Abstract
On the basis of the previous research, the Traditional Chinese Medicine theory was used to improve the drug composition for gastrointestinal acute radiation syndrome (GI-ARS). The purpose of this study was to study the therapeutic mechanism of Liangxue-Guyuan-Yishen decoction (LGYD) on GI-ARS and to provide a new scheme for the treatment of radiation injury. Here, we investigated the effects of LGYD on intestinal stem cells (ISCs) in a GI-ARS rat model. Rat health and survival and the protective efficacy of LGYD on the intestines were analyzed. The active principles in LGYD were detected using liquid chromatography-mass spectrometry (LC–MS). ISC proliferation, intestinal epithelial tight junction (TJ) protein expression and regulatory pathways were explored using immunohistochemistry, western blotting (WB) and reverse transcription quantitative polymerase chain reaction (RT-qPCR), respectively. Involvement of the WNT and MEK/ERK pathways in intestinal recovery was screened using network pharmacology analysis and validated by WB and RT-qPCR. LGYD administration significantly improved health and survival in GI-ARS rats. Pathological analysis showed that LGYD ameliorated radiation-induced intestinal injury and significantly promoted LGR5+ stem cell regeneration in the intestinal crypts, upregulated TJ protein, and accelerated crypt reconstruction in the irradiated rats. LC–MS revealed ≥13 constituents that might contribute to LGYD’s protective effects. Collectively, LGYD can promote crypt cell proliferation and ISCs after radiation damage, the above effect may be related to WNT and MEK/ERK pathway.
Keywords: Chinese herbal medicine, gastrointestinal acute radiation syndrome, intestinal stem cell, tight junction protein
INTRODUCTION
Short-term exposure to high-dose irradiation causes gastrointestinal acute radiation syndrome (GI-ARS), one of the most common complications in patients undergoing abdominal radiation therapy [1]. GI-ARS damages the intestinal structure, induces severe symptoms, and may be fatal. As very few efficacious treatment strategies are currently available for GI-ARS, symptoms are managed through fluid and electrolyte balance maintenance, blood transfusions, and antibiotics. Several chemical and biological compounds, such as insulin-like growth factor 1 [2], basic fibroblast growth factor [2], cyclin-dependent kinase 4/6 inhibitor PD0332991 [3] and antineoplastic agent BCN057 [4], have been evaluated for GI-ARS therapy. However, owing to their toxicity at effective concentrations, no drug has been approved by the US FDA [5].
Owing to their effectiveness and low toxicity, Chinese herbal prescriptions have attracted attention for the treatment of radiation-induced injury. The Chinese herbal prescription Yiqi-Jiedu decoction significantly ameliorates radiation-induced testicular injury in mice by upregulating Toll-like receptor 5 expression in the testis and mitigating spermatogenic cell apoptosis [6]. Additionally, various plant-derived products aid in the protection against radiation damage. The adenosine derivative cordycepin inhibits radiation-induced ulceration by upregulating nuclear factor erythroid 2-related factor 2 expression and preventing cell senescence [7]. Our recent research showed that natural ferulic acid extract promotes postirradiation bone defect repair by maintaining skeletal stem cell stemness [8]. Mitogen-activated protein kinase pathway activation has been implicated in the protective mechanism of ferulic acid [9, 10]. Therefore, the discovery of novel herbal medicines and elucidation of their underlying radioprotective modes of action are medically relevant pursuits.
In Traditional Chinese Medicine (TCM), the Chinese herbal Liangxue-Guyuan-Yishen decoction (LGYD) is prescribed and administered for radioprotection. Our preliminary study [11] showed that LGYD alleviates radiation-induced intestinal injury in rats partially by regulating the Toll-like receptor 4/myeloid differentiation primary response 88/nuclear factor-kappa B (NF-κB) pathway. However, the cellular and molecular mechanisms and active components contributing to the radioprotective effects of LGYD are unknown. In this study, we aimed to investigate the mechanisms underlying the beneficial effect of LGYD on intestinal stem cells (ISCs) in GI-ARS.
MATERIALS AND METHODS
Drug preparation
LGYD comprised Panax ginseng C.A.Mey. (Renshen), Hedysarum Multijugum Maxim. (Huangqi), Cornu Bubali (Shuiniujiao), Moutan officinalis (L.) Lindl. & Paxton(Mudanpi), Salvia miltiorrhiza Bunge (Danshen), Atractylis macrocephala (Koidz.) Hand.-Mazz. (Baizhu), Pueraria lobata (Willd.) Ohwi (Gegen), Rehmannia glutinosa (Gaertn.) DC. (Dihuang), Coptis chinensis Franch. (Huanglian) and Paeonia officinalis L. (Chishao). All TCM materials were purchased from Beijing Tong Ren Tang Group (Beijing, China) and decocted twice. Both decoctions were mixed, and the decocted liquid was placed in a rotary evaporator (Hei-VAP Core, Heidolph Instruments, Schwabach, Germany) and concentrated according to the corresponding proportions. The medium-concentration decoction (MD) and high-concentration decoction (HD) treatments were concentrated to 2.73 and 5.46 g/ml crude concentrations at double and quadruple the equivalent doses administered to adult humans, respectively. They were sealed and stored at 4°C, and heated to 30–40°C before intragastric administration.
The positive control treatment was glutamine (Glu) in capsule form (Jiangsu Shenhua Pharmaceutical Co., Jiangsu, China). From d1 after irradiation to before death, the above experimental animals were respectively given medium and high concentration LGYD at a dose of 10 ml/kg; the Glu group was given 0.3 g/ml Glu suspension with 0.9% (w/v) physiological saline at a dose of 10 ml/kg; the Control group and Radiation group were given 0.9% (w/v) physiological saline at a dose of 10 ml/kg. All of the above drugs were administered once a day.
Untargeted liquid chromatography-mass spectrometry analysis
The components in LGYD were analyzed using liquid chromatography-mass spectrometry (LC–MS). Methanol, acetonitrile, formic acid, and water were purchased from Merck KGaA (Darmstadt, Germany). The 2-chlorophenylalanine was obtained from GL Biochem (Shanghai, China). The devices used were: vortex oscillator (TYXH-1; ZOLLO, Shanghai, China), high-speed benchtop refrigerated centrifuge (TGL-16MS; LUXIANGYI, Shanghai, China), high-performance liquid chromatography (HPLC) system (Acquity UPLC/HPLC; Waters, USA), HPLC column (Acquity UPLC HSS T3; Waters), mass spectrometer (Q Exactive Plus Hybrid Quadrupole-Orbitrap; Thermo Fisher Scientific, Waltham, MA, USA), and centrifuge (Sigma 1-16 K, Sigma-Aldrich, Darmstadt, Germany).
Compound Discoverer v. 2.0 (Thermo Fisher Scientific) was used to extract and preprocess LC–MS detection data. The data were retrieved using the Orbitrap TCM Library (Thermo Fisher Scientific), normalized and post-edited in Microsoft Excel 2010 (Microsoft Corp., Redmond, WA, USA), and organized into a 2D data matrix comprising the molecular weight, mass/charge ratio, retention time, peak strength, and database-matching results. A TIC diagram of LC–MS in the positive and negative ion modes is shown in Supplementary Fig. S1.
Network pharmacological analysis
The absorption, distribution, metabolism and excretion model in TCMSP database was used to screen out the components with the highest content in the samples, and the components were compared and queried. Unique molecular ID and relative target information were obtained. Duplicate components and those with no corresponding ID were removed. Ingredients with a unique molecular ID in the top 50 positive and negative ion modes were obtained. Compounds were retained only if their oral bioavailability ≥ 30% and drug-likeness ≥ 0.18 based on the criteria set by the TCMSP v. 2.3 database (https://tcmsp-e.com). A TCMSP target prediction model was used to forecast the putative target proteins of each active component. The names of the target proteins were retrieved from the UniProt database (https://www.uniprot.org). The qualifier used was ‘confirmed’ and species was ‘human’. The generic names of the genes corresponding to the target proteins were identified. The data were imported into Cytoscape v. 3.8.2 (https://cytoscape.org/download.html) to construct a drug-component-target-pathway network. The name of the disease was searched in the GeneCards (https://www.genecards.org) and Online Mendelian Inheritance in Man (OMIM) (https://www.omim.org) databases to obtain the genes associated with acute intestinal radiation injury. The online Wayne figure tools (https://bioinfogp.cnb.csic.es/tools/venny/) were used to screen for intersections among the target genes of the active ingredients in the compound and genes related to acute intestinal radiation damage. The data were downloaded from the STRING database into Cytoscape v. 3.8.2 to plot a target-path network graph. The Metascape (ttps://metascape.org/gp/index.html#/main/step1) database was used for gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) analyses.
Animal groupings and radiation source
Sprague–Dawley (SD) rats (SPF; male; body weight: 280–300 g) were purchased from Beijing Biotechnology Co., Ltd (Beijing, China). They were randomly assigned to the following five groups: control (Control), model (Radiation), positive control drug (Glu), MD and HD. We used 53 rats for survival analysis, with 12 rats in each experimental group and 5 in the Control group. The subsequent experiment included 85 rats, with 20 rats in each experimental group and 5 in the Control group. After adaptive feeding for 3 days, the rats fasted for 8 h before irradiation. All experiments complied with the relevant experimental animal welfare principles, approved by the Ethics Committee of the Academy of Military Medical Science (No. IACUC-DWZX-2020-783). The γ-radiation source was 60Co and was procured from the Institute of Academy of Military Medical Science. Excluding the Control group, the rats will be placed in a irradiation box and subjected to a whole-body one-time irradiation with a 100-cm source–skin distance, 300-cGy/min radiation dose rate, and 10-Gy dose in order to prepare the rat GI-ARS model. Rats with very poor survival status and abdominal aorta aneurysm were euthanized by a peritoneal overdose of pentobarbital sodium (100 mg/kg, IV). Five rats in each group were randomly selected on Days 3, 5 and 10 postirradiation. Small intestine tissues from each rat were frozen and sliced, respectively.
Western blotting
The rat small intestine tissues were homogenized at 4°C with radioimmunoprecipitation assay buffer, protease inhibitor cocktail and phosphorylase inhibitors (Servicebio, Wuhan, China) and centrifuged at 4°C and 12 000 × g for 5 min. The proteins were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane. The membrane was incubated with anti-β-Catenin (8480, Cell Signaling Technology, Danvers, MA, USA), anti-C-MYC (185 835, Cell Signaling Technology, Danvers, MA, USA), anti-phospho-MEK (9127S, Cell Signaling Technology, Danvers, MA, USA), anti-MEK (4694S, Cell Signaling Technology, Danvers, MA, USA), anti-phospho-ERK (4370S, Cell Signaling Technology, Danvers, MA, USA), anti-ERK (4695S, Cell Signaling Technology, Danvers, MA, USA), anti-GAPDH (5174, Cell Signaling Technology, Danvers, MA, USA), anti-WNT3A (ab219412, Abcam, UK) and anti-phospho-β-Catenin (ab75777, Abcam, UK) antibodies at 4°C overnight. The membrane was then rinsed with Tris/Tween-20 buffer (Servicebio) and incubated with horseradish peroxidase-bound secondary antibodies. The bands were analyzed using a ChemiDoc Imaging System (12003153-S; Bio-Rad, USA).
Reverse transcription quantitative polymerase chain reaction
The intestinal tissue was homogenized using RNA extraction compound (G3013; Servicebio). Reverse transcription was performed using a Servicebio RT First-Strand cDNA Synthesis Kit (G3330; Servicebio) according to the manufacturer’s instructions. Primers are listed in Supplementary Table S3. The mRNA levels were normalized to glyceraldehyde-3-phosphate dehydrogenase levels.
Hematoxylin–eosin staining and immunohistochemistry
About 3 cm of the upper segment of each small intestine was immersed in 4% (v/v) paraformaldehyde for 72 h. The small intestine were sectioned into 5-μm slices, embedded in paraffin and subjected to hematoxylin–eosin (HE) staining. The small intestine paraffin sections were rehydrated in 100%, 95% (v/v) and 80% (v/v) ethanol for 10 min per treatment and rinsed with phosphate-buffered saline (PBS). The samples were incubated with 1% (v/v) bovine serum albumin, incubated with primary antibodies at 4°C overnight and washed thrice with PBS. The secondary antibody was applied at 37°C for 30 min. The samples were rinsed thrice with PBS, rinsed with distilled water and re-stained with hematoxylin. The samples were then dehydrated and sealed. Thirty intact crypts or villi were counted per section. The numbers of positive cells per crypt or villus are reported as means ± standard deviation (SD). At least five rats were used per group. The antibodies used were anti-Lgr5 (NLS1236, Novusbio, USA), anti-CyclinD1 (GB111372, Servicebio, China), anti-PCNA (GB11010, Servicebio, China), anti-Olfm4(DF13440, Affinity Biosciences, Jiangsu, China), anti-claudin-1 (GB11032, Servicebio, China) and anti-occludin (GB111401, Servicebio, China). HE staining and immunohistochemistry (IHC) staining of the small intestine were observed under a microscope (Leika, Germany). Villus length (μm) and crypt depth (μm) were measured using ImagePro Plus v. 6.0 (Media Cybernetics Inc, Rockville, MD, USA) for ≥5 sections per time point per group. The lower right corner scale was the standard. The IHC and IF results were analyzed using ImageJ (Java 1.8.0_172; National Institutes of Health, Bethesda, MD, USA). The measurements were made by three observers blinded to the treatments.
Immunofluorescence staining
The intestinal slides were dewaxed, rehydrated and immersed in EDTA antigen recovery buffer (pH 8.0). After blocking, the slides were incubated with anti-CyclinD1 (sc8396, Santa, USA) and anti-GPR49/LGR5 (bs1117R, Bioss, China). After incubating with the above antibodies overnight and washing, the intestinal section will be incubated with CY3-labeled Goat anti-mouse IgG (GB21301, Servicebio, Wuhan, China) and Alexa Fluor 488 labeled Goat Anti-Rabbit IgG (GB25303, Servicebio, Wuhan, China). After washing, DAPI reagent was added to stain the nuclei. The images were collected after the self-fluorescence quencher was added and washed. The excitation wavelength of DAPI was 330–380 nm, and the emission wavelength was 420 nm. The excitation wavelength of 488 is 465–495 nm, and the emission wavelength is 515–555 nm. The excitation wavelength of CY3 is 510–560 nm, the emission wavelength is 590 nm, the nucleus of DAPI channel is blue, the positive channel of 488 is green and the positive channel of CY3 is red. The images were analyzed using ImageJ (Java 1.8.0_172, National Institutes of Health).
Enzyme-linked immunosorbent assay
Blood (≥3 ml) was drawn from each abdominal aorta of five randomly selected rats per group and per time point. The blood was stored at room temperature and centrifuged at 4000 × g for 15 min at 4°C. The supernatants were collected and stored at −80°C until the enzyme-linked immunosorbent assay (ELISA) was performed (Rat D-Lactate ELISA Kit; Shanghai Xin Fan Biotechnology Co., Ltd, Shanghai, China). The D-lactate standard was diluted and added to the test kit plate according to the manufacturer’s directions.
Statistical analysis
SPSS v. 19.0 (IBM Co., Armonk, NY, USA) was used for statistical analysis. Data are expressed as means ± SD. P < 0.05 indicated a statistically significant difference. One-way ANOVA was used to compare the differences among the five groups at each time point. The LSD post hoc test was used when the variances were homogeneous among the groups (In order to reduce the probability of type I error in LSD method, we recalibrated the LSD test level), and the Games-Howell test was used to compare the differences between every two groups when the variances were uneven. Survival rate was determined using a Kaplan–Meier survival curve, and pairwise comparisons were made using log-rank (Mantel–Cox) and Gehan–Breslow–Wilcoxon tests. Survival analyses and graphs were plotted using GraphPad Prism v. 7 (GraphPad Software, La Jolla, CA, USA). Rat body weights were repeated-measurement data and subjected to a correlated-sample ANOVA, which was used to analyze the data at various time points per group.
RESULTS
LGYD improved the short-term survival rate and general health status of rats subjected to a lethal dose of total body irradiation
LGYD significantly prolonged short-term survival in irradiated rats (Fig. 1a; Table S1). Only one rat in each of the Radiation and Glu groups survived until Day 10, indicating a high fatality rate. Zero and two rats in the MD and HD groups, respectively, survived after 2 weeks.
Fig. 1.
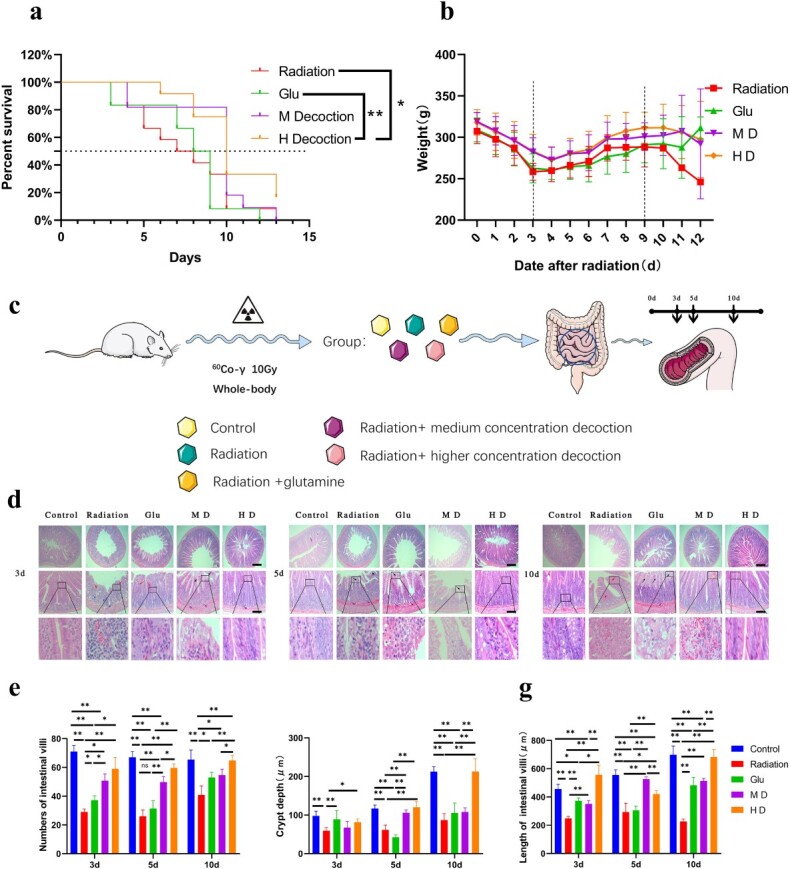
LXGYD can improve the survival status and intestinal injury state of rats after total body irradiation with a lethal dose. (a) Survival curves of rats in different treatment groups after radiation. (b) Weight change of rats in different treatment groups after radiation. (c) Rats were randomly divided into Control, Radiation, Glu, MD and HD groups. Excluding the Control, all groups were subjected to single whole-body irradiation. From Day 1 postirradiation, the Control and Radiation groups were administered normal saline. The Glu group was administered a Glu suspension and the MD and HD groups were administered 2.73 and 5.46 g/ml LXGYD, respectively. Five rats were randomly selected on Days 3, 5 and 10 postirradiation and 5 cm of small intestine, and bilateral femur was dissected and interpreted. (d) HE staining revealed that the epithelial cells of the intestinal villi of rats in the Radiation and Glu groups were enlarged and had a pale cytoplasm on Days 3, 5 and 10 postirradiation (d). Fibrous tissue proliferation was observed in the submucosa with widened gaps. After radiation, fibrous tissue proliferated in the glandular interstitium. However, in the group treated with TCM, pathological damage of the small intestine was not obvious, the epithelial cells of the intestinal villi were edematous with a light cytoplasm (d) and a relatively obvious inflammatory cell infiltration was observed in Radiation group and Glu group. A few intestinal villi exhibited microvascular hyperplasia and dilation of the lamina propria. Quantification of villus number (e), length (f) and crypt depth (g). Bars represent 200 and 100 μm, respectively, *P < 0.05, **P < 0.01.
LGYD administration improved the general health status of the irradiated rats, except for their overall survival rates. On Days 1–3, the rats were relatively lethargic, and their food and water consumption decreased sharply. Pus and blood appeared in the feces, and some animals demonstrated bleeding from the eyelids. These symptoms were severe in the Radiation group. The general health status of HD rats was marginally better than that of rats in other groups. In all groups, the average body weight decreased significantly after irradiation and reached its lowest by Day 3; it began to rise thereafter, without returning to the initial values. It continued to increase until Days 9–10. On Day 3 postirradiation, the average body weights of HD and MD rats were significantly higher than those of Radiation rats (P < 0.01). By Day 9, the average body weight of HD rats was significantly higher than that of Radiation rats (P < 0.05; Fig. 1b; Supplementary Table S2). On Days 4–8, although diarrhea was ameliorated, the rats became lethargic and bled from their eyelids or nasal cavities.
LGYD promoted small intestinal tissue repair in rats subjected to a lethal dose of radiation
HE staining revealed that the epithelial cells of the intestinal villi of rats in the Radiation and Glu groups were enlarged and had a pale cytoplasm on Days 3, 5 and 10 postirradiation (Fig. 1d; black arrow). Fibrous tissue proliferation was observed in the submucosa with widened gaps (purple arrow). Extensive necrosis of the intestinal mucosa and diffuse inflammatory cell infiltration were observed (red arrow). After radiation, the number of intestinal glands in the mucosal layer decreased significantly (P < 0.01), and fibrous tissue proliferated in the glandular interstitium (blue arrow). Fibrous tissue proliferation was observed in the submucosa with widened gaps (purple arrow). However, in the group treated with TCM, pathological damage of the small intestine was not obvious, the epithelial cells of the intestinal villi were edematous with a light cytoplasm (Fig. 1d; black arrow) and inflammatory cell infiltration was observed in certain areas (red arrow). A few intestinal villi exhibited microvascular hyperplasia and dilation of the lamina propria (yellow arrow).
We also analyzed pathological changes at various time points after irradiation (Fig. 1e–g). On Days 3, 5 and 10 postirradiation, the number of villi, crypt depth and villus length was significantly lower in Radiation rats than in Control rats (P < 0.01). Pathological damage concerning the number of villi, crypt depth and villus length was most and least severe on Days 3 and 5, respectively. Damage to the intestinal mucosa was gradually reduced in the Radiation group by Day 10. Hence, the intestine, damaged by irradiation at the experimental dose, underwent self-repair to a certain degree; however, the tissue was not restored to its normal structure.
The impact of irradiation was less severe in Glu rats than in Radiation rats, and those in the former group gradually recovered from the damage. The recovery was better in MD and HD rats than in Radiation rats. The pathological changes that occurred in the intestinal tract of MD rats gradually reversed over time. Nevertheless, some extent of inflammatory cell infiltration and vasodilation persisted. In contrast, HD rats exhibited less inflammatory cell infiltration and no apparent tube wall thickening. Vasodilation and intestinal epithelial edema were observed in a few cases. Overall, the treatment efficacy concerning the number of villi, crypt depth and villus length was high in Glu, MD and HD groups (P < 0.05); HD rats fared best among these three groups.
Active ingredients and network analysis of LGYD
The crude drug (evaporated decoction) was subjected to LC–MS (Fig. 2a); total ion chromatograms are shown in Supplementary Fig. S2. The result of LC–MS was then analyzed by network pharmacology (Fig. 2b), and the top 100 compounds identified by the semi-quantitation of positive and negative ions were selected for preliminary screening. In total, 13 effective components were obtained (Supplementary Fig. S1).
Fig. 2.
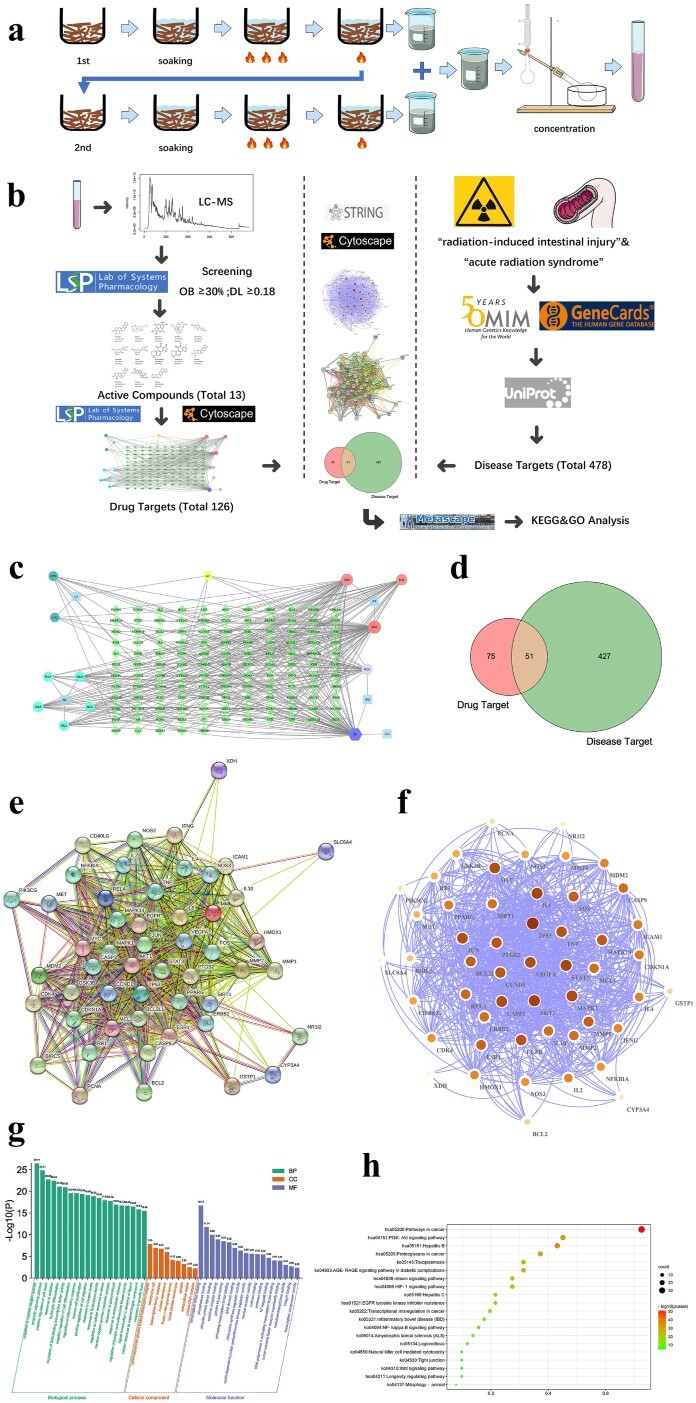
Drug preparation and network pharmacological study. (a) The drug was prepared, adjusted to the appropriate concentration, and stored at 4°C. (b) Network pharmacological procedure. (c) Target interaction relationship of core active components. Among them, light blue is the main drug (CS: red peony root, HL: coptis, DS: salvia miltiorrhiza, GG: pueraria root, HQ: Astragalus membranaceus), dark green is the active ingredient of CS (CS1: Ellagic acid, CS2: Paeoniflorin), lighter blue is the effective ingredient of HL (HL1: Berberine, HL2: Berberrubine, HL3, Palmatine, HL4, Coptisine, HL5: Berlambine), red is the DS effective components (DS1: Luteolin, DS2: Cryptotanshinone, DS3: Tanshinone IIA), lilac is the active component of HQ (HQ1: Isorhamnetin), purple is the common component of HQ and GG (B1: Formononetin) and yellow is the common component of CS and DS (A1: Baicalin). (d) The intersecting gene-disease target and core active component gene target. (e) The interaction network of the intersecting genes. Colored nodes: query proteins and first shell of interactors; white nodes: second shell of interactors; empty nodes: proteins of unknown 3D structure; filled nodes: some 3D structure is known or predicted; edges: known interactions (light blue: from curated databases; purple: experimentally determined), predicted interactions (green: gene neighborhood; red: gene fusions; blue: gene co-occurrence) and others (yellow: text mining; black: co-expression; lavender: protein homology). (f) Cytoscape map of the intersecting genes; node color and size represent how close the connections are. GO enrichment analysis (g) and KEGG enrichment analysis (h) of the intersecting genes.
We obtained 126 LGYD targets from the TCMSP database, and correlations between these targets and the core molecules were demonstrated using Cytoscape (Fig. 2c). The keywords ‘radiation-induced intestinal injury’ and ‘acute radiation syndrome’ were used to query disease-related genes using the GeneCards and OMIM databases. We obtained 478 targets after the elimination of duplicates and normalization of gene names, and the targets were converted into common gene names through the UniProt database. Common genes were identified using a Venn diagram (Fig. 2d).
We analyzed the intersecting genes corresponding to the target of the active LGYD component and genes contributing to radiation-induced intestinal damage. Fifty-one common genes were found to be potential LGYD targets (Fig. 2e), which were enriched by the STRING database and imported into Cytoscape for association analysis (Fig. 2f) and the Metascape database for GO analysis (Fig. 2g). GO BP showed 51 overlapping targets associated with the radiation-related biological processes cell cycle, differentiation, migration and inflammatory response and apoptosis. GO CC demonstrated that the targets focused on the cellular composition of enzymes regulating cyclin, cell membrane raft, transcription factors (TFs), nuclear envelope, basement membrane, spindle and extracellular matrix. GO MF revealed that the intersecting targets focused on TF, phosphorylase, ubiquitin-specific protease, cytokine receptor, NF-κB binding, protein kinase and nuclear receptor activation. KEGG enrichment analysis (Fig. 2h) revealed that at least 20 significant pathways were enriched, including PI3K, NF-κB, Wnt and TJ pathways.
LGYD might induce LGR5 + stem cell proliferation in the ileal crypts of rats via the Wnt pathway
The network pharmacological analyses suggested that the Wnt pathway played a role in intestinal crypt regeneration in irradiated rats. However, biological data are required to validate these findings. Results from IHC staining (Fig. 3a and b) showed that the expression of CyclinD1, a key protein in the WNT/β-Catenin pathway, was significantly lower in the intestinal crypts in Radiation rats than in Control rats (P < 0.01) on Day 3 postirradiation. CyclinD1 expression was higher in HD rats than in MD or Glu rats (P < 0.01) and nearly 3-fold higher than in Radiation rats (P < 0.01). CyclinD1 expression in MD rats was 2-fold higher than that in Radiation rats (P < 0.01). No significant difference between Radiation and Control rats regarding CyclinD1 expression on Day 5 postirradiation was observed. However, the former exhibited shorter intestinal villi and disordered intestinal epithelial morphology. The order of CyclinD1 expression was MD > HD > Glu. CyclinD1 expression was significantly lower in Radiation rats than in Control rats on Day 10 postirradiation (P < 0.01); however, it did not differ significantly from that in Glu rats. CyclinD1 expression significantly increased in MD and HD rats, although it was higher in HD rats than in MD rats (P < 0.01).
Fig. 3.
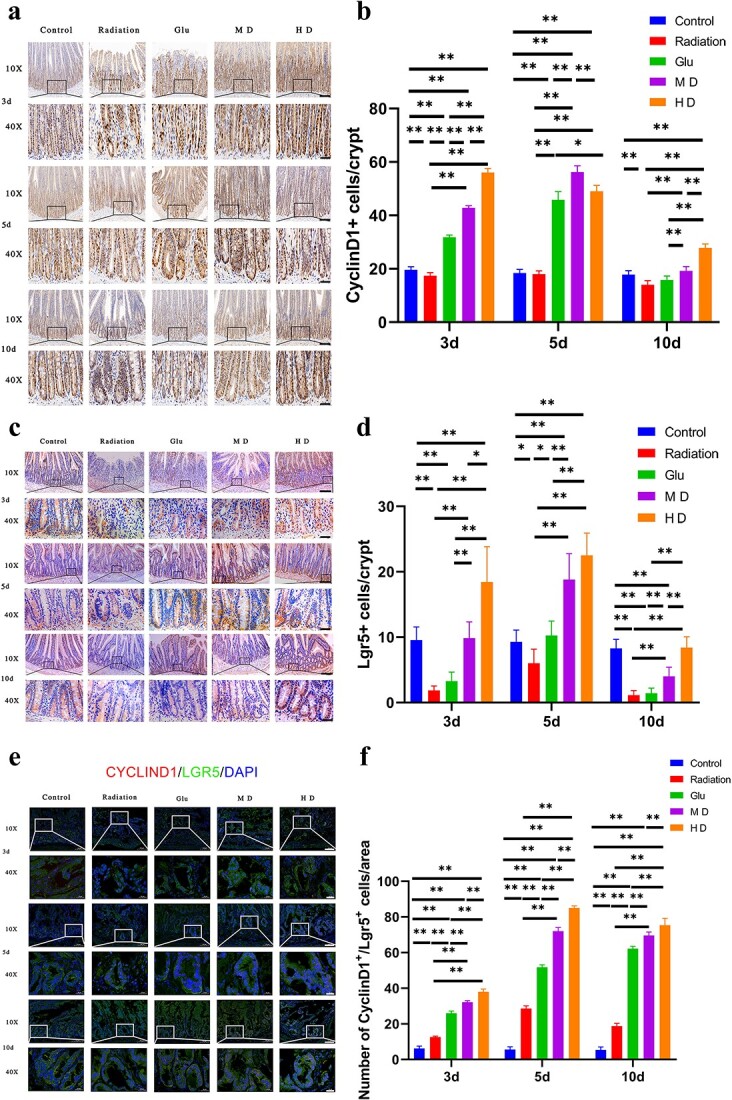
Immunohistochemical and immunofluorescence (IF) staining of Lgr5 and CyclinD1. (a, b) Immunohistochemical staining of CyclinD1 in cells at the bottom of the crypt. (c, d) Immunohistochemical staining of Lgr5-positive cells at the bottom of the crypt. (e, f) IF staining of Lgr5- and CyclinD1-positive cells in the crypt (Cyclind1 in red)/Lgr5 in green)/DAPI in blue). Bars represent 100 (10×) and 20 μm (40×), respectively; *P < 0.05, **P < 0.01.
On Days 3, 5 and 10 postradiation, the number of PCNA-positive cells in Radiation rats was significantly lower than that in Control rats (P < 0.01; Fig. 4a and b). Compared with that in the Radiation group, that in all other treatment groups improved to varying degrees. In general, HD had the most significant effect, which exceeded that in the Control group on Days 3 and 5 postradiation (P < 0.01).
Fig. 4.
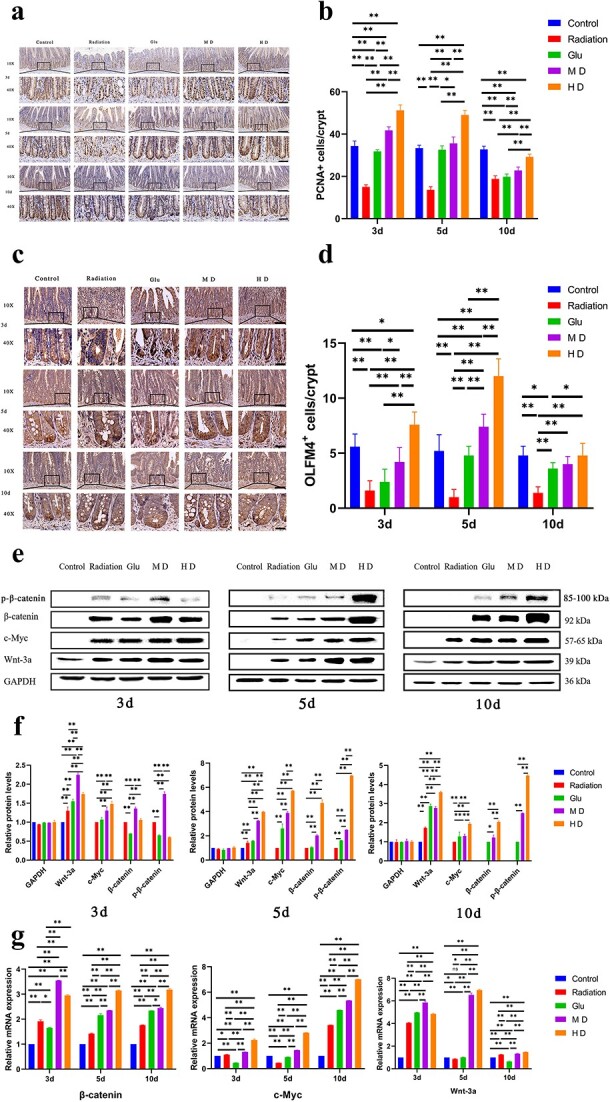
Immunohistochemical staining of PCNA and OLFM4 and WB and RT-qPCR to measure relative expression levels of β-catenin, p-β-catenin, C-MYC and WNT-3A. (a, b) Immunohistochemical staining of PCNA in cells at the bottom of the crypt. (c, d) Immunohistochemical staining of OLMF4-positive cells at the bottom of the crypt. Bars represent 100 (10×) and 20 μm (40×), respectively; *P < 0.05, **P < 0.01. (e, f) WB to measure the expression of proteins involved in the Wnt pathway. (g) RT-qPCR to measure the expression of genes involved in the Wnt pathway.
Lgr5 is involved in the Wnt pathway and is a pivotal ISC marker. IHC (Fig. 3c and d) showed that the number of Lgr5-positive cells in the intestinal crypts of Radiation rats was significantly lower than that in Control rats on Day 3 postirradiation. In contrast, Lgr5 expression was significantly upregulated in the intestinal crypts of MD and HD rats (P < 0.01). Lgr5-positive cells appeared mainly at the bottom of the crypt, and the cell numbers were similar in the MD and Control groups. Nevertheless, the number of cells in HD rats was <1.5-fold higher than that in Control rats. Radiation rats had approximately half as many Lgr5-positive cells as Control rats on Day 5 postirradiation (P < 0.05). Lgr5 expression in HD rats was 3-fold higher than that in Radiation rats. The number of Lgr5-positive cells was significantly lower in Radiation rats than in Control rats on Day 10 postirradiation (P < 0.01). MD and HD rats had significantly more Lgr5-positive cells than Radiation rats (P < 0.01), and the number of Lgr5-positive cells in HD rats was similar to that in Control rats (P > 0.05). Additionally, the expression levels of olfactomedin 4 (Olfm4), another protein involved in the Wnt pathway, were significantly decreased and remained at a relatively low level in intestinal tissues after radiation (Fig. 4c and d). The number of Olmf4-positive cells was increased to a certain extent in all three treatment groups but the recovery effect was different. In general, HD had the best effect (P < 0.05), and the number of Olmf4-positive cells in the short radiation period (0–5 days) was higher than that in the Control group (P < 0.05).
After radiation, the number of cells labeled with both CyclinD1 and Lgr5 increased significantly in the small intestine tissues (P < 0.01; Fig. 3e and f). All treatments showed a significantly increased number of labeled cells to different degrees (P < 0.01), and the effect of HD was the best (P < 0.01), followed by MD (P < 0.01); that of Glu was worse than that of MD (P < 0.01).
To verify the involvement of the Wnt pathway in intestinal repair, we measured the expression of key proteins involved in the classical Wnt pathway. At each time point, WNT3A, C-MYC, p-β-catenin and β-catenin expression were higher in irradiated rats than in Control rats (P < 0.01; Fig. 4e and f). The stimulatory effects of MD and HD on the Wnt pathway were stronger than those of Glu immediately after radiation. The stimulatory effect of HD was most evident on Days 5 and 10 postirradiation and was ~5-fold stronger than that in the Radiation group on Day 5 (P < 0.01). HD promoted the Wnt pathway more strongly than MD, especially on Days 5 and 10 postirradiation (P < 0.01).
The reverse transcription quantitative polymerase chain reaction (RT-qPCR) results were consistent with those of western blotting (WB) (Fig. 4g). The WNT-3A, C-MYC and β-catenin transcription levels were significantly higher in Radiation rats than in Control rats on Day 3 postirradiation (P < 0.05). Glu did not significantly promote WNT-3A, C-MYC or β-catenin transcription compared with that in the Radiation group; MD and HD were significantly more effective than Glu (P < 0.05). Moreover, HD was the most effective, especially on Days 5 and 10 postirradiation (P < 0.01).
LGYD promoted TJ protein expression in intestinal epithelial cells via MEK/ERK pathway activation
We measured D-lactic acid concentrations in the peripheral blood to reflect its intestinal barrier function. The D-lactic acid concentration in rats in Radiation, Glu, MD and HD groups was significantly higher than that in those in the Control group (P < 0.01; Fig. 5b). The D-lactic acid concentration in Glu, MD and HD groups was significantly lower than that in the Radiation group (P < 0.01); it was lowest in the HD group.
Fig. 5.
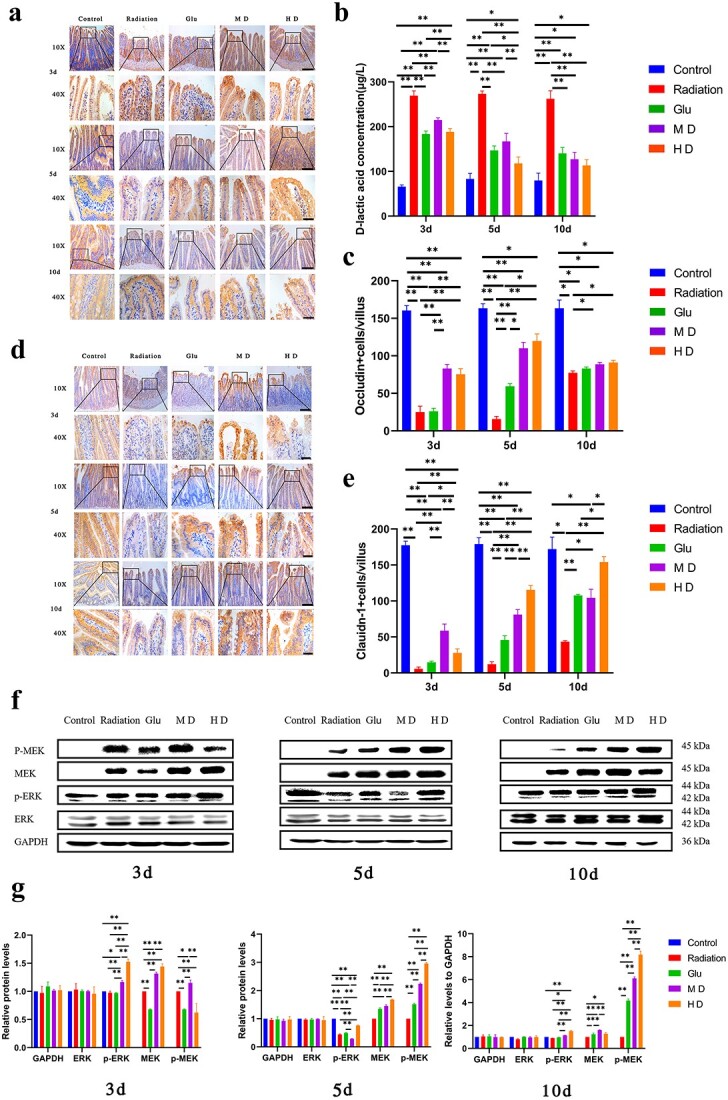
Immunohistochemical staining of occludin and claudin-1, D-lactic acid concentration and WB to measure the expression of proteins involved in the MEK/ERK pathway. (a, c, d, e) Immunohistochemical staining of occludin and claudin-1. Bars in (a) and (d) represent 100 (10×) and 20 μm (40×), respectively; *P < 0.05, **P < 0.01. (b) Rat D-Lactate ELISA Kit was used to measure D-lactic acid concentrations in the peripheral blood of rats. (f, g) Expression of key proteins involved in the MEK/ERK pathway, ERK, p-ERK, MEK and p-MEK, was measured using WB, and semi-quantitative analysis was performed.
We performed IHC staining to assess the effects of LGYD on intestinal epithelial cell TJ proteins: occludin and claudin-1 (Fig. 5a, c–e). Occludin content was significantly lower in the radiation treatment groups than in the Control group (P < 0.01). Occludin expression was significantly higher in the LGYD treatment group than in the Radiation group on Day 3 postradiation (P < 0.01); it was significantly higher in MD, HD and Glu groups on Day 5 postirradiation (P < 0.05) and in HD and MD groups on Day 10 postirradiation (P < 0.05) than in the Radiation group on these respective days.
Claudin-1 expression was significantly lower in Radiation rats than in Control rats (P < 0.01), whereas it was significantly higher in MD rats than in rats in other groups (P < 0.01) on Day 3 postirradiation. However, no difference was found in claudin-1 expression between Glu and Radiation groups (P > 0.05). Claudin-1 expression in HD rats was higher than that in MD, Glu and Radiation rats on Day 5 postirradiation (P < 0.01). HD rats had the highest claudin-1 expression, which was 2-fold greater than that in Glu rats. Claudin-1 expression was significantly higher in all treatment groups than in the Radiation group on Day 10 postirradiation (P < 0.05). At that point in time, claudin-1 expression in HD rats was approximately 4-fold higher than that in Radiation rats (P < 0.01).
The expression of key proteins involved in the MEK/ERK pathway was measured (Fig. 5f and g). No differences were found in ERK expression among the groups on Days 3, 5 and 10 postirradiation. Phosphorylated ERK expression was significantly higher in HD rats than in MD, Glu and Radiation rats (P < 0.01). MEK expression was significantly higher in MD and HD rats than in Radiation rats (P < 0.05). No significant difference was found in MEK expression between MD and HD groups. The p-MEK expression level was nearly zero in Control rats and considerably higher in HD rats than in MD, Glu and Radiation rats on Days 5 and 10 (P < 0.01).
DISCUSSION
Radiation induces severe ISC injury during pathological GI-ARS progression [12]. Restoring ISC function after irradiation may help re-establish intestinal homeostasis, alleviate acute GI-ARS symptoms and improve short-term survival [13]. In recent years, many anti-radiation drugs target small intestine stem cells, and relevant studies have revealed their mechanisms. LGYD.
Leukemia inhibitory factor (LIF) significantly affects the intestinal epithelia of both irradiated LIF-KO and WT mice [14]; however, the damage is far more severe in the former. Postirradiation LIF administration activates the AKT/GSK3β pathway and promotes ISC proliferation and intestinal epithelial regeneration. Chen et al. [15] established a GI-ARS animal model using 15 Gy local irradiation. TIGAR upregulation accelerates ISC division at the crypt bottom, thereby promoting intestinal reconstruction after lethal-dose irradiation. The combined use of podophyllotoxin and rutin alleviates radiation-induced ISC damage, possibly by upregulating the WNT/β-catenin signaling pathway [16]. In KO mice and organoid cultures, the antitumor drug BCN057 spares ISCs after lethal-dose irradiation, promotes intestinal epithelium regeneration, alleviates acute symptoms and improves short-term survival [4]. Subcutaneous Me6 injection in mice subjected to lethal-dose irradiation significantly increases intestinal crypt and villus length by promoting intestinal epithelial cell proliferation [17]. The organoid culture showed that Me6 significantly increases budding. The study indicated that β-catenin is an important downstream target of Me6 and promotes postirradiation ISC proliferation.
Our research team conducted preliminary clinical and experimental trials on the original prescription for the prevention and treatment of radiation-induced lung injury and enteritis [18]. The prescription was amended based on a Chinese herbal medicine formulated to treat radiation injury and the relevant therapeutic principles of TCM. A novel prescription (LGYD) was formulated with the intention to test its efficacy and elucidate its mechanisms in GI-ARS therapy. Our research found that LGYD could significantly relieve the symptoms of GI-ARS rats and improve the survival rate and survival status. The results showed that LGYD could promote the repair of intestinal pathological state to a certain extent, increase the number of stem cells in the small intestinal crypts after radiation, promote the proliferation of small ISCs, and upregulate the expression of tight junction protein in intestinal epithelial cells, so as to promote the reconstruction of intestinal structure, restore the intestinal mechanical barrier and other physiological functions.
There were certain limitations to the current study. Significant progress has been made in the development of drugs such as small molecule compounds and free radical scavengers to treat radiation injury. However, the US FDA has approved no drug for GI-ARS therapy. Glu is the most abundant amino acid in the serum. It is absorbed by the small intestine in response to intestinal injury and promotes intestinal epithelial cell proliferation [19]. However, it has not yet been evaluated as an independent clinical drug therapy strategy for GI-ARS. Hence, the selection of Glu as a positive drug has certain limitations. Several studies showed that it promotes Lgr5+ ISC [20]. In a Glu-deficient environment, Lgr5 + ISCs remain static while reactivated when the medium is replenished with Glu. As Glu promotes ISC proliferation, increases crypt formation and maintains intestinal homeostasis [21], we selected it as a positive drug in this study. As for the dosage of Glu, we also referred to the research with similar background (beau) and the previous research of our research group [22].
To further clarify the mechanism and target of action of LGYD in the treatment of GI-ARS, the main active components of LGYD were analyzed by LC–MS, and the possible targets of this prescription were predicted by network pharmacology. Based on the network pharmacological results, we selected both the Wnt pathway and the MEK/ERK pathway. We chose the WNT pathway because, first of all, it is closely related to the fate regulation of ISCs, and this pathway is the most important one among many pathways regulating ISCs. Second, although the correlation degree of WNT pathway is not the highest in KEGG enrichment analysis, the main objective of this study is to investigate the mechanism of LGYD on intestinal injury after radiation. In other published articles, we have studied the core factors of PI3K/AKT, NF-κB and inflammatory bowel disease pathway in enrichment analysis [11]. Therefore, to sum up, we selected the WNT pathway that is closely related to small intestine stem cells and intestinal injury for verification, and we will conduct future studies on other possible pathways.
We chose the latter as it is closely related to TJ protein expression [23,24]. The PI3K/AKT pathway was strongly correlated with the LGYD mechanism. A cross-linking effect exists between the PI3K and MEK/ERK pathways [25–29] and there is mutual MEK/ERK and Wnt pathway activation [30,31]. Hence, the MEK/ERK pathway was selected for our investigations.
The results of WB and q-PCR showed that the WNT pathway was activated to a certain extent in all groups after radiation, indicating that the small intestine tissues of rats showed a tendency of healing after radiation. Compared with the Radiation group, all treatment groups showed promoting effect on the WNT pathway, among which the Glu group was better than the Radiation group. Compared with the Glu group, the expression levels of Wnt-3a, β-catenin, p-β-catenin, c-MYC and other core molecules were more significantly upregulated after the application of LGYD, indicating that the obvious promoting effect of LGYD on WNT pathway was basically clear. This conclusion is verified by the results of Fig. 3, which showed that the number of Lgr5+ cells in the crypt of the LGYD treatment group was significantly higher than that of the Gln group and the Radiation group. It is concluded that after radiation, a large number of cells in the small intestine crypt died, ISCs with potential for proliferation and differentiation were mobilized, and WNT pathway was activated. The results showed that the compensatory increase of Lgr5+ ISCs in the crypt was increased, and the LGYD promoted the proliferation of ISCs by upregulating the WNT pathway, and finally promoted the reconstruction of small intestinal crypt structure and restored the pathological injury of small intestine. Later, we verified the effect of the compound on the MEK/ERK pathway and the expression of intestinal epithelial tight junction protein, and found that the MEK/ERK pathway, which is closely related to the expression of intestinal epithelial tight junction protein, was activated after irradiation, which may be due to the damage of intestinal epithelial tight junction protein caused by radiation and the destruction of intestinal mechanical barrier function. However, the intestine has a strong regenerative capacity, which tends to be more and more healthy after radiation, driving to express more tight junction proteins to maintain the basic structure and function of the intestinal tract. Compared with the model control group, all treatment groups promoted the upregulation of this pathway to a certain extent. Compared with Glu, LGYD could activate and upregulate the expression levels of MEK, p-MEK and p-ERK more significantly in all treatment groups, and promoted the expression of tight junction protein and approached the normal level. The promotion effect of TCM compound on the two pathways was most outstanding in the high-dose group in the early phase of radiation (3-5d), which may be due to the fact that this time point is relatively the most serious stage of radiation damage, so the repair effect in the acute phase is more obvious during this period.
CONCLUSION
In this study, we examined the active components in LGYD and verified its possible mechanism of action on ISCs and signal pathways in a GI-ARS rat model. LGYD treatment improved the recovery of GI-ARS-induced rats. Moreover, LGYD promoted ISC proliferation and TJ protein expression, potentially via the activation of the Wnt and MEK/ERK pathways. Therefore, LGYD has a positive therapeutic effect on GI-ARS and has the potential for further development and clinical application.
CONFLICT OF INTEREST
All authors disclosed no relevant relationships.
FUNDING
Authors have no funding for research.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
All experiments complied with the relevant experimental animal welfare principles, approved by the Ethics Committee of the Academy of Military Medical Science (No. IACUC-DWZX-2020-783).
CONSENT FOR PUBLICATION
All the authors agreed to be published.
DATA AVAILABILITY
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
AUTHORS’ CONTRIBUTIONS
Y.D.: Conception and design, Financial support, Administrative support, Provision of study material or patients, Final approval of manuscript
Z.Y.: Provision of study material or patients, Collection and/or assembly of data, Data analysis and interpretation, Manuscript writing
B.Y.: Provision of study material or patients, Collection and/or assembly of data, Data analysis and interpretation
Y.W.: Provision of study material or patients, Data analysis and interpretation
Z.N.: Articles editing, Data analysis and interpretation
J.F.: Collection and/or assembly of data, Data analysis and interpretation
Q.Y.: Collection and/or assembly of data, Data analysis and interpretation
X.L.: Collection and/or assembly of data, Data analysis and interpretation
H.Z.: Conception and design, Financial support, Administrative support, Provision of study material or patients
Supplementary Material
Contributor Information
Ziqiao Yan, Department of Traditional Chinese Medicine, The First Medical Center of Chinese People’s Liberation Army (PLA) General Hospital, Fuxing Road 28th, Haidian District, Beijing, 10038, China; Chinese PLA Medical School, Chinese People’s Liberation Army (PLA) General Hospital, Fuxing Road 28th, Haidian District, Beijing, 10038, China.
Bofeng Yin, Department of Experimental Hematology and Biochemistry, Beijing Institute of Radiation Medicine, Taiping Road 27th, Haidian District, Beijing, 10039, China; Beijing Key Laboratory for Radiobiology, Beijing Institute of Radiation Medicine, Taiping Road 27th, Haidian District, Beijing, 10039, China.
Yuguo Wang, Department of Traditional Chinese Medicine, The Sixth Medical Center of Chinese People’s Liberation Army (PLA) General Hospital, Fucheng Road 6th, Haidian District, Beijing, 10037, China.
Zhexin Ni, Department of Pharmaceutical Sciences, Beijing Institute of Radiation Medicine, Taiping Road 27th, Haidian District, Beijing, 10039, China.
Jian Feng, Department of Traditional Chinese Medicine, The Chinese People’s Liberation Army (PLA) 96604 Hospital, Jingningnan Road 72th, Chengguan District, Lanzhou, 730030, China.
Qianyu Yang, Graduate School of Hebei University of Chinese Medicine, Xinshinan Road 326th, Qiaoxi District, Shijiazhuang, Hebei, 050090, China.
Xiao Li, Chinese PLA Medical School, Chinese People’s Liberation Army (PLA) General Hospital, Fuxing Road 28th, Haidian District, Beijing, 10038, China.
Heng Zhu, Department of Experimental Hematology and Biochemistry, Beijing Institute of Radiation Medicine, Taiping Road 27th, Haidian District, Beijing, 10039, China; Beijing Key Laboratory for Radiobiology, Beijing Institute of Radiation Medicine, Taiping Road 27th, Haidian District, Beijing, 10039, China; Beijing Institute of Basic Medical Sciences, Taiping Road 27th, Haidian District, Beijing, 10039, China; Graduate School of Anhui Medical University, Meishan Road 69th, Shushan District, Hefei, Anhui, 230022, China.
Yongqi Dou, Department of Traditional Chinese Medicine, The First Medical Center of Chinese People’s Liberation Army (PLA) General Hospital, Fuxing Road 28th, Haidian District, Beijing, 10038, China; Chinese PLA Medical School, Chinese People’s Liberation Army (PLA) General Hospital, Fuxing Road 28th, Haidian District, Beijing, 10038, China.
REFERENCES
- 1. Chapel A, Francois S, Douay L et al. New insights for pelvic radiation disease treatment: multipotent stromal cell is a promise mainstay treatment for the restoration of abdominopelvic severe chronic damages induced by radiotherapy. World J Stem Cells 2013;5:106–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Qiu W, Leibowitz B, Zhang L, Yu J. Growth factors protect intestinal stem cells from radiation-induced apoptosis by suppressing PUMA through the PI3K/AKT/p53 axis. Oncogene 2010;29:1622–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wei L, Leibowitz BJ, Wang X et al. Inhibition of CDK4/6 protects against radiation-induced intestinal injury in mice. J Clin Invest 2016;126:4076–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bhanja P, Norris A, Gupta-Saraf P et al. BCN057 induces intestinal stem cell repair and mitigates radiation-induced intestinal injury. Stem Cell Res Ther 2018;9:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Maisin JR. Bacq and Alexander award lecture chemical radioprotection: past, present and future prospects. Int J Radiat Biol 1998;73:443–50. [DOI] [PubMed] [Google Scholar]
- 6. Wang A, Wang L, Lu X et al. A Chinese herbal prescription Yiqi Jiedu decoction attenuates irradiation induced testis injury in mice. Biomed Pharmacother 2020;123:109804. [DOI] [PubMed] [Google Scholar]
- 7. Wang Z, Chen Z, Jiang Z et al. Cordycepin prevents radiation ulcer by inhibiting cell senescence via NRF2 and AMPK in rodents. Nat Commun 2019;10:2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liang J-W, Li P-L, Wang Q et al. Ferulic acid promotes bone defect repair after radiation by maintaining the stemness of skeletal stem cells. Stem Cells Transl Med 2021;10:1217–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yin P, Zhang Z, Li J et al. Ferulic acid inhibits bovine endometrial epithelial cells against LPS-induced inflammation via suppressing NK-κB and MAPK pathway. Res Vet Sci 2019;126:164–9. [DOI] [PubMed] [Google Scholar]
- 10. Das U, Manna K, Adhikary A et al. Ferulic acid enhances the radiation sensitivity of lung and liver carcinoma cells by collapsing redox homeostasis: mechanistic involvement of Akt/p38 MAPK signalling pathway. Free Radic Res 2019;53:944–67. [DOI] [PubMed] [Google Scholar]
- 11. Wang Y, Dou Y, Feng J et al. Efficacy of Liangxue Guyuan decoction on radiation-induced intestinal injury in rats via the toll-like receptor 4/myeloid differentiation primary response 88/nuclear factor-kappa B pathway. J Tradit Chin Med 2021;41:254–61. [PubMed] [Google Scholar]
- 12. Withers HR. Regeneration of intestinal mucosa after irradiation. Cancer 1971;28:75–81. [DOI] [PubMed] [Google Scholar]
- 13. Hauer-Jensen M, Wang J, Boerma M et al. Radiation damage to the gastrointestinal tract: mechanisms, diagnosis, and management. Curr Opin Support Palliat Care 2007;1:23–9. [DOI] [PubMed] [Google Scholar]
- 14. Wang H, Wang J, Zhao Y et al. LIF is essential for ISC function and protects against radiation-induced gastrointestinal syndrome. Cell Death Dis 2020;11:588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen F, Zhang Y, Hu S et al. TIGAR/AP-1 axis accelerates the division of Lgr5- reserve intestinal stem cells to reestablish intestinal architecture after lethal radiation. Cell Death Dis 2020;11:501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kalita B, Ranjan R, Gupta ML. Combination treatment of podophyllotoxin and rutin promotes mouse Lgr5+ ve intestinal stem cells survival against lethal radiation injury through Wnt signaling. Apoptosis 2019;24:326–40. [DOI] [PubMed] [Google Scholar]
- 17. Wang S, Han Y, Zhang J et al. Me6TREN targets β-catenin signaling to stimulate intestinal stem cell regeneration after radiation. Theranostics 2020;10:10171–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
-
18.
Dou Y, Yang M, Wei Z et al. The study of early application with Dixiong decoction (
 ) for non-small cell lung cancer to decrease the incidence and severity of radiation pneumonitis: a prospective, randomized clinical trial. Chin J Integr Med 2010;16:411–6. [DOI] [PubMed] [Google Scholar]
) for non-small cell lung cancer to decrease the incidence and severity of radiation pneumonitis: a prospective, randomized clinical trial. Chin J Integr Med 2010;16:411–6. [DOI] [PubMed] [Google Scholar] - 19. Beaufrère AM, Neveux N, Patureau Mirand P et al. Long-term intermittent glutamine supplementation repairs intestinal damage (structure and functional mass) with advanced age: assessment with plasma citrulline in a rodent model. J Nutr Health Aging 2014;18:814–9. [DOI] [PubMed] [Google Scholar]
- 20. Moore SR, Guedes MM, Costa TB et al. Glutamine and alanyl-glutamine promote crypt expansion and mTOR signaling in murine enteroids. Am J Physiol Gastrointest Liver Physiol 2015;308:G831–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chen Y, Tsai Y-H, Tseng B-J, Tseng SH. Influence of growth hormone and glutamine on intestinal stem cells: a narrative review. Nutrients 2019;11:1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
-
22.
Jian F, Yongqi D, Wang Y et al. Effect of modified Xijiao Dihuang decoction (
 ) on intestinal microecology and pro-inflammatory factors after acute intestinal mucosal radiation injury in rats[J/OL]. J Tradit Chin Med 1–8 2023-04-01.36639987 [Google Scholar]
) on intestinal microecology and pro-inflammatory factors after acute intestinal mucosal radiation injury in rats[J/OL]. J Tradit Chin Med 1–8 2023-04-01.36639987 [Google Scholar] - 23. Wei G, Gao N, Chen J et al. Erk and MAPK signaling is essential for intestinal development through Wnt pathway modulation. Development 2020;147:dev185678. [DOI] [PubMed] [Google Scholar]
- 24. Miyamoto J, Mizukure T, Park S-B et al. A gut microbial metabolite of linoleic acid, 10-hydroxy-cis-12-octadecenoic acid, ameliorates intestinal epithelial barrier impairment partially via GPR40-MEK-ERK pathway. J Biol Chem 2015;290:2902–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yang R, Harada T, Li J et al. Bile modulates intestinal epithelial barrier function via an extracellular signal related kinase 1/2 dependent mechanism. Intensive Care Med 2005;31:709–17. [DOI] [PubMed] [Google Scholar]
- 26. Ersahin T, Tuncbag N, Cetin-Atalay R. The PI3K/AKT/mTOR interactive pathway. Mol BioSyst 2015;11:1946–54. [DOI] [PubMed] [Google Scholar]
- 27. Tandon M, Chen Z, Pratap J. Role of Runx2 in crosstalk between Mek/Erk and PI3K/Akt signaling in MCF-10A cells. J Cell Biochem 2014;115:2208–17. [DOI] [PubMed] [Google Scholar]
- 28. Sosa MS, Lopez-Haber C, Yang C et al. Identification of the Rac-GEF P-Rex1 as an essential mediator of ErbB signaling in breast cancer. Mol Cell 2010;40:877–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yang L, Wang R, Gao Y et al. The protective role of interleukin-11 against neutron radiation injury in mouse intestines via MEK/ERK and PI3K/Akt dependent pathways. Dig Dis Sci 2014;59:1406–14. [DOI] [PubMed] [Google Scholar]
- 30. Jeong W-J, Yoon J, Park J-C et al. Ras stabilization through aberrant activation of Wnt/β-catenin signaling promotes intestinal tumorigenesis. Sci Signal 2012;5:ra30. [DOI] [PubMed] [Google Scholar]
- 31. de Jong PR, Taniguchi K, Harris AR et al. ERK5 signalling rescues intestinal epithelial turnover and tumour cell proliferation upon ERK1/2 abrogation. Nat Commun 2016;7:11551. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


