Abstract
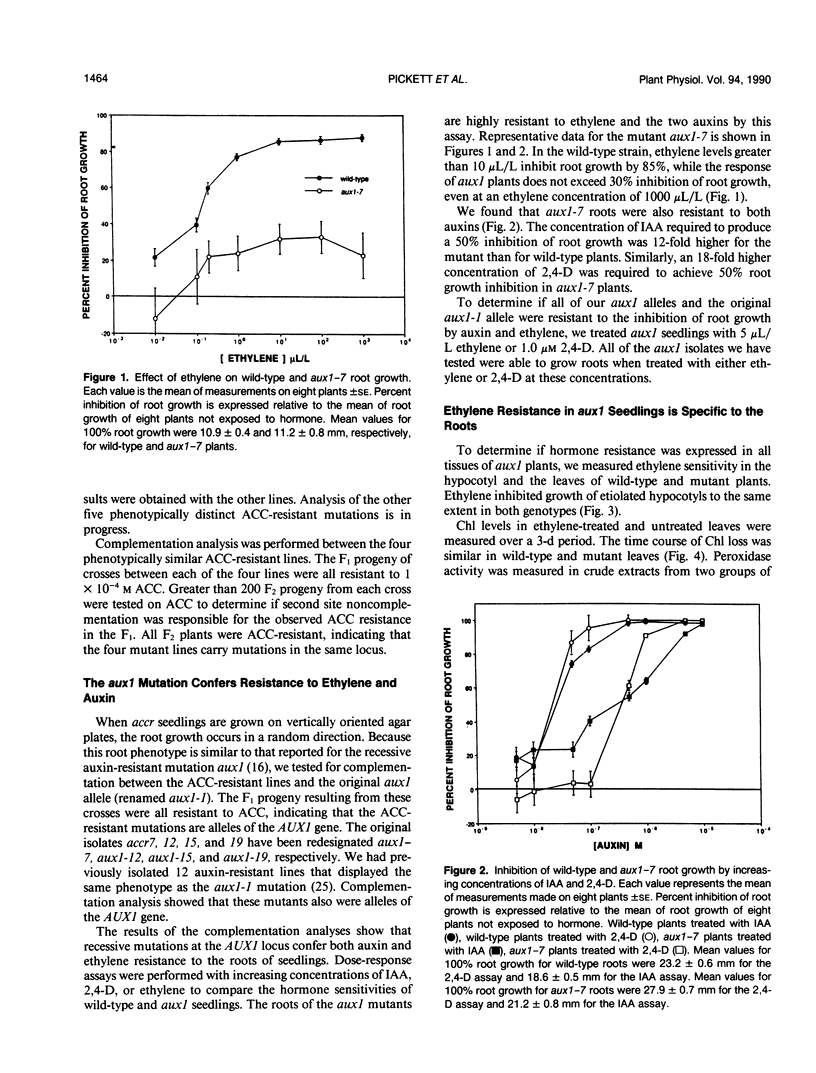
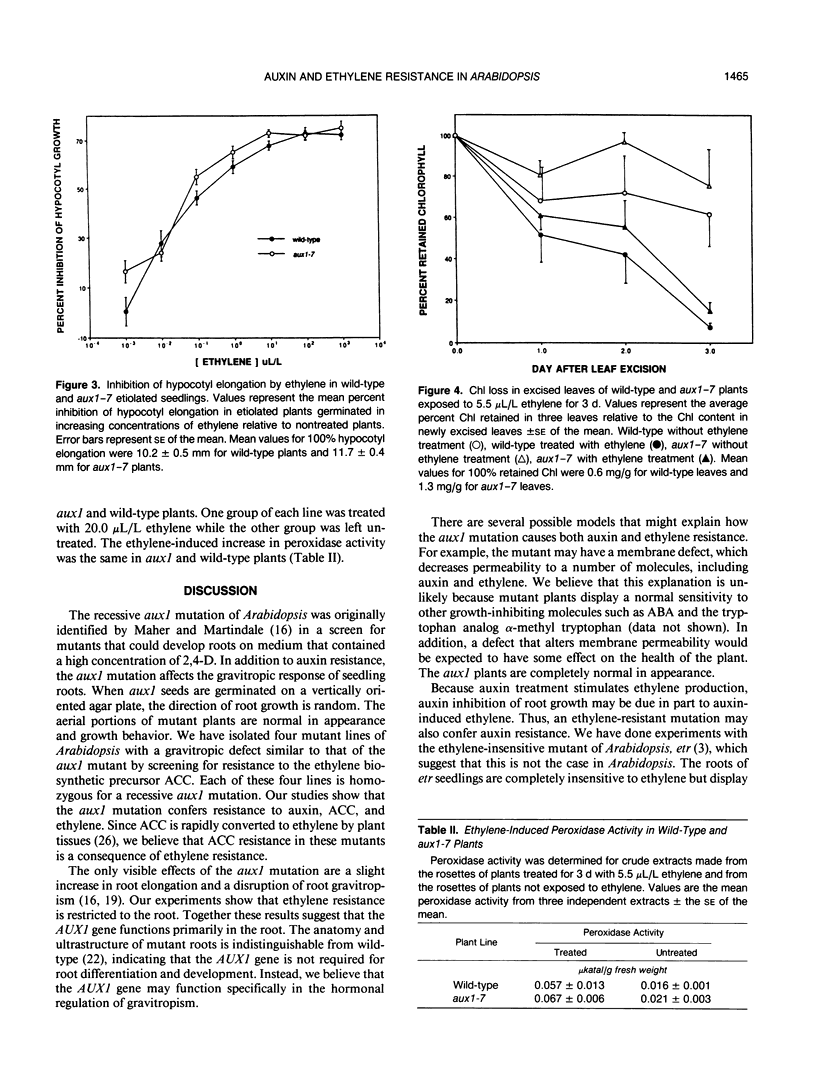
Mutagenized populations of Arabidopsis thaliana seedlings were screened for plants capable of root growth on inhibitory concentrations of the ethylene precursor 1-aminocyclopropane-1-carboxylic acid. Four of the mutant lines recovered from this screen display a defect in root gravitropism as well as hormone resistance. The aerial portions of these plants are similar to wild-type in appearance. Genetic analysis of these four mutants demonstrated that hormone resistance segregated as a recessive trait and that all four mutations were alleles of the auxin-resistant mutation aux1 [Maher HP, Martindale SJB (1980) Biochem Genet 18: 1041-1053]. These new mutants have been designated aux1-7, 1-12, 1-15, and 1-19. The sensitivity of wild-type and aux1-7 roots to indole-3-acetic acid, 2,4-dichlorophenoxyacetic acid, and ethylene was determined. The results of these assays show that aux1-7 plants require a 12-fold (indole-3-acetic acid) or 18-fold (2,4-dichlorophenoxyacetic acid) higher concentration of auxin than wild-type for a 50% inhibition of root growth. In addition, ethylene inhibition of root growth in aux1-7 plants is approximately 30% that of wild-type at saturating ethylene concentrations. These results indicate that aux1 plants are resistant to both auxin and ethylene. We have also determined the effect of ethylene treatment on chlorophyll loss and peroxidase activity in the leaves of aux1 and wild-type plants. No difference between mutant and wild-type plants was observed in these experiments, indicating that hormone resistance in aux1 plants may be limited to root growth. Our studies suggest that the AUX1 gene may have a specific function in the hormonal regulation of gravitropism.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beyer E. M. Abscission: support for a role of ethylene modification of auxin transport. Plant Physiol. 1973 Jul;52(1):1–5. doi: 10.1104/pp.52.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleecker A. B., Estelle M. A., Somerville C., Kende H. Insensitivity to Ethylene Conferred by a Dominant Mutation in Arabidopsis thaliana. Science. 1988 Aug 26;241(4869):1086–1089. doi: 10.1126/science.241.4869.1086. [DOI] [PubMed] [Google Scholar]
- Burg S. P., Burg E. A. The interaction between auxin and ethylene and its role in plant growth. Proc Natl Acad Sci U S A. 1966 Feb;55(2):262–269. doi: 10.1073/pnas.55.2.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C., Bowman J. L., DeJohn A. W., Lander E. S., Meyerowitz E. M. Restriction fragment length polymorphism linkage map for Arabidopsis thaliana. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6856–6860. doi: 10.1073/pnas.85.18.6856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliasson L., Bertell G., Bolander E. Inhibitory action of auxin on root elongation not mediated by ethylene. Plant Physiol. 1989 Sep;91(1):310–314. doi: 10.1104/pp.91.1.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kende H., Hanson A. D. Relationship between Ethylene Evolution and Senescence in Morning-Glory Flower Tissue. Plant Physiol. 1976 Apr;57(4):523–527. doi: 10.1104/pp.57.4.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King P. J. Plant hormone mutants. Trends Genet. 1988 Jun;4(6):157–162. doi: 10.1016/0168-9525(88)90021-2. [DOI] [PubMed] [Google Scholar]
- Maher E. P., Martindale S. J. Mutants of Arabidopsis thaliana with altered responses to auxins and gravity. Biochem Genet. 1980 Dec;18(11-12):1041–1053. doi: 10.1007/BF00484337. [DOI] [PubMed] [Google Scholar]
- Meyerowitz E. M. Arabidopsis thaliana. Annu Rev Genet. 1987;21:93–111. doi: 10.1146/annurev.ge.21.120187.000521. [DOI] [PubMed] [Google Scholar]
- Nam H. G., Giraudat J., Den Boer B., Moonan F., Loos WDB., Hauge B. M., Goodman H. M. Restriction Fragment Length Polymorphism Linkage Map of Arabidopsis thaliana. Plant Cell. 1989 Jul;1(7):699–705. doi: 10.1105/tpc.1.7.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen G. M., Mirza J. I., Maher E. P., Iversen T. H. Ultrastructure and movements of cell organelles in the root cap of agravitropic mutants and normal seedlings of Arabidopsis thaliana. Physiol Plant. 1984 Apr;60(4):523–531. doi: 10.1111/j.1399-3054.1984.tb04921.x. [DOI] [PubMed] [Google Scholar]
- Suttle J. C. Effect of Ethylene Treatment on Polar IAA Transport, Net IAA Uptake and Specific Binding of N-1-Naphthylphthalamic Acid in Tissues and Microsomes Isolated from Etiolated Pea Epicotyls. Plant Physiol. 1988 Nov;88(3):795–799. doi: 10.1104/pp.88.3.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson A. K., Pickett F. B., Turner J. C., Estelle M. A dominant mutation in Arabidopsis confers resistance to auxin, ethylene and abscisic acid. Mol Gen Genet. 1990 Jul;222(2-3):377–383. doi: 10.1007/BF00633843. [DOI] [PubMed] [Google Scholar]


