories, Gaithersburg, Md.) for 2 h at room temperature and then washed again with PBS-Tween. Substrate (p-nitrophenyl phosphate; Kirkegaard & Perry Laboratories) was added, and the plates were incubated in the dark for 30 min. Absorbance was read at 405 nm with a UVmax plate reader (Molecular Devices, Sunnyvale, Calif.). Antibody levels are expressed as ELISA units. The ELISA units for a given serum sample are defined as the reciprocal of the serum dilution at which the absorbance is 1.0, and this value is determined from the linear portion of an antibody titration curve.
Quantitation of antigen-specific IgG subclass antibodies was performed by solid-phase ELISA as previously described (15). Immulon-2 plates were incubated overnight with antigen in PBS (0.1 μg/50 μl) and blocked with PBS containing 0.5% casein (PBS-casein). Individual mouse sera were diluted in PBS-casein and incubated at room temperature for 4 h. Peroxidase-conjugated goat anti-mouse isotype-specific antibody (The Binding Site, San Diego, Calif.) was utilized as a secondary antibody. Standard curves for each subclass were determined by using mouse myeloma IgG1, IgG2a, IgG2b, and IgG3 (The Binding Site). Both the test sera and myeloma standards were detected by using 2,2′-azino-di(3-ethyl-benzthiazoline) sulfonic acid (Kirkegaard & Perry Laboratories) as a substrate. Absorbance was read at 405 nm. Individual antigen-specific subclasses were quantitated by using the values from the linear titration curve computed against the myeloma standard curve and were reported as micrograms per milliliter.
RESULTS
Trafficking of liposomal RTS,S versus free RTS,S in BMs.
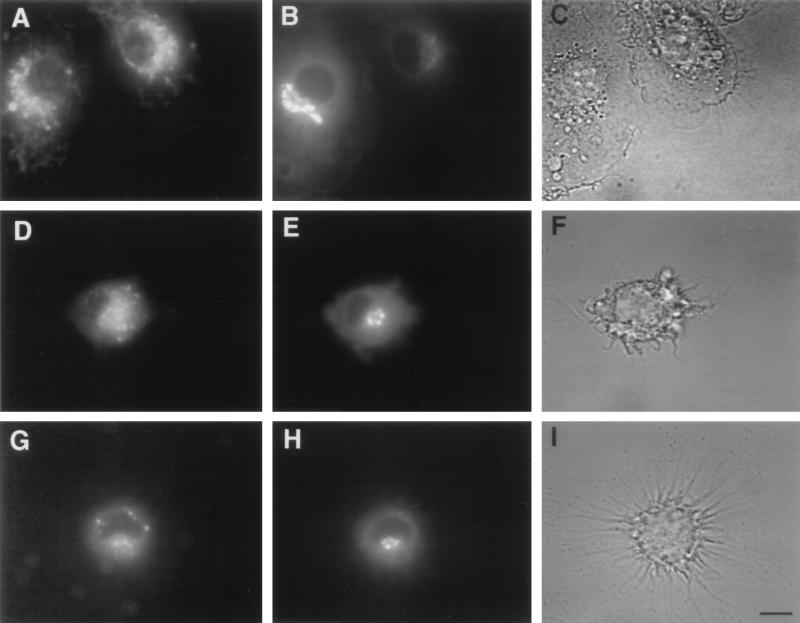
BMs were incubated with TR-RTS,S, either free or encapsulated in liposomes containing or lacking MPL. The resulting fluorescence patterns in BMs were examined to determine the intracellular trafficking of free and liposome-encapsulated RTS,S. When BMs were incubated with free (nonliposomal) TR-RTS,S, the red fluorescence initially appeared to be present throughout the cytoplasm, but after longer incubation times it also accumulated in the area of the Golgi. A similar pattern was observed when TR-RTS,S encapsulated in liposomes either containing or lacking MPL was incubated with BMs. This was verified by incubation of the BMs with C6-NBD-ceramide, a fluorophore-labeled lipid which is specific for the trans-Golgi (29), following incubation with TR-RTS,S. The green trans-Golgi-specific fluorescence colocalized in BMs with the red TR-RTS,S fluorescence regardless of whether the BMs were incubated with free (Fig. 1A and B) or with liposomal (Fig. 1D, E, G, and H) RTS,S. In most cases, the area of TR fluorescence extended beyond the borders of the C6-NBD-ceramide fluorescence, suggesting that localization of RTS,S was not limited solely to the trans-Golgi. This concentration in the Golgi has not been observed when free soluble antigens are incubated with BMs (31). The fluorescence patterns in the BMs suggested that RTS,S, both free and liposomal, went initially to the cytoplasm after uptake by the cells and later accumulated in the Golgi. This trafficking pattern is consistent with the intermediate steps that are known to be part of the processing of intracellular antigens through the MHC class I pathway.
FIG. 1.
Intracellular trafficking of liposome-encapsulated and free RTS,S in cultured BMs and localization of processed protein in the trans-Golgi area. BMs from B10.Br mice grown on coverslips were incubated with TR-RTS,S (A to C), with TR-RTS,S encapsulated in liposomes lacking MPL (D to F), or with TR-RTS,S encapsulated in liposomes containing MPL (G to I) for 90 min at 37°C and then chased for 2 h. At the end of the chase period, the cells were washed and stained with C6-NBD-ceramide, a green-fluorescent stain specific for the trans-Golgi. At the end of the incubation period, the cells were washed and mounted on a depression slide and live cells were observed under a Leitz Orthoplan microscope with an oil immersion 63× objective. The panels on the left (A, D, and G) show the TR-RTS,S fluorescence. The middle panels (B, E, and H) show the trans-Golgi fluorescence. The panels on the right (C, F, and I) show the corresponding bright-field images. Bar, 10 μm.
To ensure that the localization of TR-RTS,S in the trans-Golgi area was not just an isolated event occurring in only a few cells, BMs incubated with free TR-RTS,S were also examined at a lower magnification. The fluorescence patterns in BMs from multiple fields indicated that in 47% of 38 BMs examined, TR-RTS,S was definitely present in the region of the trans-Golgi. In the other 53% of the cells examined, TR-RTS,S was present either in the general area of the Golgi, but not localized to the trans-Golgi, or only in other areas of the BMs. It is likely that this heterogeneity of response was due to the fact that the cells were not synchronized for antigen processing.
CTL responses in mice immunized with liposomal RTS,S.
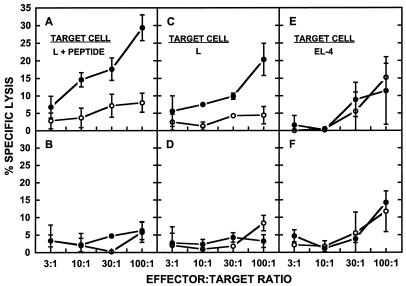
Immunization with RTS,S encapsulated in liposomes containing MPL resulted in the induction of CTLs in mice receiving a 4.2-μg dose of liposomal RTS,S (Fig. 2A, C, and E) but not in mice immunized with free RTS,S (Fig. 2B, D, and F). The response was specific for RTS,S, since the specific lysis from L929 cells pulsed with the CSP-derived 367-to-390 CTL peptide was substantially higher when these target cells were cultured with RTS,S than when they were cultured with medium alone (Fig. 2A). Although specific lysis greater than 30% was not observed in these experiments, similar levels of specific lysis have been reported previously as representing a positive CTL response (18, 26, 33, 44). Control L929 cells also gave somewhat higher lysis with effector cells cultured with RTS,S than with effector cells cultured with medium (Fig. 2C), but the lysis of the control L929 cells due to RTS,S-cultured effector cells was always lower than that obtained from CTL peptide-pulsed L929 cells, whereas the lysis levels of both control and peptide-pulsed L929 cells were similar with control effector cells (cultured with medium alone) (Fig. 2A and C). The RTS,S-specific cytolytic activity was restricted by MHC class I molecules, since only peptide-pulsed L929 cells (Fig. 2A), expressing H-2k, and not peptide-pulsed EL-4 cells (Fig. 2E), expressing H-2b, were lysed. The MHC class I restriction of the RTS,S-specific cytolytic activity was further confirmed by abrogation of the antigen-specific cytolytic activity when anti-class I antibodies and anti-CD8 antibodies, but not anti-class II antibodies or anti-CD4 antibodies, were added to the cultures (data not shown).
FIG. 2.
Cytotoxic T-lymphocyte response in mice immunized with liposomal and free RTS,S. Mice (five per group) were immunized intraperitoneally at 0 and 4 weeks with RTS,S, either encapsulated in liposomes containing MPL at 4.2 μg of RTS,S per dose (A, C, and E) or free at 10 μg of RTS,S per dose (B, D, and F). Spleens were harvested at 2 weeks after the boosting immunization, and the effector cells obtained as described in Materials and Methods were cultured with either RTS,S (solid circles) or medium (open circles). Target cells were either L929 cells pulsed with the 367-to-390 CTL peptide (L + PEPTIDE) (A and B), control L929 cells (L) (C and D), or EL-4 cells pulsed with the 367-to-390 CTL peptide (EL-4) (E and F). The percent specific lysis was calculated as described in Materials and Methods. The spontaneous release was less than 23% of the total release in all cases. Each error bar represents the standard deviation of the means of triplicate samples. The data presented, although from a single experiment, are representative of the data from multiple repetitions of the experiment.
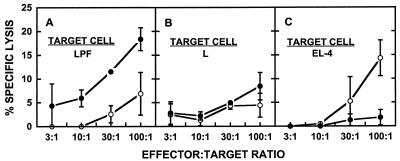
The CTLs induced in the mice immunized with liposomal RTS,S recognized the 367-to-390 CTL epitope of the P. falciparum CSP, since specific lysis was observed when effector cells from the mice immunized with liposomal RTS,S which had been cultured with the 367-to-390 peptide were incubated with L929 cells transfected with the P. falciparum gene (Fig. 3A) but not after incubation with control L929 cells (Fig. 3B). This activity also was MHC class I restricted, since no antigen-specific lysis was observed when EL-4 cells pulsed with the 367-to-390 peptide were the target cells (Fig. 3C).
FIG. 3.
Cytotoxic T-lymphocyte response against the CSP-derived 367-to-390 CTL peptide in mice immunized with liposomal RTS,S. Mice (five per group) were immunized with RTS,S encapsulated in liposomes containing MPL as described in the legend to Fig. 2. Effector cells obtained as described in the legend to Fig. 2 were cultured with either the CSP-derived 367-to-390 CTL peptide (solid circles) or medium (open circles). The target cells were L929 cells transfected with the P. falciparum gene (LPF) (A), control L929 cells (L) (B), or EL-4 cells pulsed with the 367-to-390 CTL peptide (EL-4) (C). The percent specific lysis was calculated as described in Materials and Methods. The spontaneous release was less than 24% of the total release in all cases. Each error bar represents the standard deviation of the mean of triplicate samples. The data presented, although from a single experiment, are representative of the data from multiple repetitions of the experiment.
A CTL response against the 367-to-390 CTL epitope was induced only when lipid A was present in the liposomes (Table 1). Specific lysis was observed when effector cells from mice immunized with RTS,S encapsulated in liposomes containing MPL which had been cultured with the 367-to-390 peptide were incubated with L929 cells pulsed with the 367-to-390 peptide as the targets, but not with effector cells from mice immunized with RTS,S encapsulated in liposomes lacking MPL. No specific lysis was obtained when L929 cells pulsed with medium alone were used as the targets (data not shown).
TABLE 1.
Effect of liposomal lipid A on the induction of cytotoxic T-cell responses in mice immunized with RTS,S encapsulated in liposomes containing or lacking lipid A
| Immunogena | E/Tb | % Specific lysisc
|
|
|---|---|---|---|
| Peptide | Medium | ||
| L(MPL + RTS,S) | 30:1 | 15.0 ± 6.7 | 3.9 ± 4.0 |
| 10:1 | 8.5 ± 4.9 | 3.8 ± 3.8 | |
| 3:1 | 4.8 ± 4.8 | 1.4 ± 1.4 | |
| L(RTS,S) | 30:1 | 2.9 ± 1.5 | 7.8 ± 4.3 |
| 10:1 | 0.8 ± 0.6 | 2.4 ± 2.4 | |
| 3:1 | 4.7 ± 4.2 | 4.0 ± 2.0 | |
Mice (three per group) were immunized as described in Materials and Methods with RTS,S (4 μg/dose) encapsulated in liposomes either containing lipid A [L(MPL + RTS,S)] or lacking lipid A [L(RTS,S)].
E/T, effector-to-target-cell ratio. Effector cells were obtained from the spleens of the immunized mice as indicated in Materials and Methods. The target cells were L929 cells pulsed with the 367-to-390 CTL peptide.
Percent specific lysis by effector cells cultured in the presence (Peptide) or absence (Medium) of the 367-to-390 CTL peptide was calculated as described in Materials and Methods. Each value represents percent specific lysis ± the standard error of the mean of triplicate samples.
NANP-specific IgG antibody response to liposomal RTS,S in mice.
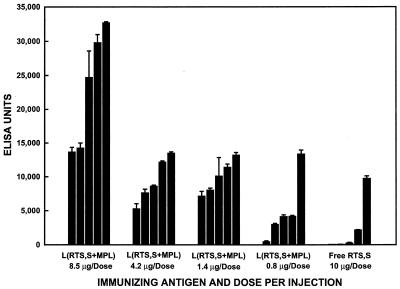
Immunization of B10.Br mice with different doses of liposomal RTS,S resulted in the production of NANP-specific IgG antibodies at all four RTS,S doses tested. As can be seen in Fig. 4, the NANP-specific IgG response at 6 weeks after the primary immunization (2 weeks after the secondary immunization) increased with increasing RTS,S doses. All five mice receiving the highest liposomal RTS,S dose (8.5 μg) gave high IgG titers, and these titers were as high as or higher than any obtained in the mice receiving the lower doses of liposomal RTS,S. At both the 4.2- and 1.4-μg doses, all five mice responded, and the titers were similar for the two doses. At the 0.8-μg dose, only four of the five mice responded, and of these four, only one had a high titer. With all four doses of liposomal RTS,S, however, the IgG responses were much higher than those obtained after immunization with a 10-μg dose of free RTS,S, with the exception of one of the five mice immunized with free RTS,S, which had an IgG titer comparable to the titers obtained with the lower doses of liposomal RTS,S.
FIG. 4.
Effect of antigen dose on the R32-specific IgG antibody response of individual mice immunized with free RTS,S or RTS,S encapsulated in liposomes containing MPL [L(RTS,S+MPL)] at 6 weeks after the primary immunization. Mice (five per group) were immunized intraperitoneally at 0 and 4 weeks with RTS,S at the indicated dose, either free or encapsulated in liposomes containing MPL. Each bar represents the mean ELISA units obtained at 6 weeks after the primary immunization for an individual mouse. Each error bar represents the standard deviation of the mean of triplicate samples.
IgG subclass pattern in mice immunized with liposomal or free RTS,S.
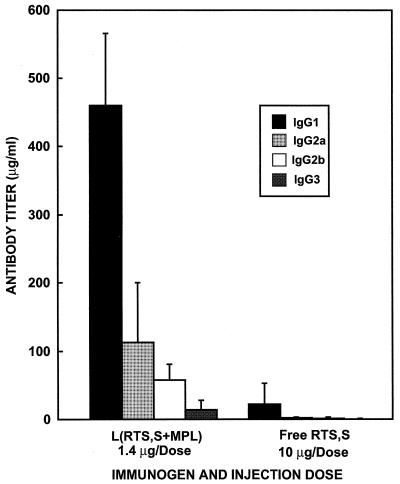
The IgG subclass distribution was also examined in the mice immunized with RTS,S, either free or encapsulated in liposomes containing MPL. The subclass distribution patterns were similar for all four doses of liposomal RTS,S. The predominant subclasses observed after immunization with liposomal RTS,S were IgG1 and IgG2a (Fig. 5). Since the mice immunized with free RTS,S gave much lower antibody titers, the IgG subclass pattern was difficult to determine (Fig. 5). However, the subclass distribution pattern was consistent with that obtained in the mice immunized with liposomal RTS,S.
FIG. 5.
IgG subclass distribution at 6 weeks after the primary immunization in mice immunized with liposomal or free RTS,S. Mice were immunized as indicated in the legend to Fig. 4. IgG subclass levels were determined quantitatively by ELISA as described in Materials and Methods. Each bar represents the mean IgG antibody subclass titer of the group of five mice at 6 weeks after the primary immunization. Each error bar represents the standard deviation of the mean.
DISCUSSION
The intracellular trafficking in BMs of RTS,S, an antigen containing P. falciparum sporozoite epitopes attached to hepatitis B surface protein particles, both free and encapsulated in liposomes, has been examined. As would be expected based on our previous results (31), TR-RTS,S fluorescence was observed first in the cytoplasm and subsequently in the trans-Golgi area of BMs that were incubated with liposome-encapsulated TR-RTS,S (Fig. 1D, E, G, and H). It is interesting that free RTS,S gave this same pattern of fluorescence (Fig. 1A and B), but this may not be surprising, since RTS,S contains lipid and has physical properties similar to those of the lipoprotein particles of HBsAg isolated from human serum (35, 40). The intracellular fluorescence patterns observed for RTS,S, whether free or encapsulated in liposomes, are consistent with the intermediate steps that are known to be part of the processing of intracellular antigens through the MHC class I pathway.
Because RTS,S appeared to be processed by the class I pathway, mice were immunized with free or liposomal RTS,S and tested for the induction of a CTL response against CSP epitopes. Previous studies showed that encapsulation of antigens containing the T-cell epitope of the P. falciparum CSP, amino acids 367 to 390 (22), in liposomes containing lipid A resulted in the induction of MHC class I-restricted CTL responses specific for the 367-to-390 epitope (4, 19, 44). As would be expected from these previous results, an MHC class I-restricted CD8+ CTL response against the 367-to-390 epitope was obtained in the mice immunized with RTS,S encapsulated in lipid A-containing liposomes (Fig. 2 and 3). No CTL response against the 367-to-390 epitope was detected, however, in mice immunized with RTS,S encapsulated in liposomes lacking lipid A (Table 1). In other studies, a strong class I-restricted CTL response was observed against HBsAg, whether administered alone or in liposomes lacking lipid A (13). Formulations of RTS,S with alum alone or with added MPL or in oil-in-water emulsions with or without added adjuvants have been tested in mice and humans (16, 38). Although a CTL response against the CSP epitopes of RTS,S has been reported in only one human vaccine recipient (16, 38), several of these RTS,S formulations have been shown to induce strong CTL responses against CSP epitopes in mice (11). In the present study, no CTL responses specific for the CSP epitopes of RTS,S were detected in the mice immunized with free RTS,S (Fig. 2B, D, and F) despite the evidence for class I processing of free RTS,S in APCs (Fig. 1A and B). These data suggest that processing by the class I pathway, although generally believed to be required for induction of a CTL response, does not necessarily result in a CTL response.
Since liposomal antigens have been reported to be processed by both the class I and class II pathways (31), the IgG antibody response against the NANP epitope of RTS,S was also measured in mice immunized with free or liposomal RTS,S. As has been observed for a variety of other antigens (2, 3, 5, 7, 43), the NANP-specific antibody response to RTS,S encapsulated in liposomes containing MPL was markedly enhanced compared to the response to free RTS,S (Fig. 4). The NANP-specific IgG response to liposomal RTS,S was dependent on the RTS,S dose in the dose range studied.
Different adjuvants have been shown to have specific effects on the pattern of IgG antibody subclasses obtained (1, 20). Murine antibody responses to soluble protein antigens are generally restricted to the IgG1 subclass despite the fact that IgG2a represents the major component of mouse serum IgG (28) and is the most effective activator of complement (20). Immunization with a recombinant secreted hepatitis B virus antigen has been found to result in a predominance of IgG1, whereas immunization with a core antigen elicited primarily IgG2a and IgG2b antibodies (25). A predominance of IgG1 was also obtained after immunization with DNA encoding the S region of HBsAg when encapsulated in cationic liposomes (17). IgG1 was the predominant subclass observed after immunization with RTS,S encapsulated in liposomes containing MPL, although there was a substantial amount of IgG2a obtained as well. Although the IgG titers were low after immunization with free RTS,S, the IgG subclass pattern appeared to be similar to that obtained after immunization with liposomal RTS,S. It is possible that, in the case of RTS,S, IgG1 remained predominant after encapsulation in liposomes containing lipid A, because even the free antigen exists as a lipid-containing particle. This is in contrast to our previous results, which showed that when cholera toxin or a synthetic malaria antigen (SPf66) was present in liposomes containing lipid A, the predominating IgG subclass shifted from IgG1 to IgG2a (15). These results suggest that the effect of encapsulating antigens in liposomes containing lipid A on the IgG subclass pattern is dependent on the antigen.
The data presented here suggest that for antigens such as RTS,S, which are lipid-containing particles, class I processing may not result in a CTL response unless an additional adjuvant, such as liposomes containing lipid A, is utilized. As has been found previously for many other antigens, the IgG antibody response to RTS,S also was markedly enhanced by encapsulation in liposomes containing lipid A, whereas the amount of IgG2a relative to that of IgG1 appeared unchanged after encapsulation in liposomes containing lipid A, in contrast to what was observed previously with other antigens (15).
ACKNOWLEDGMENTS
We are grateful to SmithKline Beecham Biologicals, Rixensart, Belgium, for providing the RTS,S antigen used in this study.
We acknowledge the excellent technical assistance of Elaine Morrison in performing all immunization and bleeding procedures.
REFERENCES
- 1.Allison A C, Byars N E. An adjuvant formulation that selectively elicits the formation of antibodies of protective isotypes and of cell-mediated immunity. J Immunol Methods. 1986;95:157–168. doi: 10.1016/0022-1759(86)90402-3. [DOI] [PubMed] [Google Scholar]
- 2.Alving C R. Liposomes as carriers of antigens and adjuvants. J Immunol Methods. 1991;140:1–13. doi: 10.1016/0022-1759(91)90120-5. [DOI] [PubMed] [Google Scholar]
- 3.Alving C R. Lipopolysaccharide, lipid A, and liposomes containing lipid A as immunologic adjuvants. Immunobiology. 1993;187:430–466. doi: 10.1016/S0171-2985(11)80355-4. [DOI] [PubMed] [Google Scholar]
- 4.Alving C R, Koulchin V, Glenn G M, Rao M. Liposomes as carriers of peptide antigens: induction of antibodies and cytotoxic T lymphocytes to conjugated and unconjugated peptides. Immunol Rev. 1995;145:5–31. doi: 10.1111/j.1600-065x.1995.tb00075.x. [DOI] [PubMed] [Google Scholar]
- 5.Alving C R, Shichijo S, Mattsby-Baltzer I, Richards R L, Wassef N M. Preparation and use of liposomes in immunological studies. In: Gregoriadis G, editor. Liposome technology. 2nd ed. III. Boca Raton, Fla: CRC Press, Inc.; 1993. pp. 317–339. [Google Scholar]
- 6.Alving C R, Wassef N M. Cytotoxic T lymphocytes induced by liposomal antigens: mechanisms of immunological presentation. AIDS Res Hum Retroviruses. 1994;10:S91–S94. [PubMed] [Google Scholar]
- 7.Alving C R, Wassef N M, Richards R L. Use of adjuvants for enhancement of antibody responses. In: Weir D, Blackwell C, Herzenberg L, Herzenberg L, editors. Handbook of experimental immunology. 5th ed. 2A. The lymphoid system, sect. 3. Antibody responses and affinity maturation. Oxford, England: Blackwell Scientific Publications; 1996. pp. 87.1–87.10. [Google Scholar]
- 8.Attaya M, Jameson S, Martinez C K, Hermel E, Aldrich C, Forman J, Lindahl K F, Bevan M, Monaco J J. Ham-2 corrects the class I antigen-processing defect in RMA-S cells. Nature. 1992;355:647–649. doi: 10.1038/355647a0. [DOI] [PubMed] [Google Scholar]
- 9.Ballou W R, Hoffman S L, Sherwood J A, Hollingdale M R, Neva F A, Hockmeyer W T, Gordon D M, Schneider I, Wirtz R A, Young J F, Wasserman G F, Reeve P, Diggs C L, Chulay J D. Safety and efficacy of a recombinant DNA Plasmodium falciparum sporozoite vaccine. Lancet. 1987;i:1277–1281. doi: 10.1016/s0140-6736(87)90540-x. [DOI] [PubMed] [Google Scholar]
- 10.Braciale T J, Morrison L A, Sweeter M T, Sambrook J, Gething M J, Braciale V L. Antigen presentation pathways to class I and class II MHC-restricted T lymphocytes. Immunol Rev. 1987;98:95–114. doi: 10.1111/j.1600-065x.1987.tb00521.x. [DOI] [PubMed] [Google Scholar]
- 11.Cohen, J. Personal communication.
- 12.Deres K, Schild H, Wiesmüller K-H, Jung G, Rammensee H-G. In vivo priming of virus-specific cytotoxic T lymphocytes with synthetic lipopeptide vaccine. Nature. 1989;342:561–564. doi: 10.1038/342561a0. [DOI] [PubMed] [Google Scholar]
- 13.Diminsky D, Reimann Z J, Schirmbeck R, Barenholz Y. Structural and functional characterization of liposomal recombinant hepatitis B vaccine. J Liposome Res. 1996;6:289–304. [Google Scholar]
- 14.Germain R N, Margoulies D H. The biochemistry and cell biology of antigen processing and presentation. Annu Rev Immunol. 1993;11:403–450. doi: 10.1146/annurev.iy.11.040193.002155. [DOI] [PubMed] [Google Scholar]
- 15.Glenn G M, Rao M, Richards R L, Matyas G R, Alving C R. Murine IgG subclass antibodies to antigens incorporated in liposomes containing lipid A. Immunol Lett. 1995;47:73–78. doi: 10.1016/0165-2478(95)00069-h. [DOI] [PubMed] [Google Scholar]
- 16.Gordon D M, McGovern T W, Krzych U, Cohen J C, Schneider I, LaChance R, Heppner D G, Yuan G, Hollingdale M, Slaoui M, Hauser P, Voet P, Sadoff J C, Ballou W R. Safety, immunogenicity, and efficacy of a recombinantly produced Plasmodium falciparum circumsporozoite protein-hepatitis B surface antigen subunit vaccine. J Infect Dis. 1995;171:1576–1585. doi: 10.1093/infdis/171.6.1576. [DOI] [PubMed] [Google Scholar]
- 17.Gregoriadis G, Saffie R, de Souza J B. Liposome-mediated DNA vaccination. FEBS Lett. 1997;402:107–110. doi: 10.1016/s0014-5793(96)01507-4. [DOI] [PubMed] [Google Scholar]
- 18.Harding C V, Song R. Phagocytic processing of exogenous particulate antigens by macrophages for presentation by class I MHC molecules. J Immunol. 1994;153:4925–4933. [PubMed] [Google Scholar]
- 19a.Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council. Guide for the care and use of laboratory animals. Washington, D.C: National Academy Press; 1996. [Google Scholar]
- 19.Heppner G D, Gordon D M, Gross M, Wellde B, Leitner W, Krzych U, Schneider I, Wirtz R A, Richards R L, Trofa A, Hall T, Sadoff J C, Boerger P, Alving C R, Sylvester D R, Porter T G, Ballou W R. Safety, immunogenicity and efficacy of Plasmodium falciparum repeatless circumsporozoite protein vaccine encapsulated in liposomes. J Infect Dis. 1996;174:361–366. doi: 10.1093/infdis/174.2.361. [DOI] [PubMed] [Google Scholar]
- 20.Kenney J S, Hughes B W, Masada M P, Allison A C. Influence of adjuvants on the quantity, affinity, isotype and epitope specificity of murine antibodies. J Immunol Methods. 1989;121:157–166. doi: 10.1016/0022-1759(89)90156-7. [DOI] [PubMed] [Google Scholar]
- 21.Kovacsovics-Bankowski M, Rock K L. A phagosome-to-cytosol pathway for exogenous antigens presented on MHC class I molecules. Science. 1995;267:243–246. doi: 10.1126/science.7809629. [DOI] [PubMed] [Google Scholar]
- 22.Kumar S, Miller L H, Quakyi I A, Keister D B, Houghten R A, Maloy W L, Moss B, Berzofsky J A, Good M F. Cytotoxic T cells specific for the circumsporozoite protein of Plasmodium falciparum. Nature. 1988;334:258–260. doi: 10.1038/334258a0. [DOI] [PubMed] [Google Scholar]
- 23.Lopes L M, Chain B M. Liposome-mediated delivery stimulates a class I-restricted cytotoxic T cell response to soluble antigen. Eur J Immunol. 1992;22:287–290. doi: 10.1002/eji.1830220143. [DOI] [PubMed] [Google Scholar]
- 24.Martinon F, Gras-Masse H, Boutillon C, Chirat F, Deprez B, Guillet J-G, Gomard E, Tartar A, Levy J-P. Immunization of mice with lipopeptides bypasses the prerequisite for adjuvant. Immune response of BALB/c mice to human immunodeficiency virus envelope glycoprotein. J Immunol. 1992;149:3416–3422. [PubMed] [Google Scholar]
- 25.Milich D R, Schödel F, Hughes J L, Jones J E, Peterson D L. The hepatitis B virus core and e antigens elicit different Th cell subsets: antigen structure can affect Th cell phenotype. J Virol. 1997;71:2192–2201. doi: 10.1128/jvi.71.3.2192-2201.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller M D, Gould-Fogerite S, Shen L, Woods R M, Koenig S, Mannino R J, Letvin N L. Vaccination of rhesus monkeys with synthetic peptide in a fusogenic proteoliposome elicits simian immunodeficiency virus-specific CD8+ cytotoxic T lymphocytes. J Exp Med. 1992;176:1739–1744. doi: 10.1084/jem.176.6.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moore M W, Carbone F R, Bevan M J. Introduction of soluble protein into the class I pathway of antigen processing and presentation. Cell. 1988;54:777. doi: 10.1016/s0092-8674(88)91043-4. [DOI] [PubMed] [Google Scholar]
- 28.Natsuume-Sakai S, Motonishi K, Migita S. Quantitative estimations of five classes of immunoglobulins in inbred mouse strains. Immunology. 1977;32:861–866. [PMC free article] [PubMed] [Google Scholar]
- 29.Pagano R E, Sepanski M A, Martin O C. Molecular trapping of a fluorescent ceramide analogue at the Golgi apparatus of fixed cells: interaction with endogenous lipids provides a trans-Golgi marker for both light and electron microscopy. J Cell Biol. 1989;109:2067–2079. doi: 10.1083/jcb.109.5.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rao, M., and C. R. Alving. Class I presentation of liposomal antigens. In D. D. Lasic and D. Papahadjopoulos (ed.), Medical applications of liposomes, in press. Elsevier Press, New York, N.Y.
- 31.Rao M, Rothwell S W, Wassef N M, Pagano R E, Alving C R. Visualization of peptides derived from liposome-encapsulated proteins in the trans-Golgi area of macrophages. Immunol Lett. 1997;59:55–105. doi: 10.1016/s0165-2478(97)00107-7. [DOI] [PubMed] [Google Scholar]
- 32.Rao M, Wassef N M, Alving C R, Krzych U. Intracellular processing of liposome-encapsulated antigens by macrophages depends upon the antigen. Infect Immun. 1995;63:2396–2402. doi: 10.1128/iai.63.7.2396-2402.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reddy R, Zhou F, Nair S, Huang L, Rouse B T. In vivo cytotoxic T lymphocyte induction with soluble proteins administered in liposomes. J Immunol. 1992;148:1585–1589. [PubMed] [Google Scholar]
- 34.Richards R L, Alving C R, Wassef N M. Liposomal subunit vaccines: effects of lipid A and aluminum hydroxide on immunogenicity. J Pharm Sci. 1996;85:1286–1289. doi: 10.1021/js9601593. [DOI] [PubMed] [Google Scholar]
- 35.Rutgers T, Gordon D, Gathoye A M, Hollingdale M, Hockmeyer W, Rosenberg M, De Wilde M. Hepatitis B surface antigen as carrier matrix for the repetitive epitope of the circumsporozoite protein of Plasmodium falciparum. Bio/Technology. 1988;6:1065–1070. [Google Scholar]
- 36.Schild H, Norda M, Deres K, Falk K, Rötzschke O, Wiesmüller K-H, Jung G, Rammensee H-G. Fine specificity of cytotoxic T lymphocytes primed in vivo either with virus or synthetic lipopeptide vaccine or primed in vitro with peptide. J Exp Med. 1991;174:1665–1668. doi: 10.1084/jem.174.6.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spies T, DeMars R. Restored expression of major histocompatibility class I molecules by gene transfer of a putative peptide transporter. Nature. 1991;351:323–324. doi: 10.1038/351323a0. [DOI] [PubMed] [Google Scholar]
- 38.Stoute J A, Slaoui M, Heppner D G, Momin P, Kester K E, Desmons P, Wellde B T, Garçon N, Krzych U, Marchand M, Ballou W R, Cohen J D. A preliminary evaluation of a recombinant circumsporozoite protein vaccine against Plasmodium falciparum malaria. N Engl J Med. 1997;336:86–91. doi: 10.1056/NEJM199701093360202. [DOI] [PubMed] [Google Scholar]
- 39.Titus J A, Haugland R, Sharrow S O, Segal D M. Texas Red, a hydrophilic, red-emitting fluorophore for use with fluorescein in dual parameter flow microfluorometric and fluorescence microscopic studies. J Immunol Methods. 1982;50:193–204. doi: 10.1016/0022-1759(82)90225-3. [DOI] [PubMed] [Google Scholar]
- 40.Valenzuela P, Medina A, Rutter W J, Ammerer G, Hall B D. Synthesis and assembly of hepatitis B virus surface antigen particles in yeast. Nature. 1982;298:347–350. doi: 10.1038/298347a0. [DOI] [PubMed] [Google Scholar]
- 41.Verma J N, Rao M, Amselem S, Krzych U, Alving C R, Green S J, Wassef N M. Adjuvant effects of liposomes containing lipid A: enhancement of liposomal antigen presentation and recruitment of macrophages. Infect Immun. 1992;60:2438–2444. doi: 10.1128/iai.60.6.2438-2444.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vogel F R, Powell M F. Section on Walter Reed liposomes. In: Powell M F, Neman M J, editors. Vaccine design: the subunit and adjuvant approach. New York, N.Y: Plenum Press; 1995. pp. 226–227. [Google Scholar]
- 43.Wassef N M, Alving C R, Richards R L. Liposomes as carriers for vaccines. ImmunoMethods. 1994;4:217–222. doi: 10.1006/immu.1994.1023. [DOI] [PubMed] [Google Scholar]
- 44.White K, Krzych U, Gordon D M, Porter T G, Richards R L, Alving C R, Deal C D, Hollingdale M, Silverman C, Sylvester D R, Ballou W R, Gross M. Induction of cytolytic and antibody responses using Plasmodium falciparum repeatless circumsporozoite protein encapsulated in liposomes. Vaccine. 1993;11:1341–1346. doi: 10.1016/0264-410x(93)90105-7. [DOI] [PubMed] [Google Scholar]
- 45.White W I, Cassatt D R, Madsen J, Burke S J, Woods R M, Wassef N M, Alving C R, Koenig S. Antibody and cytotoxic T-lymphocyte responses to a single liposome-associated peptide antigen. Vaccine. 1995;13:1111–1122. doi: 10.1016/0264-410x(94)00058-u. [DOI] [PubMed] [Google Scholar]