Abstract
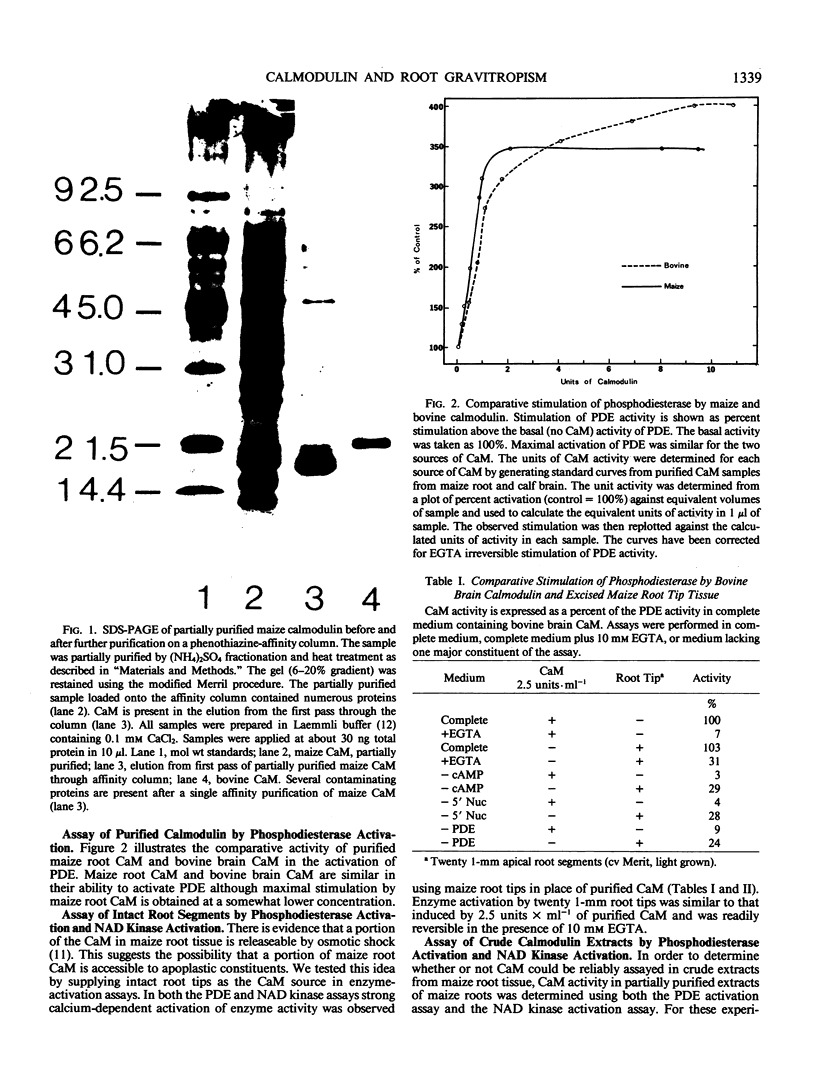
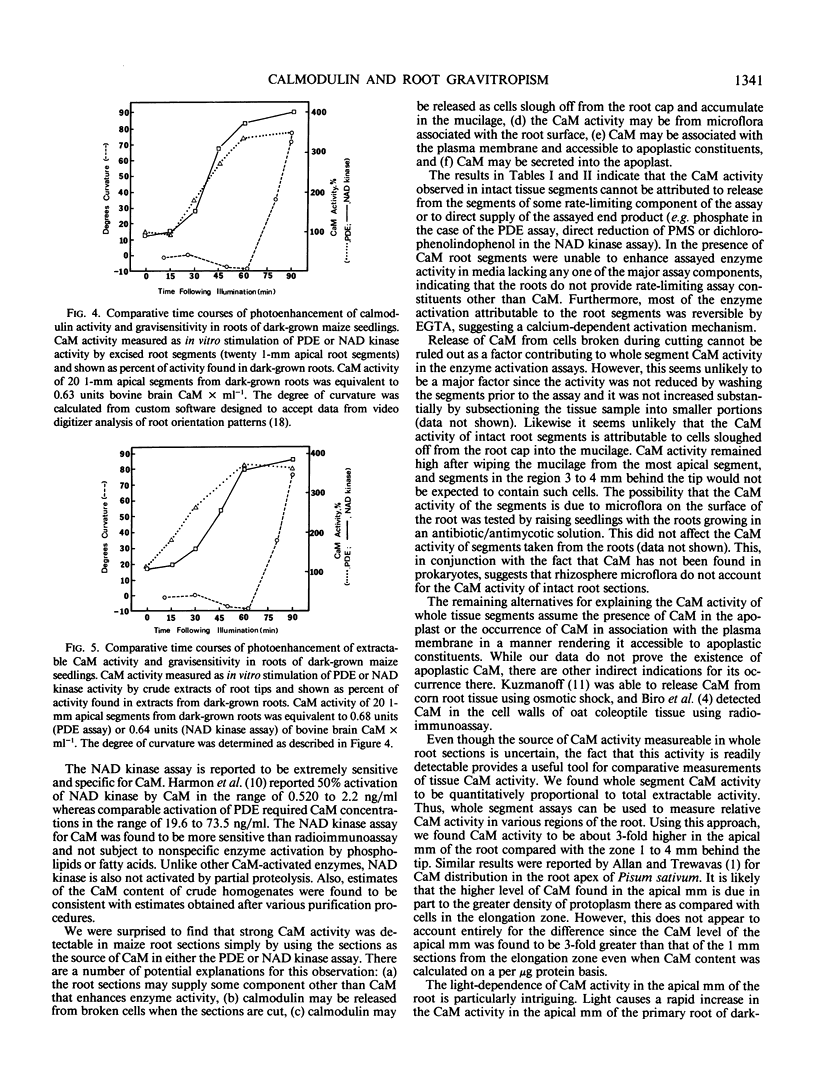
Recent evidence indicates a role for calcium and calmodulin in the gravitropic response of primary roots of maize (Zea mays, L.). We examined this possibility by testing the relationship between calmodulin activity and gravitropic sensitivity in roots of the maize cultivars Merit and B73 × Missouri 17. Roots of the Merit cultivar require light to be gravitropically competent. The gravitropic response of the Missouri cultivar is independent of light. The occurrence of calmodulin in primary roots of these maize cultivars was tested by affinity gel chromatography followed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis with bovine brain calmodulin as standard. The distribution of calmodulin activity was measured using both the phosphodiesterase and NAD kinase assays for calmodulin. These assays were performed on whole tissue segments, crude extracts, and purified extracts. In light-grown seedlings of the Merit cultivar or in either dark- or light-grown seedlings of the Missouri cultivar, calmodulin activity per millimeter of root tissue was about 4-fold higher in the apical millimeter than in the subtending 3 millimeters. Calmodulin activity was very low in the apical millimeter of roots of dark-grown (gravitropically nonresponsive) seedlings of the Merit cultivar. Upon illumination, the calmodulin activity in the apical millimeter increased to a level comparable to that of light-grown seedlings and the roots became gravitropically competent. The time course of the development of gravitropic sensitivity following illumination paralleled the time course of the increase in calmodulin activity in the apical millimeter of the root. The results are consistent with the suggestion that calmodulin plays an important role in the gravitropic response of roots.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biro R. L., Daye S., Serlin B. S., Terry M. E., Datta N., Sopory S. K., Roux S. J. Characterization of oat calmodulin and radioimmunoassay of its subcellular distribution. Plant Physiol. 1984 Jun;75(2):382–386. doi: 10.1104/pp.75.2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Dauwalder M., Roux S. J., Hardison L. Distribution of calmodulin in pea seedlings: immunocytochemical localization in plumules and root apices. Planta. 1986;168:461–470. [PubMed] [Google Scholar]
- Daye S., Biro R. L., Roux S. J. Inhibition of gravitropism in oat coleoptiles by the calcium chelator, ethyleneglycol-bis-(beta-aminoethyl ether)-N,N'-tetraacetic acid. Physiol Plant. 1984 Jul;61(3):449–454. doi: 10.1111/j.1399-3054.1984.tb06354.x. [DOI] [PubMed] [Google Scholar]
- Feldman L. J., Gildow V. Effects of light on protein patterns in gravitropically stimulated root caps of corn. Plant Physiol. 1984 Feb;74(2):208–212. doi: 10.1104/pp.74.2.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon A. C., Jarrett H. W., Cormier M. J. An enzymatic assay for calmodulins based on plant NAD kinase activity. Anal Biochem. 1984 Aug 15;141(1):168–178. doi: 10.1016/0003-2697(84)90441-x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee J. S., Evans M. L. Polar transport of 45Ca2+ across the elongation zone of gravistimulated roots. Plant Cell Physiol. 1985;26(8):1587–1595. [PubMed] [Google Scholar]
- Lee J. S., Mulkey T. J., Evans M. L. Gravity-Induced Polar Transport of Calcium across Root Tips of Maize. Plant Physiol. 1983 Dec;73(4):874–876. doi: 10.1104/pp.73.4.874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. S., Mulkey T. J., Evans M. L. Reversible loss of gravitropic sensitivity in maize roots after tip application of calcium chelators. Science. 1983 Jun 24;220(4604):1375–1376. doi: 10.1126/science.220.4604.1375. [DOI] [PubMed] [Google Scholar]
- Merril C. R., Goldman D., Sedman S. A., Ebert M. H. Ultrasensitive stain for proteins in polyacrylamide gels shows regional variation in cerebrospinal fluid proteins. Science. 1981 Mar 27;211(4489):1437–1438. doi: 10.1126/science.6162199. [DOI] [PubMed] [Google Scholar]
- Nelson A. J., Evans M. L. Analysis of growth patterns during gravitropic curvature in roots of Zea mays by use of a computer-based video digitizer. J Plant Growth Regul. 1986;5:73–83. doi: 10.1007/BF02025958. [DOI] [PubMed] [Google Scholar]
- Sharma R. K., Wang J. H. Preparation and assay of the Ca2+--dependent modulator protein. Adv Cyclic Nucleotide Res. 1979;10:187–198. [PubMed] [Google Scholar]
- Watterson D. M., Iverson D. B., Van Eldik L. J. Spinach calmodulin: isolation, characterization, and comparison with vertebrate calmodulins. Biochemistry. 1980 Dec 9;19(25):5762–5768. doi: 10.1021/bi00566a015. [DOI] [PubMed] [Google Scholar]