Abstract
Most cases of tuberculosis are due to reactivation of endogenous infection which may have lain quiescent or dormant for decades. How Mycobacterium tuberculosis survives for this length of time is unknown, but it is hypothesized that reduced oxygen tension may trigger the tubercle bacillus to enter a state of dormancy. Mycobacterium bovis BCG and M. tuberculosis H37Rv were cultured under aerobic, microaerobic, and anaerobic conditions. Their ultrastructural morphology was analyzed by transmission electron microscopy (TEM), and protein expression profiles were compared by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). TEM revealed that the microaerobically and anaerobically cultured bacilli but not the aerobically cultured bacilli developed a strikingly thickened cell wall outer layer. The thickening was not observed in aerobically cultured stationary-phase bacilli or in anaerobically cultured Mycobacterium smegmatis. A highly expressed protein was detected by SDS-PAGE in microaerobic and anaerobic cultures and was identified as the 16-kDa small heat shock protein or α-crystallin homolog. Immunolocalization by colloidal gold immunoelectron microscopy identified three patterns of protein distribution in M. bovis BCG cultured under low oxygen tension. The 16-kDa protein was strongly associated with the cell envelope, fibrous peptidoglycan-like structures, and intracellular and peripheral clusters. These results suggest that tubercle bacilli may adapt to low-oxygen conditions by developing a thickened cell wall and that the 16-kDa protein may play a role in stabilizing cell structures during long-term survival, thus helping the bacilli survive the low oxygen tension in granulomas. As such, the cell wall thickening and the 16-kDa protein may be markers for the dormant state of M. tuberculosis.
Mycobacterium tuberculosis, the causative agent of tuberculosis, accounts for more deaths in the world than any other pathogen (2, 25). One of the major contributing factors to the success of M. tuberculosis as a human pathogen is its ability to persist in the face of the immune response, only to reactivate later and cause disease. Most cases of tuberculosis are the result of reactivation of preexisting infection rather than reinfection (25), and one-third of the world’s population has been estimated to be latently infected (39). The 6-month treatment time (so called short-term therapy) needed to achieve a cure can lead to patient noncompliance resulting in a poor outcome, further spread of the disease, and the development of drug-resistant strains. A drug which can selectively kill dormant bacilli is urgently needed. The combination of such a drug and existing drugs that target actively replicating bacilli would rapidly kill both populations, drastically reduce the treatment time, and eliminate persisters and their potential for reactivation.
How the tubercle bacillus survives during the dormant stage of infection is largely unknown. Following initial infection, the bacilli typically replicate inside host macrophages until an effective immune response is mounted and the bacilli become restricted to granulomas and the progression of the disease is halted. The internal environment of the granuloma is considered to be a hostile one, characterized by low O2 levels and high CO2 levels, acidic pH, and the presence of aliphatic organic acids, such that it was assumed that the bacilli died within a short period of time (11–13). However, it was later demonstrated that bacilli were not necessarily killed and could survive for many years (44). Using an in vitro model whereby M. tuberculosis bacilli were grown in a nonagitated culture, Wayne found that in the microaerophilic sedimented layer of the medium, nondividing, yet viable, bacilli were present (41). These nondividing-but-viable bacilli led Wayne to suggest that they may be analogous to tubercle bacilli that persist in vivo during quiescent tuberculosis. Microaerophilically cultured bacilli were found to undergo an orderly metabolic shiftdown, evidenced by an apparent shift into the glyoxylate cycle, permitting adaptation to the usually lethal effects of anaerobiosis (43). Tolerance of anaerobic conditions was further supported by the finding that the dormant bacilli were partially or completely resistant to isoniazid and rifampin yet, unlike aerobically cultured bacilli, were susceptible to metronidazole, a drug typically directed against anaerobic bacteria (45).
The experimental work by Wayne and coworkers clearly implies that low oxygen tension may play a role in mycobacterial dormancy and that adaptation to anaerobiosis may be a feature of persisting tubercle bacilli. In this study we sought to determine the adaptations mycobacteria may make at both the morphological and molecular level in response to low-oxygen culture conditions. We report that there is a very marked thickening of the cell wall outer electron-opaque layer in Mycobacterium bovis BCG and M. tuberculosis when the bacteria are cultured under either microaerobic or anaerobic conditions. We also identified a protein in M. bovis BCG bacilli which was upregulated under microaerobic and anaerobic conditions, and we describe the localization of this 16-kDa protein and its patterns of distribution, as determined by immunogold electron microscopy. The significance of these findings to the dormant phase of M. tuberculosis and to the latency of human tuberculosis are discussed.
MATERIALS AND METHODS
Organisms and culture conditions.
M. bovis BCG (Statens Seruminstitut strain ST1077), M. tuberculosis H37Rv (ATCC 9360), and Mycobacterium smegmatis mc2155 were grown in Middlebrook 7H9 medium supplemented with albumin-dextrose-catalase (ADC; Difco Laboratories Ltd., West Molesey, United Kingdom) and 0.05% (vol/vol) Tween 80 (Sigma, Poole, United Kingdom). In some experiments Tween 80 was replaced, where stated, with 0.2% (vol/vol) glycerol. Either sterile polystyrene universals or 100-ml glass bottles containing 10 or 50 ml of 7H9–ADC–Tween 80 broth, respectively, were inoculated with bacilli and cultured in a shaking 37°C incubator until slight turbidity was achieved (usually at an optical density at 600 nm [OD600] of ∼0.4). The culture vessels were then placed in a standard nonshaking 37°C incubator (the M. tuberculosis cultures were placed in a nonshaking CO2 incubator with 5% CO2) under either microaerobic or anaerobic conditions. To obtain a microaerobic environment, a self-induced oxygen gradient was permitted to form as the bacilli settled to the bottom of the culture vessel. The screw caps were loosened to allow gaseous exchange between the environment and the media. For anaerobic conditions, the culture was overlaid with filter-sterilized mineral oil (molecular biology grade; Sigma) and the tube caps were tightened. In some experiments M. bovis BCG was cultured in an anaerobic cabinet in the absence of mineral oil. The microaerobic and anaerobic cultures were left undisturbed for periods of up to 6 months before harvesting. Controls comprised bacilli cultured aerobically with agitation to an OD600 of 0.07 to 0.4 (low-OD control) and bacilli cultured aerobically with shaking for 6 weeks to an OD600 of >1.0 (nutrient-limited stationary-phase control [NLSPC]). Aerobic cultures were coprocessed with the anaerobic samples. Cultures were routinely checked for contaminants and were examined by Ziehl-Neelsen staining.
TEM.
Cells were harvested by low-speed centrifugation, washed in sterile phosphate-buffered saline (PBS), and fixed for a minimum of 1 h in 2.5% (vol/vol) glutaraldehyde in 0.1 M sodium phosphate. Cells were then pelleted, the supernatant was removed, and the pellet was resuspended in 1% (wt/vol) osmium tetroxide for 1 h. Cells were then dehydrated through a graded ethanol series (50, 70, 90, and 100%; each level was applied twice for 5 min each time) and propylene oxide (twice for 15 min) and infiltrated with, unless otherwise stated, agar 100 (Agar, Stansted, United Kingdom). Resin blocks were polymerized at 60°C for 24 h. Sections were cut on a Reichert-Jung Ultracut E ultratome to silver/gold interference, picked up on Formvar-coated grids, and stained with 30% (wt/vol) uranyl acetate in 70% (vol/vol) methanol and counterstained with Reynold’s lead citrate (34) before examination on a Jeol JEM-100CXII TEM at an acceleration voltage of 80 kV. Under some circumstances, where stated, one or more of the above fixation or staining steps were omitted. Cells were also prepared for TEM by the method of progressive lowering of temperature (PLT) (see below), following which grids were stained with osmium tetroxide vapor by placing them in a petri dish for 3 h with an osmium tetroxide crystal. Qualitative X-ray microprobe analysis was used to help determine which heavy metal was responsible for the contrast observed in the thickened cell wall. Briefly, routinely prepared sections were analyzed in the electron microscope fitted with a high-resolution scanning attachment, a LaB6 filament, and a Link ISIS 30-mm2 Si(Li) detector, atmosphere thin window, and multichannel analyzer (Oxford Instruments, High Wycombe, United Kingdom).
Protein extraction and SDS-PAGE analysis.
Bacilli were harvested, washed, and resuspended in 1 ml of PBS containing 0.4 ml of sterile glass beads (0.1 mm in diameter) before disruption in a Mini Beadbeater (Stratech Scientific, Luton, United Kingdom) by three 60-s bursts at 50,000 rpm. Protein concentration was determined by the bicinchoninic acid assay (BCA kit; Pierce and Warriner, Chester, United Kingdom). Proteins were separated on a 12% denaturing polyacrylamide gel by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (26) using a Mini-Protean II minigel apparatus (Bio-Rad, Hemel Hempstead, United Kingdom) and were visualized by 0.5% Coomassie brilliant blue staining. A protein band of interest was excised, and the N-terminal sequence was determined at the Glaxo Wellcome Medicines Research Centre, Stevenage, United Kingdom.
Cloning and sequencing of the M. bovis BCG 16-kDa α-crystallin homolog.
Oligonucleotide primers based on the N-terminal sequence of the M. bovis BCG 16-kDa protein and the nucleotide sequence of the gene encoding the M. tuberculosis 16-kDa α-crystallin homolog (GenBank accession no., S79751) were designed. Two primers, 5′-ATGGCCACCACCCTTCCCGT-3′ and 5′-CAGTTGGTGGACCGGATCTG-3′, were used to amplify the M. bovis BCG 16-kDa α-crystallin gene with TaqI polymerase (Boehringer Mannheim, Lewes, United Kingdom) by PCR. The PCR product was purified (Geneclean Spin kit; Anachem, Luton, United Kingdom), polished with PfuI polymerase, blunt-end cloned into pCR-ScriptSK(+) (Stratagene, Cambridge, United Kingdom), and transformed into Escherichia coli SURE cells (Stratagene). Transformants were selected for ampicillin resistance with blue/white color selection. Plasmid DNA containing the appropriate insert was sequenced on an automated sequencer (ABI 373) by Alta Bioscience, University of Birmingham, United Kingdom, by the Taq Dye Deoxy terminator cycle method (Applied Biosystems Ltd., Warrington, United Kingdom).
Immunoelectron microscopy.
Bacilli were prepared for immunogold analysis by the PLT method (35). Samples were fixed for 60 min in 2% paraformaldehyde–0.05% glutaraldehyde in 0.1 M phosphate buffer. Samples were dehydrated through a graded ethanol series (from 30 to 100% ethanol) from 0 to −50°C. The samples were then infiltrated with Lowicryl HM20 for 24 h at −50°C prior to embedding and polymerization with ultraviolet light in fresh Lowicryl HM20, first for 48 h at −50°C and then for 24 h at 15°C. Silver/gold sections were cut as described above and labelled by blocking with 1.0% bovine serum albumin in PBS for 45 min at room temperature before exposure to a pooled polyclonal murine anti-M. tuberculosis recombinant 16-kDa α-crystallin antibody (a kind gift from J. Ivanyi [MRC Clinical Sciences Centre, Tuberculosis and Related Infections Unit, Imperial College School of Medicine, Hammersmith Campus, London, United Kingdom]) diluted in blocking solution for 3 h at 37°C. The grids were washed three times for 5 min in blocking solution before the addition of the immunogold conjugate, a 1:50 dilution of 10-nm colloidal gold-labelled goat anti-mouse antibody (British Biocell International, Cardiff, United Kingdom). The grids were washed a further three times for 5 min in blocking solution and three times for 1 min in distilled water before being stained with 30% uranyl acetate in 70% methanol. Controls of preimmune murine serum and conjugate, as well as conjugate only, were incorporated to ensure that low background labelling and high specificity of labelling were achieved. Sections were visualized with a Jeol JEM-100CXII TEM at an acceleration voltage of 80 kV.
RESULTS
Changes in cell wall electron density in M. bovis BCG and M. tuberculosis are associated with decreased oxygen tension.
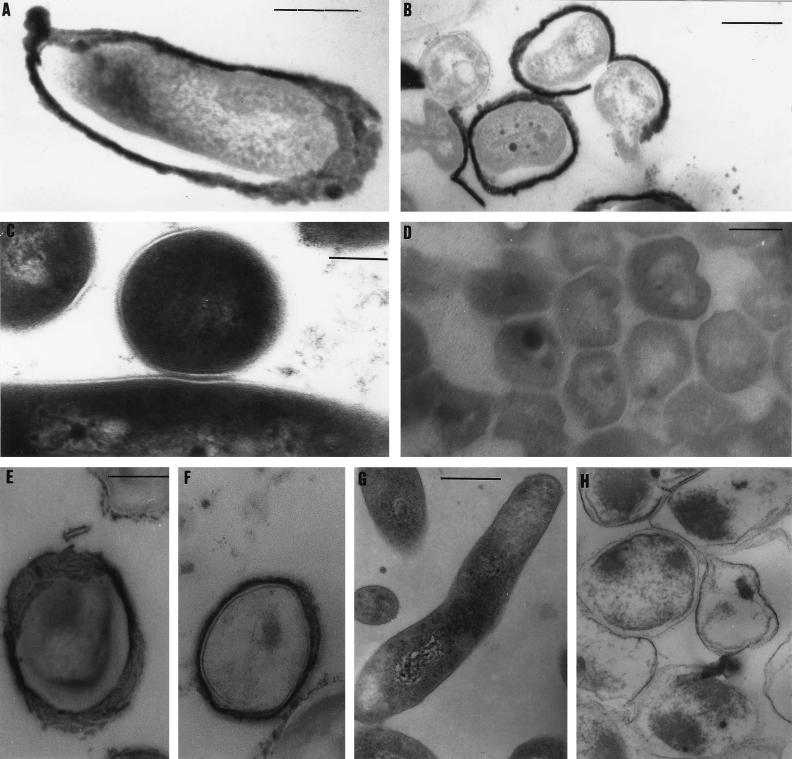
Analysis by TEM of M. bovis BCG cultured under anaerobic conditions for 6 months revealed a marked thickening of the cell wall (Fig. 1A and B). The main feature of these cells was the extremely dense electron staining of the cell walls, which in most cases homogeneously covered the surfaces of the bacilli. In some cases the thickened cell wall appeared to have ruptured, to have “peeled” off some cells, and other cells appeared to be denuded of part of their thickened cell walls, probably due to the processing of the cells for TEM. Despite the long period of culture under these conditions, the internal architecture of the cells appeared to be preserved. The same cell wall thickening was also seen in microaerobic cultures, although less frequently. However, this phenomenon was not observed in aerobically grown (low-OD control) bacilli (Fig. 1C) or in bacilli cultured aerobically with continuous agitation for 6 weeks, which would have entered stationary phase (NLSPC) (Fig. 1D). The bacilli cultured under microaerobic and anaerobic conditions for 6 months could be resuscitated by inoculation into fresh media (7H9–ADC–glycerol broth) under aerobic growth conditions. Only a loopful of the 6-month cultures was inoculated, and the results were merely recorded as positive growth. We did not ascertain how many cells in the inoculum were viable.
FIG. 1.
Effect of anaerobic culture on the morphology of M. bovis BCG, M. tuberculosis, and M. smegmatis. (A) Longitudinal section of an M. bovis BCG bacillus cultured anaerobically for 6 months showing pronounced cell wall thickening (some cytoplasmic dehydration is evident). (B) Transverse sections of M. bovis BCG bacilli cultured anaerobically for 6 months. (C) Lack of cell wall thickening in low-OD aerobically cultured M. bovis BCG bacilli. (D) NLSPC M. bovis BCG bacilli showing the absence of cell wall thickening. (E and F) Cell wall thickening in M. tuberculosis following anaerobic culture for 1 month under mineral oil. (G) Aerobically cultured M. smegmatis. (H) M. smegmatis bacilli exhibiting cell lysis following 35 days of anaerobic culture. Bars, 200 nm except for panels C (100 nm) and G (400 nm). The bar in panel A is also valid for panel F; the bar in panel D is also valid for panel H.
To determine whether this cell wall thickening could be observed in a pathogenic mycobacterial species, M. tuberculosis bacilli were cultured under anaerobic conditions in the same way for 1 month. TEM analysis showed that M. tuberculosis also developed a thickened cell wall associated with a decreased availability of oxygen (Fig. 1E and F). This was not observed in aerobically grown M. tuberculosis. Morphologically, the thickened cell wall appeared to be similar in M. tuberculosis and M. bovis BCG. In contrast, the fast-growing saprophytic species, M. smegmatis, did not develop the thickened cell wall after 17 and 35 days of anaerobic culture. In the 35-day-old anaerobic culture less than 1% of the bacilli remained intact. Of those which were intact, none exhibited the cell wall thickening (Fig. 1H).
Confirmation of observations: elimination of influence of mineral oil and Tween 80.
It was not possible that the presence of the mineral oil used to induce anaerobic conditions was responsible for the thickening as it was also seen in microaerobic cultures (cultured in the absence of mineral oil). Bacilli cultured in an anaerobic cabinet without mineral oil developed the thickened cell wall from 2 weeks onwards. Moreover, when M. bovis BCG cells were grown as described for the aerobic cultures, overlaid with mineral oil prior to being washed and processed for TEM analysis, and treated in the same manner as for the anaerobic cultures, thickened cell walls were not observed (only M. bovis BCG was used in these experiments as facilities for standard anaerobic culture of M. tuberculosis were not available). To eliminate the possibility that this was a Tween 80-dependent phenomenon, M. bovis BCG cells were cultured under mineral oil for 6 weeks in 7H9–ADC broth with 0.2% (vol/vol) glycerol replacing Tween 80. The same thickened cell walls were observed, showing that Tween 80 was not necessary for the thickened cell walls to be formed.
Osmium tetroxide postfixation and staining is primarily responsible for the detection of the cell wall thickening.
Osmium tetroxide was shown, by a number of experiments, to be necessary for the postfixation of the cell wall in samples prepared for routine TEM and for enhancing the contrast of the cell wall in samples prepared for TEM by the PLT method. In one experiment, anaerobic cultures were prepared for routine TEM; however, half of the culture was not postfixed in osmium tetroxide but was treated identically in every other respect to the remainder of the culture. When sections from the nonpostfixed specimen were examined, it was found that the thick cell wall was not visualized. This contrasted starkly with the osmium tetroxide-postfixed cell preparation in which the thick cell wall was evident even in the absence of further staining with lead or uranium salts. To provide further evidence that osmium tetroxide was responsible for the staining, X-ray microprobe analysis was undertaken to compare the cell wall to the background. This analysis confirmed the presence of osmium in the cell wall. No common coprecipitants such as phosphate (as illustrated by the presence of phosphorus) were identified. To further confirm that osmium tetroxide was responsible for the staining, some cells were prepared for TEM by the PLT method. This method of preparation permits structural integrity to survive the preparation process but yields poor ultrastructural detail. Sections prepared in this manner were stained with osmium vapor after processing. The detection of the unusual cell wall morphology in cells prepared and stained in this manner suggests that the osmium was not altering the cell wall during processing for standard TEM analysis.
Cell wall thickness and cell size.
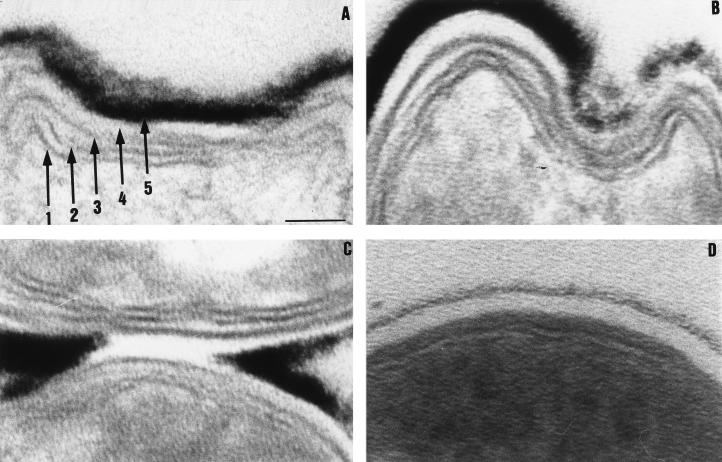
Image enhancement of electron micrographs taken of the thickened cell wall (Fig. 2) showed that the thickening was restricted to the cell wall outer electron-opaque layer. The other components of the cell wall appeared not to be enhanced in any observable way. The thickness of the cell wall was measured for anaerobically cultured bacilli. Measurements were only taken on transverse sections of the cell wall at the thinnest point. Anaerobic culture of M. bovis BCG in the presence or absence of mineral oil did not alter the degree of thickening of the cell wall. The cell wall was thicker in anaerobically cultured M. tuberculosis than in anaerobically cultured M. bovis BCG (21.2 ± 2.04 nm [n = 9] versus 16.1 ± 1.03 nm [n = 49], respectively). The internal diameters of the bacilli (i.e., excluding the cell wall) were also measured by measuring the diameters of the cells at the narrowest axes of transverse sections. The cell diameter was used as opposed to the cell length as it was easier to measure and more likely to reflect a valid and reproducible measurement. The anaerobically cultured M. bovis BCG cells were much thinner (255 ± 4.35 nm [n = 52]) than the aerobically grown controls (284 ± 6.20 nm [n = 29]), and these differences were highly significant (P < 0.001). The same pattern was also observed for M. tuberculosis, where the anaerobically cultured bacilli were thinner (278 ± 22.5 nm [n = 9]) than the aerobically grown bacilli (338 ± 18.9 nm [n = 10]; P < 0.05).
FIG. 2.
Cell wall architecture of anaerobically cultured M. bovis BCG (A to C) compared to that of aerobically cultured M. bovis BCG (D) showing that only the outer electron-opaque layer is thickened in response to low oxygen tension. The numbers in panel A refer to the individual cell wall envelope components, as interpreted by Brennan and Nikaido (3). From the cytoplasm outwards, the numbers indicate the following: 1 and 2, the plasma membrane; 3, the electron-dense layer (peptidoglycan); 4, the electron-transparent layer; 5, the outer electron-opaque layer. The thickened outer electron-opaque layer and cell envelope appear to exhibit plasticity (B). Note the absence of the outer electron-opaque layer where two bacilli are in direct physical contact (C). (D) Low-OD aerobically cultured bacillus. Bar, 30 nm (for all panels).
Identification of the M. bovis BCG 16-kDa α-crystallin homolog.
SDS-PAGE analysis of protein extracted from M. bovis BCG cultured microaerobically and anaerobically for 1, 2, 4, 12, and 26 weeks (6 months) showed the presence of a protein of approximately 18 kDa (data not shown). This protein appeared to reach maximal expression, as a proportion of total protein, after 2 weeks of either microaerobic or anaerobic culture, and this level of expression was maintained for up to 26 weeks of culture. The protein appeared to be poorly expressed in the low-OD aerobic controls, but greater expression was noted in the NLSPC and probably reflects its induction by oxygen deficiency due to cell clumping. The N-terminal sequence of this protein—ATTLPVQRHPRSLFP—had 100% homology with the previously described M. tuberculosis 16-kDa small heat shock protein (smHSP) or α-crystallin homolog (27, 40).
A partial nucleotide sequence of the gene encoding the M. bovis BCG 16-kDa α-crystallin protein is 100% identical to that of the equivalent region in the M. tuberculosis homolog.
PCR amplification of M. bovis BCG genomic DNA with primers based on the M. tuberculosis gene sequence encoding the 16-kDa protein generated a product 434 bp in size. Excluding the primer sequences, 394 bases were sequenced and were found to be 100% identical to those of the equivalent region in M. tuberculosis.
Immunolocalization of the M. bovis BCG 16-kDa α-crystallin protein.
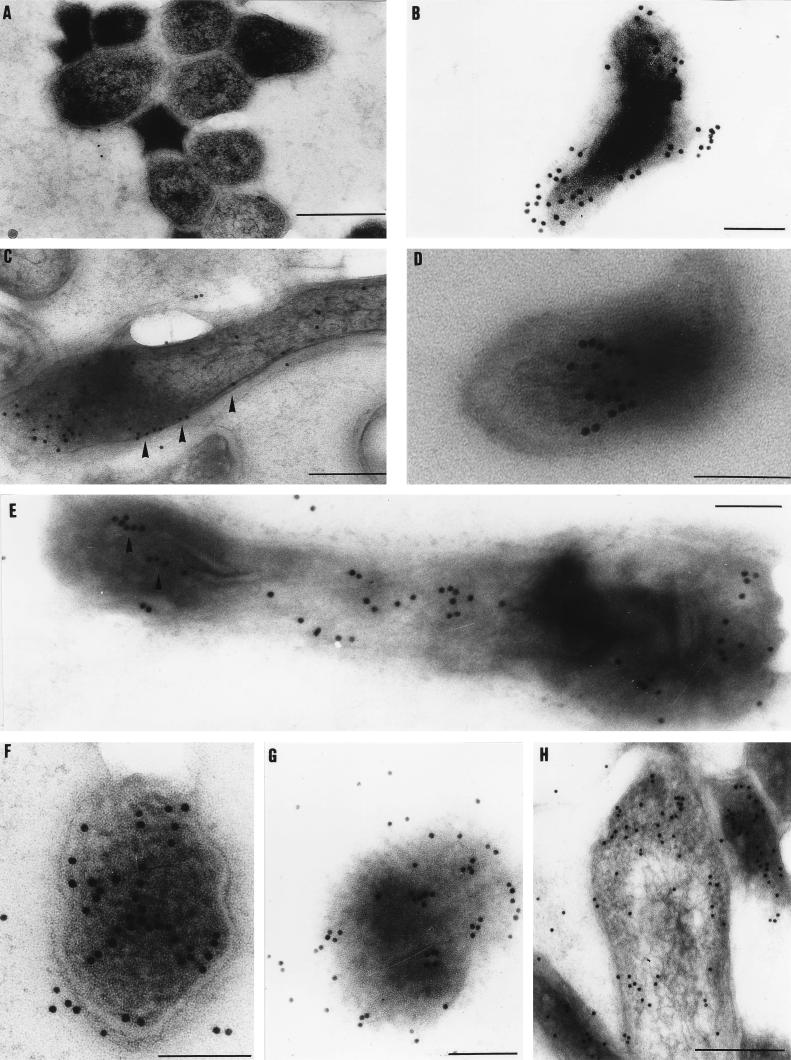
Immunoelectron microscopy with a specific polyclonal antiserum to the 16 kDa protein was used to determine the distribution of the protein. An examination of aerobically cultured low-OD bacilli revealed poor expression of the 16-kDa protein (Fig. 3A). However, a high degree of specific labelling was observed for the microaerobic and anaerobic cultures from 2 to 26 weeks, and three patterns of 16-kDa protein distribution were observed.
FIG. 3.
Immunogold electron microscopy localization of the 16-kDa α-crystallin protein of M. bovis BCG. (A) Low level of prevalence of the 16-kDa protein in the low-OD aerobic control. (B) Bacillus from a 12-week anaerobic culture showing localization to the periphery of the cell. (C) Twenty-six-week anaerobically cultured bacillus showing localization of the 16-kDa protein to the cell wall (marked by the arrowheads). (D) Twenty-six-week anaerobically cultured bacillus showing the 16-kDa protein associated with a splayed arrangement of fibrous structures. (E) Bacillus cultured for 26 weeks microaerobically showing localization of the protein to fibrils thought to be peptidoglycan (indicated by arrowheads). (F) Four-week anaerobically cultured bacillus; lines of colloidal gold labelling are seen despite the absence of detailed fibril ultrastructure. (G) Four-week anaerobically cultured bacillus showing clusters both associated with the intracellular environment and at the cell periphery. (H) Bacillus cultured for 2 weeks anaerobically. Note the lack of localization to the chromosome in the center of the cell. Bars, 100 nm except for panel A (300 nm) and panels C and H (200 nm).
Sixteen-kilodalton protein pattern of distribution I: localization to the cell periphery.
The protein was frequently found to be localized to the peripheral regions of M. bovis BCG bacilli (Fig. 3B), where the label could clearly be seen to follow the contours of the cell. The 16-kDa protein was also located to the cell membrane and cell wall skeleton and less frequently the outer regions of the cell wall (Fig. 3C), suggesting that the 16-kDa protein may be associated with maintaining the structural integrity of the cell.
Sixteen-kilodalton protein pattern of distribution II: localization to fibrous structures.
The second type of label distribution showed that there were internal line structures along which the 16-kDa protein was associated (Fig. 3D and E). These lines could be frequently visualized as structural details of varying electron density on the electron micrographs and probably represent peptidoglycan located in the cell wall (1, 22). These fibrous structures were most frequently seen in longitudinal sections, probably due to the plane of sectioning. However, this pattern of label distribution was also seen in the absence of detailed fibril ultrastructure (Fig. 3F).
Sixteen-kilodalton protein pattern of distribution III: localization to intracellular and peripheral clusters.
The 16-kDa protein was observed to form clusters (Fig. 3G and H) which did not exceed 40 nm in width. These clusters were located both inside the cell and at its periphery, but which cellular components the clusters were associated with could not be determined. However, little or no label appeared to be associated with chromosomal DNA (Fig. 3H).
DISCUSSION
This is the first time changes in mycobacterial ultrastructural morphology have been correlated to reduced oxygen tension. We have shown that there is an expansion of the outer electron-opaque layer of the cell wall in anaerobically and microaerobically cultured bacilli concomitant with a reduction in the diameter of the cell as determined by measuring transverse sections. The outer electron-opaque layer has only been clearly identified as a distinct entity relatively recently but was found to be much thinner than that described here (32).
The cell wall thickening could only be observed in cells prepared for routine TEM which had been postfixed with osmium tetroxide, suggesting that the cell wall thickening was extractable in organic solvents at room temperature (used in the subsequent steps in routine TEM sample preparation). Moreover, when cells prepared for electron microscopy by the PLT method were stained by osmium tetroxide vapor, the cell wall thickening was much more readily observed than it was in unstained cells prepared by this method. Rastogi and colleagues, who first described the outer layer by ruthenium red staining (32), suggested that it consisted of polysaccharides because ruthenium red binds to acidic polysaccharides (16). However, ruthenium red can also bind to lipids (3). Ruthenium red staining in the absence of osmium tetroxide and the silver proteinate staining of our samples did not reveal any differences between aerobically and anaerobically cultured bacilli (data not shown).
The thickened walls of M. bovis BCG and M. tuberculosis cells reported here may help the bacilli to survive in oxygen-deficient conditions in vivo. Although M. tuberculosis is known to survive within the body for long periods, much less is known about M. bovis BCG. However, the report of an AIDS patient developing M. bovis BCG adenitis 30 years after being vaccinated with BCG as a child (33) indicates that M. bovis BCG can persist for many years. Just how tubercle bacilli survive in vivo and in what form have been subject to conjecture for many years. The finding that M. tuberculosis has a sigma factor similar to the SigF sporulation sigma factor from Streptomyces coelicolor has led to the suggestion that M. tuberculosis may enter a spore-like state during persistent infection (9). However, it has been previously proposed that the thick nature of the mycobacterial wall may negate the necessity for these bacteria to sporulate (29), and the thickened cell wall reported here may support that hypothesis. We did not find any evidence for a spore-like or coccoid morphology. Scanning electron microscopy of our bacilli showed that they had a typical rod-shaped morphology, and Ziehl-Neelsen staining showed that they were acid fast (data not shown). However, we frequently noticed by TEM that the anaerobically cultured bacilli appeared shorter in length, although it was not possible to assess this quantitatively. The cell wall thickening may be the manifestation of the dormant state of M. tuberculosis, and investigations are under way to determine the clinical significance of these findings by TEM analysis of granulomas.
The thickened wall described in this report may act as a protective coat or mantle and may offer protection against hostile environments such as the toxic conditions associated with granulomas. This is significant when one considers that the oxidative stress response in M. tuberculosis is dysfunctional with an inactive oxyR gene (10, 38) and that the quantities or activities of catalase and superoxide dismutase of anaerobically cultured bacilli are decreased (43). It was particularly interesting to see that at the point where two bacilli were in direct physical contact (as are the two pairs of bacilli in Fig. 1B) the thickened outer layer was absent (see also Fig. 2C) but that cells in close contact (Fig. 1B) clearly had generated their own thickened outer layers. The direct contact between the electron-transparent layers, which is considered the most hydrophobic domain (3), would create a hydrophobic focus, thereby possibly negating the need to develop the outer layer.
The fact that the aerobically grown stationary-phase cultures of M. bovis BCG did not develop the thick cell wall underlines the importance of oxygen deficiency as the trigger for its synthesis. However, it is possible that lower oxygen tension is only a proxy for the stimulus that causes the thickened cell wall to develop. As most tuberculosis is due to reactivation of infection this cell wall thickening could be an important virulence and survival determinant. The facts that M. smegmatis survives poorly under low oxygen tension and does not develop a thick cell wall suggest that such a morphological adaptation is important in anaerobic survival. It is intriguing that M. smegmatis does not possess the 16-kDa α-crystallin homolog (46), and it will be interesting to determine whether this has some bearing on the inability of M. smegmatis to survive prolonged anaerobic culture and generate the thickened outer layer. The ability to survive anaerobiosis may only be a feature of the slow-growing pathogenic mycobacteria and may reflect their successful evolution in adapting to the low oxygen potential of host tissues. Previous studies have suggested that the ability to tolerate anaerobic conditions may be pivotal to the pathogenicity of M. tuberculosis. M. tuberculosis H37Rv has a lower respiratory rate than avirulent strain M. tuberculosis H37Ra, and the rate of respiration is less inhibited by lower oxygen tension in H37Rv than in H37Ra (20). It could also be argued that a lower respiratory rate may provide the bacilli with sufficient time to adapt to lower oxygen tension in preparation for entering a state of dormancy, and the ordered metabolic shutdown observed by Wayne and Lin (43) probably reflects this. Moreover, during granuloma formation the rate of oxygen depletion is likely to be slow enough to permit adaptation to an anaerobic environment.
Comparing protein expression profiles between the three culture states in M. bovis BCG revealed the upregulation of the 16-kDa smHSP or α-crystallin homolog in response to low oxygen tension. During the course of our work Yuan et al. published a report which showed that the 16-kDa protein was upregulated by M. tuberculosis during stationary phase or under reduced oxygen tension (46). M. bovis BCG and M. tuberculosis 16-kDa smHSPs are members of the smHSP superfamily (5) and have homology in an 80-amino-acid region at the C terminus known as the α-crystallin domain. They also have a tendency to aggregate; Chang and coworkers (6) showed that the 16-kDa protein aggregates as an oligomer with a molecular mass of 149 ± 8 kDa (consisting of nine monomers, in a trimer-of-trimers organization). These proteins also prevent the thermal aggregation of other proteins (6, 21, 23, 46), but they do not prevent the loss of enzymic activity. The smHSPs act in an ATP-independent manner unlike the GroEL and DnaK HSPs (4, 6, 15, 23, 30). This is significant because they are frequently developmentally regulated, usually when and where metabolic activity is minimal such as in the eye lens (4) and, intriguingly, they are increasingly being discovered in sporulating microorganisms (17, 19, 36, 37). Interestingly, there appears to be an association between the expression of these proteins and quiescence induced by oxygen limitation. For example, the M. tuberculosis 16-kDa protein was found to have ∼41% similarity at the amino acid level (24) with the 21-kDa spore protein (SP21) or the low-molecular-weight HSP of Stigmatella aurantiaca (17), which is synthesized during fruiting body and spore formation but which is not found in vegetative cells. Spore production could be induced by anoxia (14), and oxygen limitation was found to induce the synthesis of this protein (18). The encysted embryos of the brine shrimp Artemia franciscana, which can survive continuous anoxia for more than 4 years and which have an undetectable metabolic rate during that time, also highly express a smHSP (7).
The three patterns of 16-kDa protein localization identified suggest that this protein is likely to have multiple targets throughout the cell and that it is integral to the stabilization of cellular structure during the dormant state. As the 16-kDa protein was localized to the cell wall, it is possible that some moieties the 16-kDa protein chaperones are also localized to this region. The only other prokaryotic smHSP to be studied in terms of immunoelectron microscopy localization is the SP21 protein of S. aurantiaca (28). This protein has many of the patterns of distribution reported here, although what the SP21 protein binds to was not identified. It is likely that the 16-kDa protein interacts in some way with the peptidoglycan layer of the mycobacterial cell, as indicated by the fact that it was observed to localize to fibrous structures. The clustering in the cytoplasm we observed was similar to the cytoplasmic clustering seen in S. aurantiaca (28). It has been proposed that the SP21 protein of S. aurantiaca may protect RNA species required for germination from being degraded (18), and as the smHSPs may be able to bind RNA (31), one could speculate that the mycobacterial 16-kDa protein may play a role in the protection of RNA. For example, Wayne (42) described how microaerobically cultured bacilli were able to multiply in a synchronous manner 8 h after reaeration of the culture; this suggests that much of the machinery required for this round of replication, including specific RNA and proteins, must already be in place. These would require protection from damage during dormancy and this protection could involve the chaperone function of the 16-kDa protein. However, the finding that there was little or no association of clusters with chromosomal material suggests that the protein does not play a role in protecting DNA during dormancy.
Yuan et al.’s data and our findings strongly suggest that oxygen deficiency is an important trigger for the synthesis of the 16-kDa protein. Therefore, its expression is not likely to be part of a general stress response. This agrees with our reverse-transcription PCR findings, which show that whereas the mycobacterial groEL and dnaK analogs are poorly transcribed after 2 weeks of microaerobic and anaerobic culture (with expression levels lowest in the anaerobic culture), the 16-kDa protein gene was highly expressed (8).
These results suggest that tubercle bacilli may adapt to low oxygen conditions by developing a thickened cell wall and that the 16-kDa protein confers an advantage on the bacilli during its dormant phase by stabilizing and protecting cell structures. The fact that both these phenomena were detected after 2 weeks of culture under low oxygen suggests that they may be developmentally regulated by similar mechanisms. Experiments to determine the biochemical components of the thickened outer layer and the cellular components the 16-kDa protein binds to are in progress. Understanding the roles that cell wall thickening and the expression of the 16-kDa protein play in mycobacterial dormancy may lead to novel strategies to kill persisters or prevent the reactivation of disease.
ACKNOWLEDGMENTS
This work was funded by the Glaxo Wellcome Action TB initiative.
We thank Paul Stanley and Lesley Tompkins for preparing the samples for electron microscopy and for their expert technical advice. We also thank Paul Stanley and Peter Whittle for preparing the electron micrographs. We thank Mike Osborne and Kerstin Williams (Birmingham) for their constructive comments regarding the TEM work, and we particularly thank Philip Draper (NIMR, London) for his helpful advice and suggestions. We also thank Neil Freeman (Glaxo Wellcome) for performing the N-terminal sequencing and Ken Duncan, Karen Kempsell, and Pauline Lukey (Glaxo Wellcome, Stevenage) for their support, many discussions, and helpful advice.
REFERENCES
- 1.Barksdale L, Kim K-S. Mycobacterium. Bacteriol Rev. 1977;41:217–372. doi: 10.1128/br.41.1.217-372.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bloom B R, Murray J L. Tuberculosis: commentary on a reemergent killer. Science. 1992;257:1055–1064. doi: 10.1126/science.257.5073.1055. [DOI] [PubMed] [Google Scholar]
- 3.Brennan P, Nikaido H. The envelope of mycobacteria. Annu Rev Biochem. 1995;64:29–63. doi: 10.1146/annurev.bi.64.070195.000333. [DOI] [PubMed] [Google Scholar]
- 4.Buchner J. Supervising the fold: functional principles of molecular chaperones. FASEB J. 1996;10:10–19. [PubMed] [Google Scholar]
- 5.Caspers G-J, Leunissen J A M, de Jong W W. The expanding small heat shock family, and structure predictions of the conserved “α-crystallin domain”. J Mol Evol. 1995;40:238–248. doi: 10.1007/BF00163229. [DOI] [PubMed] [Google Scholar]
- 6.Chang Z, Primm T P, Jakana J, Lee I H, Serysheva I, Chiu W, Gilbert H F, Quiocho F A. Mycobacterium tuberculosis 16-kDa antigen (hsp16.3) functions as an oligomeric structure in vitro to suppress thermal aggregation. J Biol Chem. 1996;271:7218–7223. [PubMed] [Google Scholar]
- 7.Clegg J. Embryos of Artemia franciscana survive four years of continuous anoxia: the case for complete metabolic rate depression. J Exp Biol. 1997;200:467–475. doi: 10.1242/jeb.200.3.467. [DOI] [PubMed] [Google Scholar]
- 8.Cunningham, A. F., and C. L. Spreadbury. Unpublished data.
- 9.DeMaio J, Zhang Y, Ko C, Zhang D B, Bishai W R. A stationary phase, stress response sigma factor from Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 1996;93:2790–2794. doi: 10.1073/pnas.93.7.2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deretic V, Philipp W, Dhandayuthapani S, Mudd M H, Curcic R, Garbe T, Heym B, Via L E, Cole S T. Mycobacterium tuberculosis is a natural mutant with an inactivated oxidative-stress regulatory gene: implications for sensitivity to isoniazid. Mol Microbiol. 1995;17:889–900. doi: 10.1111/j.1365-2958.1995.mmi_17050889.x. [DOI] [PubMed] [Google Scholar]
- 11.Dubos R J. The effect of organic acids on mammalian tubercle bacilli. J Exp Med. 1950;92:319. doi: 10.1084/jem.92.4.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dubos R J. Effect of the composition of the gaseous and aqueous environment on the survival of tubercle bacilli in vitro. J Exp Med. 1953;97:357. doi: 10.1084/jem.97.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dubos R J. Effect of ketone bodies and other metabolites on the survival and multiplication of staphylococci and tubercle bacilli. J Exp Med. 1953;98:145. doi: 10.1084/jem.98.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gerth K, Metzger R, Reichenbach H. Induction of myxospores in Stigmatella aurantiaca (Myxobacteria)—inducers and inhibitors of myxospore formation, and mutants with a changed sporulation behaviour. J Gen Microbiol. 1993;139:865–871. [Google Scholar]
- 15.Groenen P J T A, Merck K B, de Jong W W, Bloemendal H. Structure and modification of the junior chaperone α-crystallin—from lens transparency to molecular pathology. Eur J Biochem. 1994;225:1–19. doi: 10.1111/j.1432-1033.1994.00001.x. [DOI] [PubMed] [Google Scholar]
- 16.Hayat M A. Principles and techniques of electron microscopy. 3rd ed. United Kingdom: Macmillan, Basingstoke; 1989. [Google Scholar]
- 17.Heidelbach M, Skladny H, Schairer H U. Purification and characterization of SP21, a development-specific protein of the myxobacterium Stigmatella aurantiaca. J Bacteriol. 1993;175:905–908. doi: 10.1128/jb.175.3.905-908.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heidelbach M, Skladny H, Schairer H U. Heat shock and development induce synthesis of a low-molecular-weight stress-responsive protein in the myxobacterium Stigmatella aurantiaca. J Bacteriol. 1993;175:7479–7482. doi: 10.1128/jb.175.22.7479-7482.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Henriques A O, Beall B W, Moran C P., Jr CotM of Bacillus subtilis, a member of the α-crystallin family of stress proteins, is induced during development and participates in spore outer coat formation. J Bacteriol. 1997;179:1887–1897. doi: 10.1128/jb.179.6.1887-1897.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heplar J Q, Clifton C E, Raffel S, Futrelle C M. Virulence of the tubercle bacillus. I. Effect of oxygen tension upon respiration of virulent and avirulent bacilli. J Infect Dis. 1954;94:90–98. doi: 10.1093/infdis/94.1.90. [DOI] [PubMed] [Google Scholar]
- 21.Horwitz J. α-Crystallin can act as a molecular chaperone. Proc Natl Acad Sci USA. 1992;89:10449–10453. doi: 10.1073/pnas.89.21.10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Imaeda T, Kanetsuna F, Galindo B. Ultrastructure of cell walls of genus Mycobacterium. J Ultrastruct Res. 1968;25:46–63. doi: 10.1016/s0022-5320(68)80059-0. [DOI] [PubMed] [Google Scholar]
- 23.Jakob U, Gaestel M, Engel K, Buchner J. Small heat shock proteins are molecular chaperones. J Biol Chem. 1992;268:1517–1520. [PubMed] [Google Scholar]
- 24.Kempsell, K. E. Personal communication.
- 25.Kochi A. The global tuberculosis situation and the new control strategy of the World Health Organization. Tubercle. 1991;72:1–6. doi: 10.1016/0041-3879(91)90017-m. [DOI] [PubMed] [Google Scholar]
- 26.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 27.Lee B-Y, Hefta S A, Brennan P J. Characterization of the major membrane protein of virulent Mycobacterium tuberculosis. Infect Immun. 1992;60:2066–2074. doi: 10.1128/iai.60.5.2066-2074.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lünsdorf H, Schairer H U, Heidelbach M. Localization of the stress protein SP21 in indole-induced spores, fruiting bodies, and heat-shocked cells of Stigmatella aurantiaca. J Bacteriol. 1995;177:7092–7099. doi: 10.1128/jb.177.24.7092-7099.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Minnikin D E. Lipids: complex lipids, their chemistry, biosynthesis and roles. In: Ratledge C, Stanford J, editors. The biology of the mycobacteria. 1. Physiology, identification and classification. London, United Kingdom: Academic Press, Inc.; 1982. pp. 95–184. [Google Scholar]
- 30.Nicholl I D, Quinlan R A. Chaperone activity of α-crystallin modulates intermediate filament assembly. EMBO J. 1994;13:945–953. doi: 10.1002/j.1460-2075.1994.tb06339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nover L, Scharf K D, Neumann D. Cytoplasmic heat shock granules are formed from precursor particles and are associated with a particular set of mRNA. Mol Cell Biol. 1989;9:1298–1308. doi: 10.1128/mcb.9.3.1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rastogi N, Fréhel C, David H L. Triple-layered structure of mycobacterial cell wall: evidence for the existence of a polysaccharide-rich outer layer in 18 mycobacterial species. Curr Microbiol. 1986;13:237–242. [Google Scholar]
- 33.Reynes J, Perez C, Lamaury I, Janbon F, Bertrand A. Bacille Calmette-Guerin adenitis 30 years after immunization in a patient with AIDS. J Infect Dis. 1989;160:727. doi: 10.1093/infdis/160.4.727. [DOI] [PubMed] [Google Scholar]
- 34.Reynolds E S. The use of lead citrate at high pH as an electron opaque stain in electron microscopy. J Cell Biol. 1963;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Robertson D, Monaghan P, Clarke C, Atherton A J. An appraisal of low-temperature embedding by progressive lowering of temperature into Lowicryl HM20 for immunocytochemical studies. J Microsc. 1992;168:85–100. doi: 10.1111/j.1365-2818.1992.tb03253.x. [DOI] [PubMed] [Google Scholar]
- 36.Sauer U, Dürre P. Sequence and molecular characterization of a DNA region encoding a small heat shock protein of Clostridium acetobutylicum. J Bacteriol. 1993;175:3394–3400. doi: 10.1128/jb.175.11.3394-3400.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Servant P, Mazodier P. Characterization of Streptomyces albus 18-kilodalton heat shock-responsive protein. J Bacteriol. 1995;177:2998–3003. doi: 10.1128/jb.177.11.2998-3003.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sherman D R, Sabo P J, Hickey M J, Arain T M, Mahairas G G, Yuan Y, Barry III C E, Stover C K. Disparate responses to oxidative stress in saprophytic and pathogenic mycobacteria. Proc Natl Acad Sci USA. 1995;92:6625–6629. doi: 10.1073/pnas.92.14.6625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sudre P, ten Dam G, Kochi A. Tuberculosis: a global review of the situation today. Bull W H O. 1992;70:149–159. [PMC free article] [PubMed] [Google Scholar]
- 40.Verbon A, Hartskeerl R A, Schuitema A, Kolk A H J, Young D B, Lathigra R. The 14,000-molecular weight antigen of Mycobacterium tuberculosis is related to the alpha-crystallin family of low-molecular-weight heat shock proteins. J Bacteriol. 1992;174:1352–1359. doi: 10.1128/jb.174.4.1352-1359.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wayne L G. Dynamics of submerged growth of Mycobacterium tuberculosis under aerobic and microaerophilic conditions. Am Rev Respir Dis. 1976;114:807–811. doi: 10.1164/arrd.1976.114.4.807. [DOI] [PubMed] [Google Scholar]
- 42.Wayne L G. Synchronized replication of Mycobacterium tuberculosis. Infect Immun. 1977;17:528–530. doi: 10.1128/iai.17.3.528-530.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wayne L G, Lin K. Glyoxylate metabolism and adaptation of Mycobacterium tuberculosis to survival under anaerobic conditions. Infect Immun. 1982;37:1042–1049. doi: 10.1128/iai.37.3.1042-1049.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wayne L G, Salkin D. The bacteriology of resected tuberculous pulmonary lesions. I. The effect of interval between reversal of infectiousness and subsequent surgery. Am Rev Tuberc Pulm Dis. 1956;74:376–387. doi: 10.1164/artpd.1956.74.3.376. [DOI] [PubMed] [Google Scholar]
- 45.Wayne L G, Sramek H A. Metronidazole is bactericidal to dormant cells of Mycobacterium tuberculosis. Antimicrob Agents Chemother. 1994;38:2054–2058. doi: 10.1128/aac.38.9.2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yuan Y, Crane D D, Barry C E., III Stationary phase-associated protein expression in Mycobacterium tuberculosis: function of the mycobacterial α-crystallin homolog. J Bacteriol. 1996;178:4484–4492. doi: 10.1128/jb.178.15.4484-4492.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]