Significance
In plants, SERRATE (SE) phase separates to drive dicing body (D-body) assembly and promote miRNA processing. However, how SE phase separation is regulated remains unknown. Here, we identified that Cyclophilin71 (CYP71), a peptidyl-prolyl isomerase (PPIase), positively regulates miRNA processing through a forward genetic screening. Interestingly, CYP71 interacts with SE and enhances its phase separation to promote the formation of D-bodies and increase the activity of the Dicing complex, thereby promoting miRNA processing. The function of CYP71 in miRNA processing is dependent on its PPIase activity. These findings reveal orchestration of miRNA processing by a cyclophilin protein and suggest regulation of SE phase separation by peptidyl-prolyl cis–trans isomerization, a structural mechanism.
Keywords: miRNA, phase separation, Dicing complex, Cyclophilin71, PPIase
Abstract
MicroRNAs (miRNAs) play an important role in gene regulation. In Arabidopsis, mature miRNAs are processed from primary miRNA transcripts by the Dicing complex that contains Dicer-like 1 (DCL1), SERRATE (SE), and Hyponastic Leaves 1 (HYL1). The Dicing complex can form nuclear dicing bodies (D-bodies) through SE phase separation. Here, we report that Cyclophilin71 (CYP71), a peptidyl-prolyl isomerase (PPIase), positively regulates miRNA processing. We show that CYP71 directly interacts with SE and enhances its phase separation, thereby promoting the formation of D-body and increasing the activity of the Dicing complex. We further show that the PPIase activity is important for the function of CYP71 in miRNA production. Our findings reveal orchestration of miRNA processing by a cyclophilin protein and suggest the involvement of peptidyl-prolyl cis–trans isomerization, a structural mechanism, in SE phase separation and miRNA processing.
MicroRNAs (miRNAs) are 21–24-nucleotide (nt) small RNAs that play important regulatory roles in plant development and responses to biotic and abiotic stresses (1, 2). 21-nt miRNAs are associated with ARGONAUTE 1 (AGO1) to down-regulate target gene expression through mRNA cleavage and/or translation inhibition (3), whereas 24-nt long miRNAs are associated with AGO4 to repress target gene transcription through DNA methylation (4, 5).
In plants, the biogenesis of 21-nt miRNAs entails the transcription of MIR genes by RNA Polymerase II (Pol II) to generate primary miRNA transcripts (pri-miRNAs) that contain stem-loop structures (6, 7). The majority of pri-miRNAs are processed from base to loop, although some are processed from loop to base or bidirectionally (8, 9). pri-miRNAs are cotranscriptionally or posttranscriptionally cleaved by Dicer-like 1 (DCL1) into stem-loop-structured precursor miRNAs (pre-miRNAs), which are further cleaved by DCL1 into 21-nt miRNAs (10–12). DCL1 interacts with a double-stranded RNA-binding protein Hyponastic Leaves 1 (HYL1) (13, 14) and a zinc-finger protein SERRATE (SE) (15, 16) and forms dicing bodies (D-bodies) in the nucleoplasm (17, 18). Recently, we have shown that SE drives the formation of D-bodies through liquid–liquid phase separation. Pri-miRNAs are processed into mature miRNAs at a high efficiency in D-bodies, and after processing, miRNAs are bound by HYL1 and released from D-bodies (19). How D-body formation and its role in miRNA biogenesis are regulated remains elusive.
Cyclophilins, a subgroup of immunophilins identified as cellular targets of the immunosuppressive drug Cyclosporin A (CsA), are conserved in diverse organisms, including microbes, animals, and plants (20, 21). Many cyclophilins have peptidyl-prolyl isomerase (PPIase) activity and catalyze the cis–trans isomerization of peptide bonds preceding proline residues. Cyclophilins can regulate gene transcription and splicing, modulate the functions of proteins and participate in signal transduction (20, 21). In Arabidopsis thaliana, there are at least 29 cyclophilins (22). They are localized in different cellular compartments and regulate plant growth and development, hormonal signaling, and stress responses (21). Cyclophilin71 (CYP71) is a nucleus-localized cyclophilin. It facilitates H3K27me3 deposition at homeotic genes and represses their expression (23), which requires its PPIase activity (24). It is also involved in the recruitment of FASCIATA1 (FAS1) and Like Heterochromatin Protein1 (LHP1) to chromatin for the maintenance of chromatin remodeling and transcriptional silencing (25).
In this study, we found that CYP71 promotes miRNA biogenesis. CYP71 facilitates SE phase separation and D-body formation to enhance Dicing complex activity, thus promoting miRNA processing. The function of CYP71 in miRNA processing requires its PPIase activity. We propose that CYP71-catalyzed peptidyl-prolyl isomerization of SE regulates its phase separation and miRNA processing.
Results
CYP71 Is Required for miRNA Production in Arabidopsis.
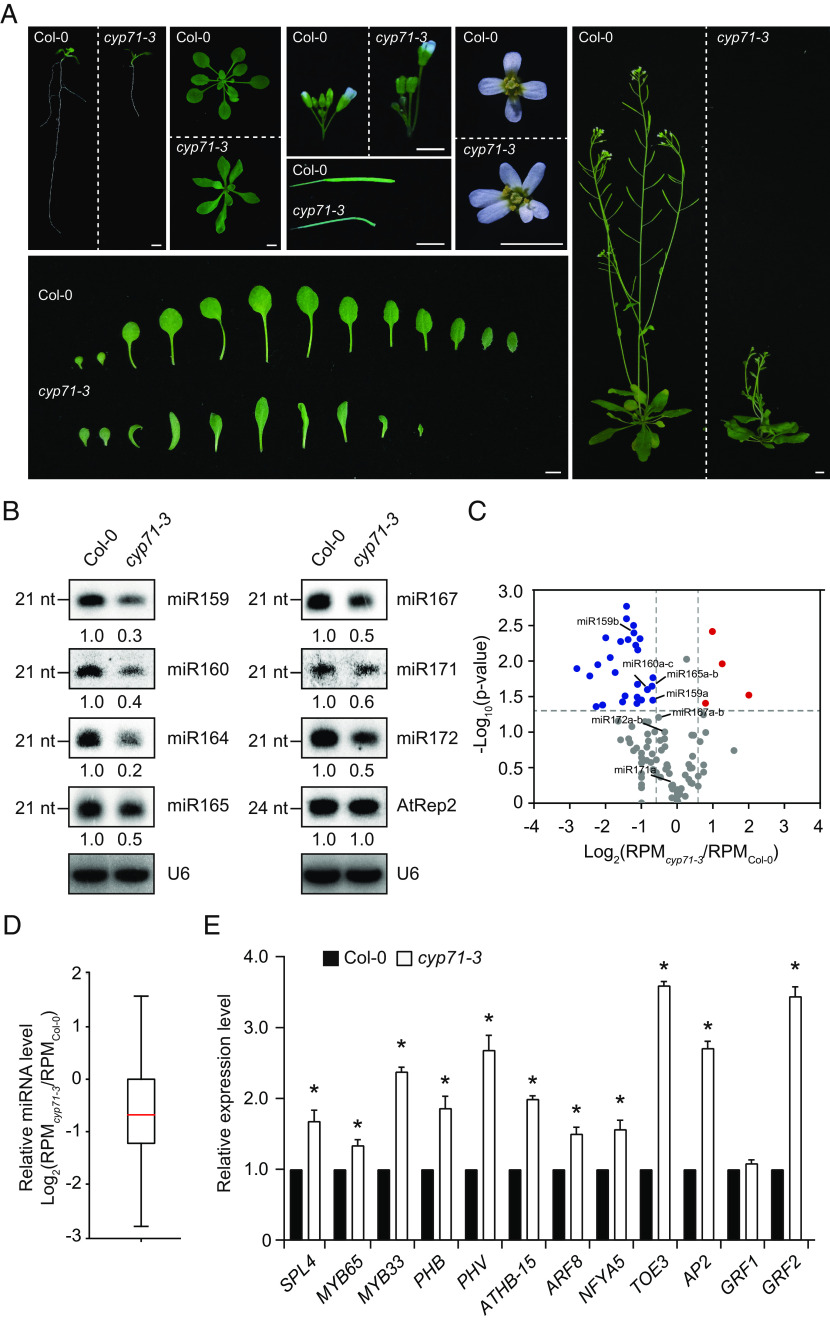
We have previously used ema1-1, which has highly clustered trichomes due to enhanced activity of an artificial miRNA (amiR-trichome) (26), for a suppressor screen and identified the suppressor of ema1 6 (soe6) mutant (10). Trichomes became nonclustered on the leaves of soe6 seedlings (SI Appendix, Fig. S1A). In soe6, the accumulation of amiR-trichome and multiple endogenous miRNAs was reduced (SI Appendix, Fig. S1B), and correspondingly, their target genes were derepressed (SI Appendix, Fig. S1C). Map-based cloning and whole-genome sequencing revealed that soe6 carries a C to T transition in the fifth exon of CYP71 (AT3G44600), which creates a premature stop codon (SI Appendix, Fig. S1D). Expression of full-length CYP71 complemented the phenotypes of soe6 (SI Appendix, Fig. S1 A–C). These results suggest that CYP71 positively regulates miRNA production.
To further confirm the role of CYP71 in miRNA production, soe6 was backcrossed to wild-type Col-0 three times to remove the amiR-trichome transgene and the ema1-1 mutation, and the resulting mutant was designated as cyp71-3 (SI Appendix, Fig. S1D). cyp71-3 exhibited pleiotropic developmental defects, including retardation of root and shoot growth, development of narrow and deformed leaves, premature termination of primary inflorescence, development of flowers with fused petals, and reduced fertility (Fig. 1A). These phenotypes were similar to those displayed by the T-DNA insertional mutants cyp71-1 and cyp71-2 (23). In cyp71-3 and cyp71-2, the expression level of CYP71 was decreased by 90% and 80%, respectively (SI Appendix, Fig. S2A). Like in soe6 seedlings, the accumulation of multiple endogenous miRNAs, including miR159, miR160, miR164, miR165, miR167, miR171, and miR172, was markedly reduced in cyp71-3 and cyp71-2 (Fig. 1B and SI Appendix, Fig. S2B). To provide a global view of miRNAs affected by loss of CYP71, we performed small RNA sequencing using Col-0, cyp71-3, and cyp71-2 seedlings (Datasets S1 and S2). The results showed that many miRNAs had reduced accumulation in cyp71-3 and cyp71-2 (Fig. 1 C and D and SI Appendix, Fig. S2 C and D). In total, we identified 20 and 17 miRNAs that had significantly reduced accumulation (Fold change ≥ 1.5, P < 0.05) in cyp71-3 and cyp71-2, with 16 being overlapping (SI Appendix, Fig. S2E and Dataset S2). A few miRNAs somehow had increased accumulation in cyp71-3 and cyp71-2 (SI Appendix, Fig. S2E and Dataset S2).
Fig. 1.
CYP71 positively regulates miRNA production. (A) Pleiotropic developmental defects of the cyp71-3 mutant. (Scale bars, 5 mm.) (B) Northern blot results showing miRNA accumulation levels in Col-0 and cyp71-3. U6 was probed as loading controls. The signals are quantified, and relative intensities are shown. See SI Appendix, Fig. S2B, for one additional replicate. (C) Volcano plot of the differentially expressed miRNAs in cyp71-3 as determined by small RNA sequencing. miRNAs showing ≥1.5-fold reduction and increase in cyp71-3 (P < 0.05, Welch’s t test) are highlighted in blue and red, respectively, and representative miRNAs are indicated. (D) Box plot showing the log2-transformed fold change of miRNA abundances in cyp71-3 as determined by small RNA sequencing. The horizontal mark indicates the median, and the bottom and top edges of the box indicate the 25th and 75th percentiles, respectively. The whiskers extend to the most extreme data points. (E) qRT-PCR analysis of the expression levels of miRNA targets in Col-0 and cyp71-3. Error bars represent SD (n = 3). Asterisks indicate significant differences (P < 0.05, t test).
To assess the global effects of cyp71 mutations on miRNA target gene expression, we performed RNA sequencing using Col-0, cyp71-3, and se-1 seedlings (Datasets S1 and S3). We found that 64 miRNA target genes were significantly derepressed (Q < 0.05) in se-1. Thirty eight were significantly derepressed (Q < 0.05) in cyp71-3, with 25 being overlapping with those derepressed in se-1 (SI Appendix, Fig. S2F and Dataset S3). Statistical analysis revealed that the sharing is much greater than expected by chance (P < 0.05). The derepression of a panel of miRNA target genes was confirmed by qRT-PCR (Fig. 1E).
These results together demonstrate a role for CYP71 in miRNA production.
CYP71 Does Not Regulate the Transcription of MIR Loci.
We next sought to investigate which step CYP71 regulates in the miRNA biogenesis pathway. We first detected the levels of pri-miRNAs in cyp71-3 by performing qRT-PCR. We found that the levels of multiple pri-miRNAs were elevated in cyp71-3 (Fig. 2A). Disruption of CYP71 function may up-regulate gene transcription, as CYP71 mediates H3K27me3-dependent gene silencing and chromatin remodeling (23, 25). Thus, it was possible that the increase in pri-miRNA levels in cyp71-3 was a result of enhanced MIR transcription. To test this, we analyzed the effect of the cyp71-3 mutation on the activities of MIR159b and MIR167a promoters, which were fused to a GUS reporter gene. As measured by histochemical staining and qRT-PCR, GUS expression levels were comparable in the wild-type and cyp71-3 plants (Fig. 2B and SI Appendix, Fig. S3A). We further examined the occupancy of Pol II at multiple MIR loci by performing chromatin immunoprecipitation (ChIP). No alteration of Pol II occupancy at these MIR loci was observed in cyp71-3 (Fig. 2C and SI Appendix, Fig. S3 B and C). We finally performed the nuclear run-on assay to directly measure the transcription activities in Col-0 and cyp71-3 seedlings. We found that the transcription rate of MIR genes in cyp71-3 was not different from that in Col-0 (Fig. 2D). Our data together suggest that CYP71 does not regulate MIR transcription.
Fig. 2.
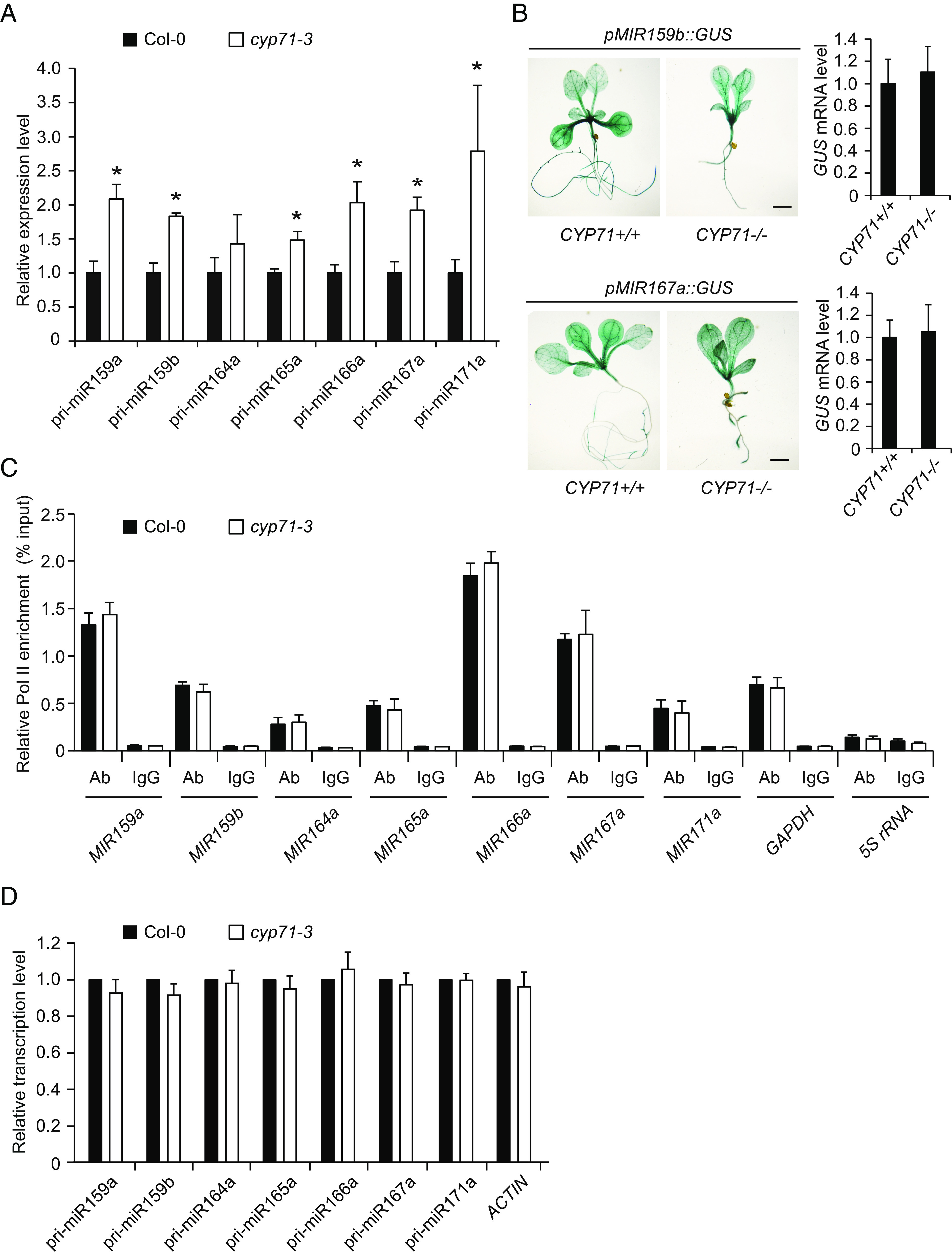
CYP71 does not regulate MIR transcription. (A) qRT-PCR analysis of pri-miRNA levels in Col-0 and cyp71-3. Error bars represent SD (n = 3). Asterisks indicate significant differences (P < 0.05, t test). (B) Representative GUS staining images of 2-wk-old pMIR159b::GUS and pMIR167a::GUS transgenic plants in the wild-type (CYP71+/+) and mutant (CYP71−/−) backgrounds, respectively. (Scale bars, 2 mm.) GUS mRNA levels were determined by qRT-PCR. Error bars represent SD (n = 3). No significant difference was detected (P < 0.05, t test). See SI Appendix, Fig. S3A, for additional GUS staining images. (C) ChIP analysis of Pol II occupancy at the indicated MIR loci in Col-0 and cyp71-3. Error bars represent SD (n = 3). No significant difference was detected (P < 0.05, t test). (D) Transcription rates of MIR genes in Col-0 and cyp71-3 as measured by nuclear run-on. The transcription rates of MIR genes were normalized against GAPDH. Error bars represent SD (n = 3). No significant difference was detected (P < 0.05, t test).
CYP71 Interacts with the Dicing Complex.
The facts that pri-miRNA levels were elevated, miRNA accumulation was reduced, but MIR transcription remained unaltered in cyp71-3 suggest that CYP71 may positively regulate miRNA processing. We thus examined whether CYP71 interacts with the components of the Dicing complex. We first performed bimolecular fluorescence complementation (BiFC) assay in Nicotiana benthamiana leaves. As previously reported (10, 17), the interactions between DCL1, HYL1, and SE were primarily detected in D-bodies (Fig. 3A and SI Appendix, Fig. S4A). CYP71 was found to interact with DCL1, HYL1, and SE in D-bodies, as well as in the nucleoplasm (Fig. 3A and SI Appendix, Fig. S4A). In yeast two-hybrid assay, however, the interaction was only detected between CYP71 and SE (Fig. 3B and SI Appendix, Fig. S4B), suggesting direct binding of CYP71 to SE. Supporting the yeast two-hybrid results, in the in vitro pulldown experiments using recombinant proteins, CYP71 interacted with SE, but not with DCL1 and HYL1 (Fig. 3C and SI Appendix, Fig. S4C). However, when we incubated CYP71 with in vitro reconstituted Dicing complex that contains DCL1, HYL1, and SE and performed DCL1 pulldown, CYP71 was retrieved as SE and HYL1 (Fig. 3D and SI Appendix, Fig. S4C). SE drives the formation of D-bodies through liquid–liquid phase separation (19). When we incubated Cy5-labeled Flag-CYP71 with in vitro reconstituted D-bodies that contained BFP-SE, DCL1-GFP, and mCherry-HYL1, we found that CYP71 was incorporated into D-bodies (Fig. 3E and SI Appendix, Fig. S4C). These results suggest that CYP71 is associated with the Dicing complex via SE.
Fig. 3.
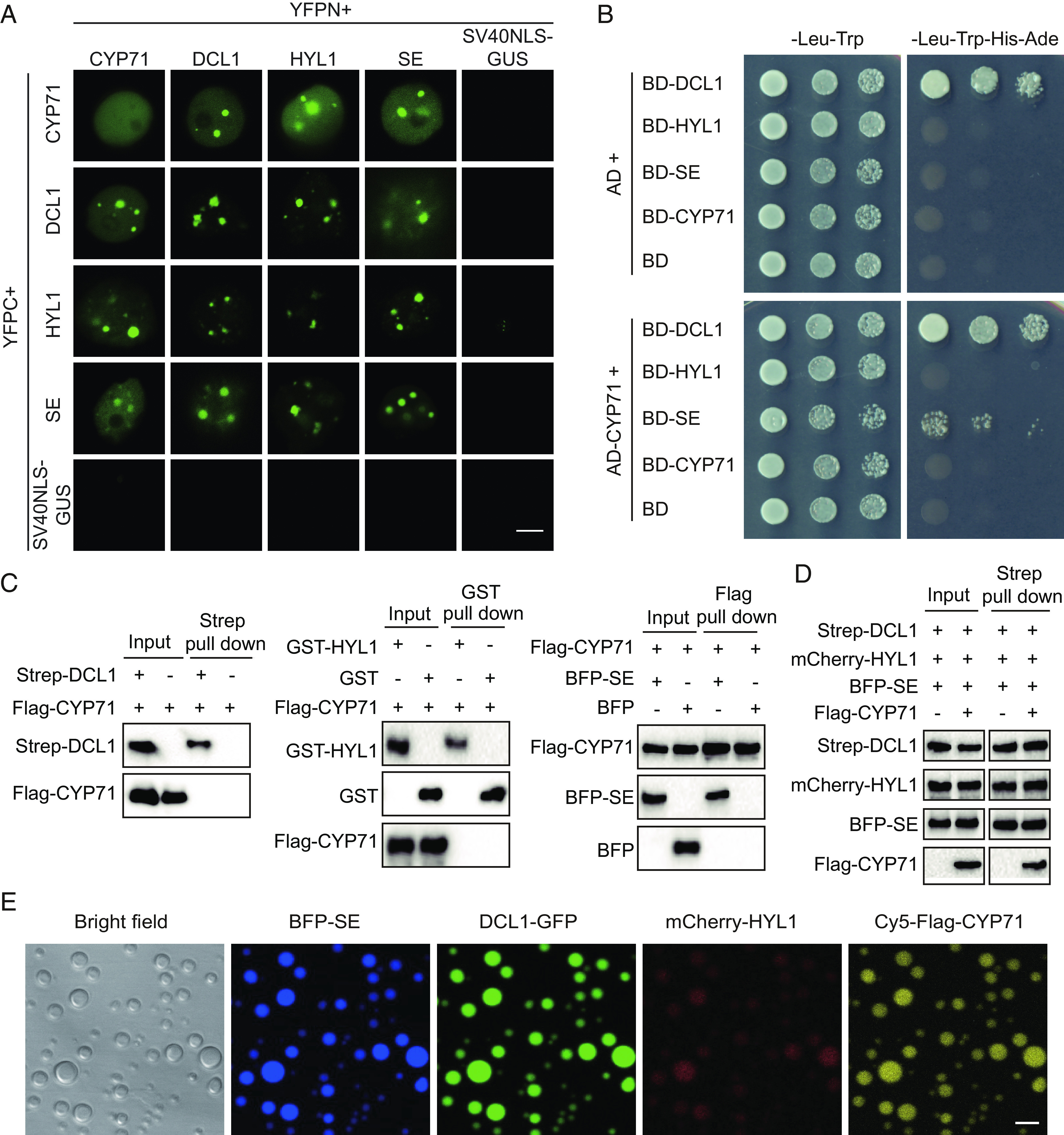
CYP71 is associated with the Dicing complex via SE. (A) Detection of the interactions between CYP71 and Dicing complex components in N. benthamiana leaves by BiFC assay. SV40NLS-GUS was used as a negative control. (Scale bar, 10 μm.) See SI Appendix, Fig. S4A, for additional replicate. (B) Detection of the interactions between CYP71 and Dicing complex components by yeast two-hybrid assay. AD, GAL4-activation domain; BD, GAL4-DNA-binding domain. See SI Appendix, Fig. S4B, for additional replicate. (C) Detection of the interactions between CYP71 and Dicing complex components by in vitro pulldown assay. (D) Detection of the association of CYP71 with the Dicing complex by in vitro pulldown assay. (E) Representative images showing the incorporation of Cy5-labeled Flag-CYP71 into reconstituted D-bodies. D-bodies were reconstituted with DCL1-GFP (0.05 μM), mCherry-HYL1 (0.1 μM), BFP-SE (2 μM), and pre-miR172b (0.3 μM). (Scale bar, 5 μm.)
CYP71 Promotes SE Phase Separation and D-body Formation.
The interaction of CYP71 with SE prompted us to examine whether CYP71 has an effect on phase separation of SE and the formation of D-bodies. We assessed the ability of BFP-SE to condense into droplets in the absence or presence of Cy5-labeled Flag-CYP71. We found that the addition of Cy5-labeled Flag-CYP71 significantly increased BFP-SE droplet formation, suggesting that CYP71 can facilitate SE phase separation (Fig. 4 A and B). When D-bodies were reconstituted using various concentrations of BFP-SE in the absence or presence of Cy5-labeled Flag-CYP71, we found that the addition of CYP71 significantly increased the formation of reconstituted D-bodies (Fig. 4 C and D), suggesting that CYP71 promotes D-body formation in vitro.
Fig. 4.
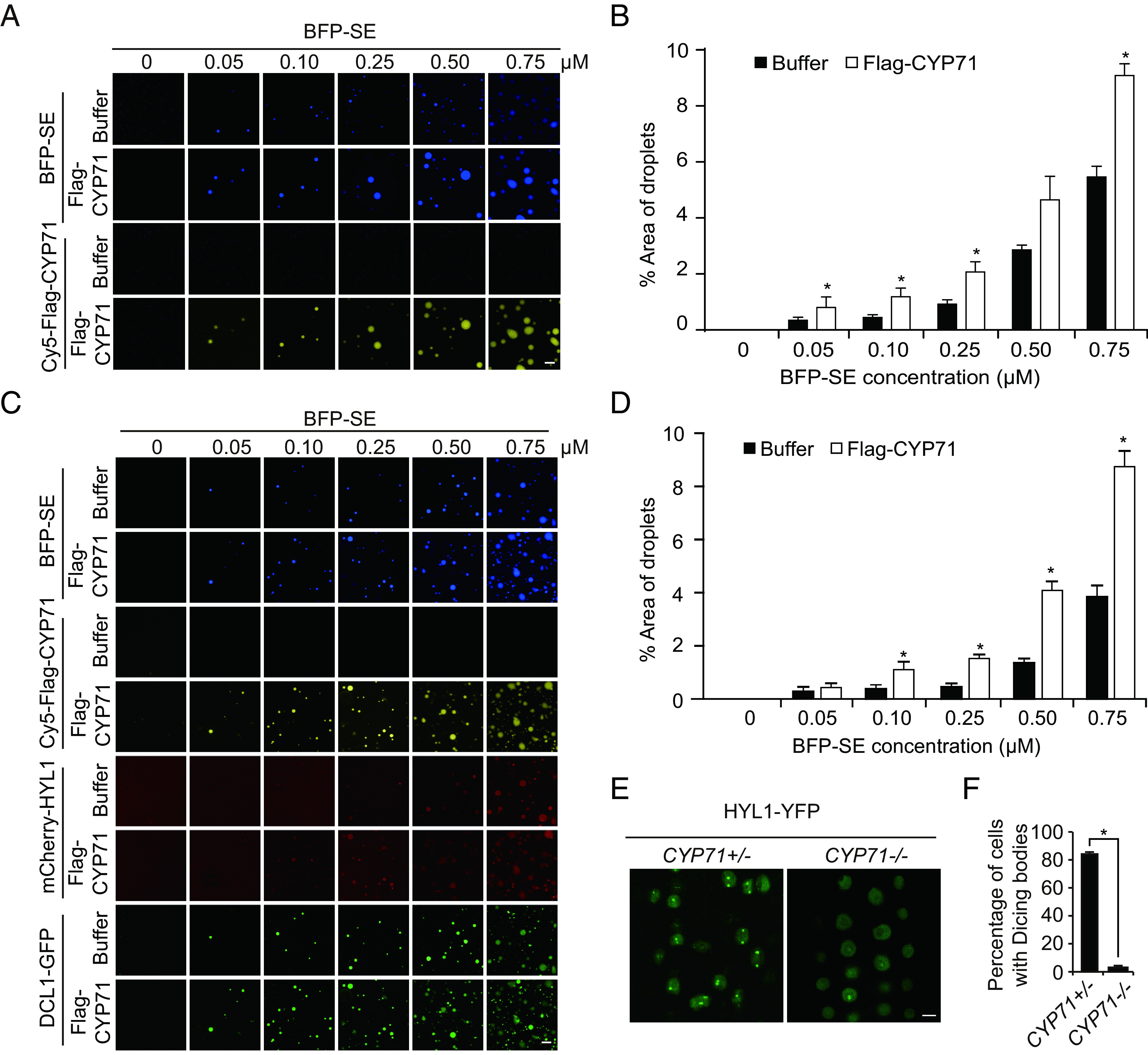
CYP71 promotes SE phase separation, D-body formation, and Dicing complex activity. (A) Representative images showing SE droplet formation at increasing concentrations (0 to 0.75 μM) of BFP-SE in the absence or presence of Cy5-labeled Flag-CYP71 (0.25 μM). (Scale bar, 5 μm.) (B) Percentage of the area occupied by SE droplets in a field of view in the absence or presence of Cy5-labeled Flag-CYP71 (0.25 μM). Error bars represent SD (n = 3). Asterisks indicate significant differences (P < 0.05, t test). (C) Representative images showing the formation of reconstituted D-bodies in the absence or presence of Cy5-labeled Flag-CYP71 (0.25 μM). D-bodies were reconstituted with DCL1-GFP (0.05 μM), mCherry-HYL1 (0.1 μM), BFP-SE (0 to 0.75 μM), and pre-miR172b (0.1 μM). (Scale bar, 5 μm.) (D) Percentage of the area occupied by reconstituted D-bodies in a field of view in the absence or presence of Cy5-labeled Flag-CYP71 (0.25 μM). Error bars represent SD (n = 3). Asterisks indicate significant differences (P < 0.05, t test). (E) Representative images of D-bodies in root tip cells of the wild-type Col-0 and cyp71-3 mutant. (Scale bar, 5 μm.) D-bodies are marked with HYL1-YFP. (F) Percentages of nuclei containing D-bodies in different genotypes. Six root tips were analyzed. More than 50 nuclei in each root tip were examined. Data are expressed as mean ± SD. The asterisk indicates a significant difference (P < 0.05, t test). See SI Appendix, Fig. S4D, for source data.
To examine whether CYP71 plays a role in D-body formation in vivo, the cyp71-3 mutation was introduced into a transgenic line expressing HYL1-YFP which marks D-bodies (17), by genetic crossing. As SE plays a broad role in RNA metabolism and other processes (27–30) and is distributed in a heterogeneous subnuclear pattern depending on the cell type (17), SE was not chosen to mark D-bodies. We found that most root cells contain one or two D-bodies in the wild-type plants (Fig. 4 E and F and SI Appendix, Fig. S4D), as previously reported (17, 19). However, in cyp71-3 plants, the majority of root cells lacked discernable D-bodies (Fig. 4 E and F and SI Appendix, Fig. S4D). Transgenic HYL1-YFP was expressed at comparable levels in wild-type and cyp71-3 plants (SI Appendix, Fig. S4E), excluding the possibility that failure of D-body formation in cyp71-3 is caused by defects in HYL1-YFP expression. These data together indicate a role for CYP71 in D-body formation.
The PPIase Activity Is Important for CYP71 to Promote SE Phase Separation and D-body Formation.
CYP71 contains an N-terminal WD40 repeat domain and a C-terminal CYCLOPHILIN domain (SI Appendix, Fig. S5A). The CYCLOPHILIN domain, also known as the PPIase domain, harbors PPIase activity (24). To investigate the importance of the PPIase activity for CYP71 to function, we sought to generate CYP71 variants that lose the PPIase activity. Sequence alignment of the CYCLOPHILIN domains of CYP71 homologues and human Cyclophilin A, the prototypical member of the cyclophilin family enzymes (31, 32), revealed that R55 and F60, two residues essential for the PPIase activity of CypA (33), are highly conserved among CYP71 homologues across kingdoms (SI Appendix, Fig. S5B). The corresponding residues, R519 and F524 in CYP71, were therefore subjected to site-directed mutagenesis (SI Appendix, Fig. S5 A and B). Notably, although the cyp71-2 mutation is located after the R519 and R524 residues (SI Appendix, Fig. S1D), the functions of these two residues were also disrupted in cyp71-2, like in cyp71-3, because the expression level of CYP71 was remarkably decreased in these mutants, likely due to non-sense-mediated mRNA decay (SI Appendix, Fig. S2A). Results of the peptidyl-prolyl isomerization assay validated that the PPIase domains of CYP71R519A and CYP71F524A had reduced PPIase activity (SI Appendix, Fig. S5 C–E). In vitro pulldown experiments showed that the interactions of CYP71R519A and CYP71F524A with SE were not compromised compared to that of CYP71 (Fig. 5A).
Fig. 5.
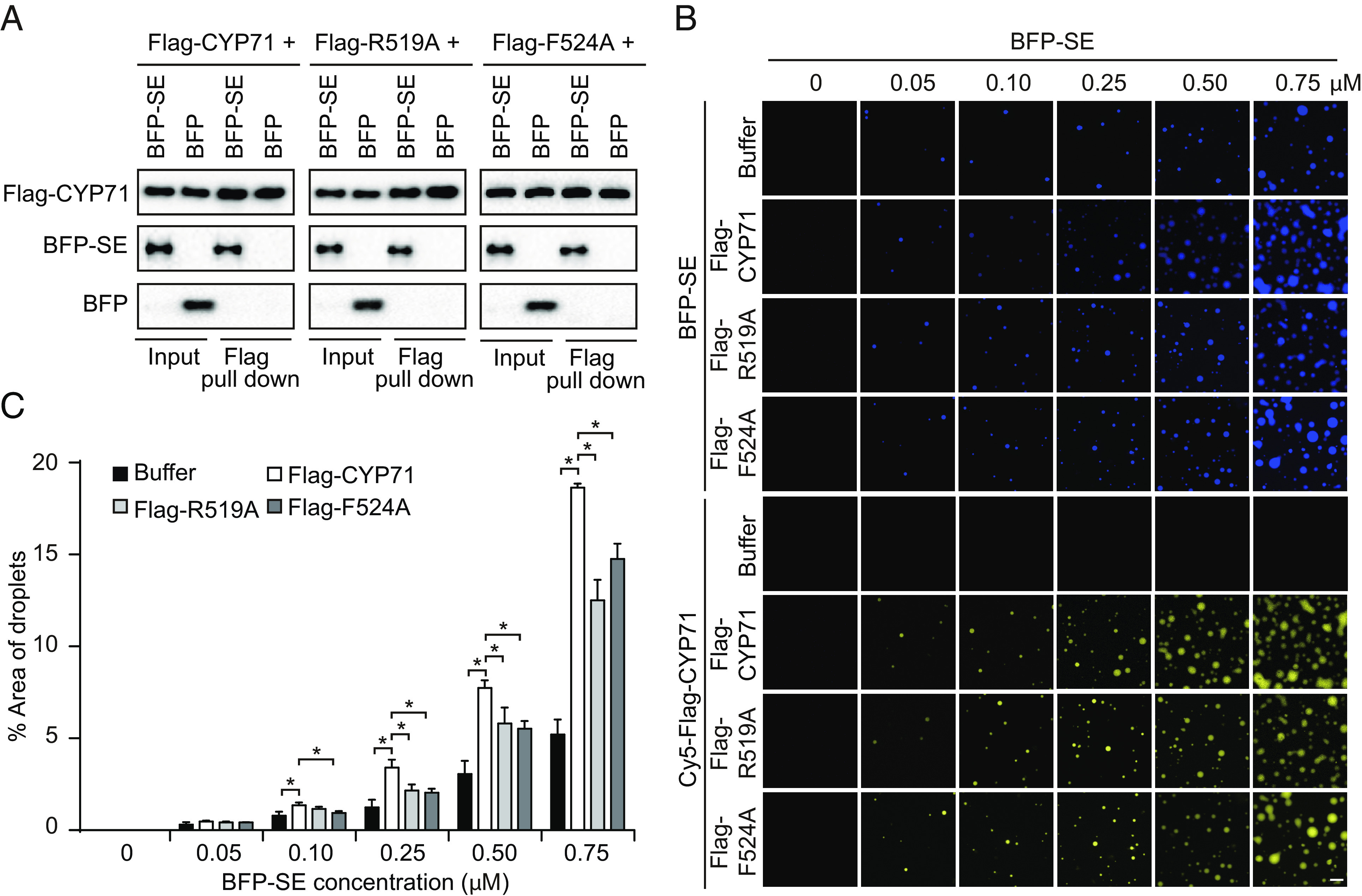
PPIase activity is important for CYP71 to promote SE phase separation and D-body formation. (A) Detection of the interactions of Flag-CYP71, Flag-CYP71R519A (Flag-R519A), or Flag-CYP71F524A (Flag-F524A) with BFP-SE by in vitro pulldown assay. (B) Representative images showing SE droplet formation at increasing concentrations (0 to 0.75 μM) of BFP-SE in the presence of Cy5-labeled Flag-CYP71, Flag-CYP71R519A (Flag-R519A), or Flag-CYP71F524A (Flag-F524A) (0.25 μM). (Scale bar, 5 μm.) (C) Percentage of the area occupied by droplets in a field of view in the presence of Cy5-labeled Flag-CYP71, Flag-CYP71R519A (Flag-R519A) or Flag-CYP71F524A (Flag-F524A) (0.25 μM). Error bars represent SD (n = 3). Asterisks indicate significant differences (P < 0.05, t test).
We next examined whether the PPIase activity of CYP71 is important for CYP71 to promote SE phase separation. We measured the levels of BFP-SE droplet formation at various concentrations with the supplement of Cy5-labeled Flag-CYP71, Flag-CYP71R519A or Flag-CYP71F524A. The results showed that Flag-CYP71R519A or Flag-CYP71F524A had weaker ability to promote SE droplet formation compared to Flag-CYP71 (Fig. 5 B and C). Similarly, we found that Flag-CYP71R519A and Flag-CYP71F524A had reduced ability to promote the formation of D-bodies (SI Appendix, Fig. S6 A and B). Together, our data suggest that the PPIase activity is important for CYP71 to promote SE phase separation and D-body formation.
The PPIase Activity of CYP71 Enhances Dicing Complex Activity.
SE phase separation and D-body formation is required for highly efficient miRNA processing (19). We next investigated the importance of CYP71 for miRNA processing by performing in vitro dicing activity assay. Cy5-internally-labeled pre-miR172b was incubated with the in vitro reconstituted Dicing complex (DCL1-GFP, mCherry-HYL1, and BFP-SE) in the absence or presence of Flag-CYP71. The percentage of pre-miR172b that is processed into miR172b/* was measured at multiple time points (Fig. 6A and SI Appendix, Fig. S7). The results showed that the percentage of pre-miR172b that is processed into miR172b/* was significantly higher when CYP71 was added, especially at later time points (Fig. 6B). We calculated the initial processing rate based on the results and found that the initial processing rate of the Dicing complex was enhanced ~1.4 fold by Flag-CYP71 (Fig. 6C). Our results suggest that CYP71 promotes Dicing complex activity. To determine whether the PPIase activity of CYP71 is important for CYP71 to boost Dicing complex activity, we compared the activity of Flag-CYP71, Flag-CYP71R519A, and Flag-CYP71F524A in in vitro dicing activity assay (Fig. 6A and SI Appendix, Fig. S7). We found that Flag-CYP71R519A and Flag-CYP71F524A were barely able to increase the percentage of pri-miR172b that is processed into miR172b/* at each time point (Fig. 6B). Thus, the initial processing rate of the Dicing complex was barely enhanced by Flag-CYP71R519A and Flag-CYP71F524A (Fig. 6C). These results suggest that the PPIase activity of CYP71 is important for CYP71 to boost Dicing complex activity.
Fig. 6.
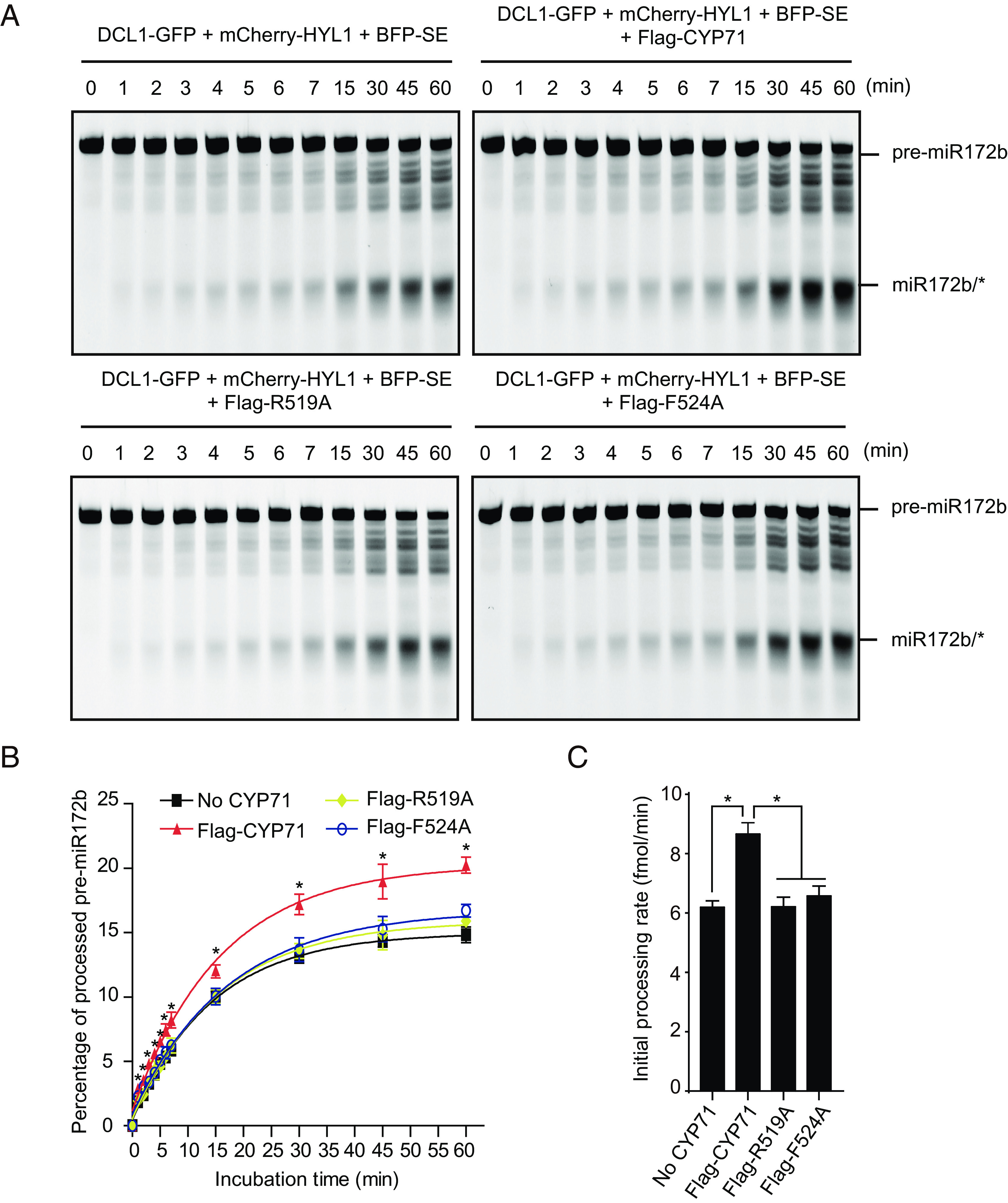
PPIase activity of CYP71 is important for miRNA processing in vitro. (A) Gel image of pre-miR172 processing by the Dicing complex without or with Flag-CYP71 or its variants at different time points. DCL1-GFP (0.05 μM), mCherry-HYL1 (0.1 μM), and BFP-SE (0.2 μM) were incubated with pre-miR172b (0.03 μM) in the absence or presence of Flag-CYP71 (0.5 μM), Flag-CYP71R519A (Flag-R519A) (0.5 μM) or Flag-CYP71F524A (Flag-F524A) (0.5 μM). See SI Appendix, Fig. S7, for two additional replicates. (B) Curves showing the percentages of pre-miR172b that is processed into miR172b/* by the Dicing complex without or with Flag-CYP71 or its variants at different time points. Data were fit to the exponential equation S=(a-b)exp(-kobsdt)+b. S represents the fraction of pre-miR172b cleaved at each time point, a and b are the fractions of pre-miR172b cleaved at the beginning and plateau (t → ∞) stages of the reaction, respectively. kobsd is the observed rate constant. Error bars represent SD (n = 3). Asterisks indicate significant differences (P < 0.05, t test). (C) Initial processing rates of the reactions in (A) was determined by linear regression (from 0 to 5 min). Error bars represent SD (n = 3). Asterisks indicate significant differences (P < 0.05, t test).
The PPIase Activity of CYP71 Is Important for miRNA Production In Vivo.
Finally, we examined whether the PPIase activity of CYP71 is important for miRNA production in vivo, we complemented the cyp71-3 mutant with CYP71-YFP, CYP71R519A-YFP, or CYP71F524A-YFP, which are expressed under the control of the native CYP71 promoter. We found that CYP71-YFP could fully rescue the developmental defects of cyp71-3 (Fig. 7A). CYP71R519A-YFP and CYP71F524A-YFP barely rescued the developmental defects of cyp71-3 (Fig. 7A), although their expression levels were comparable to that of CYP71-YFP (SI Appendix, Fig. S8A). The cyp71-3 mutant had reduced expression of DCL1. Although complementation with CYP71R519A-YFP or CYP71F524A-YFP somehow increased DCL1 expression to a higher-than-normal level (SI Appendix, Fig. S8B), the overaccumulation of pri-miRNAs in cyp71-3 was not or only slightly alleviated (SI Appendix, Fig. S8C). We further detected the accumulation levels of multiple miRNAs. Unlike CYP71-YFP, which fully restores miRNA accumulation in cyp71-3, CYP71R519A-YFP and CYP71F524A-YFP did not restore or only partially restored the accumulation of detected miRNAs in cyp71-3 (Fig. 7B). Correspondingly, CYP71R519A-YFP and CYP71F524A-YFP did not restore or only partially restored target gene repression in cyp71-3 (Fig. 7C). Taken together, our results indicate that the PPIase activity of CYP71 is important for miRNA production and target gene repression in vivo.
Fig. 7.
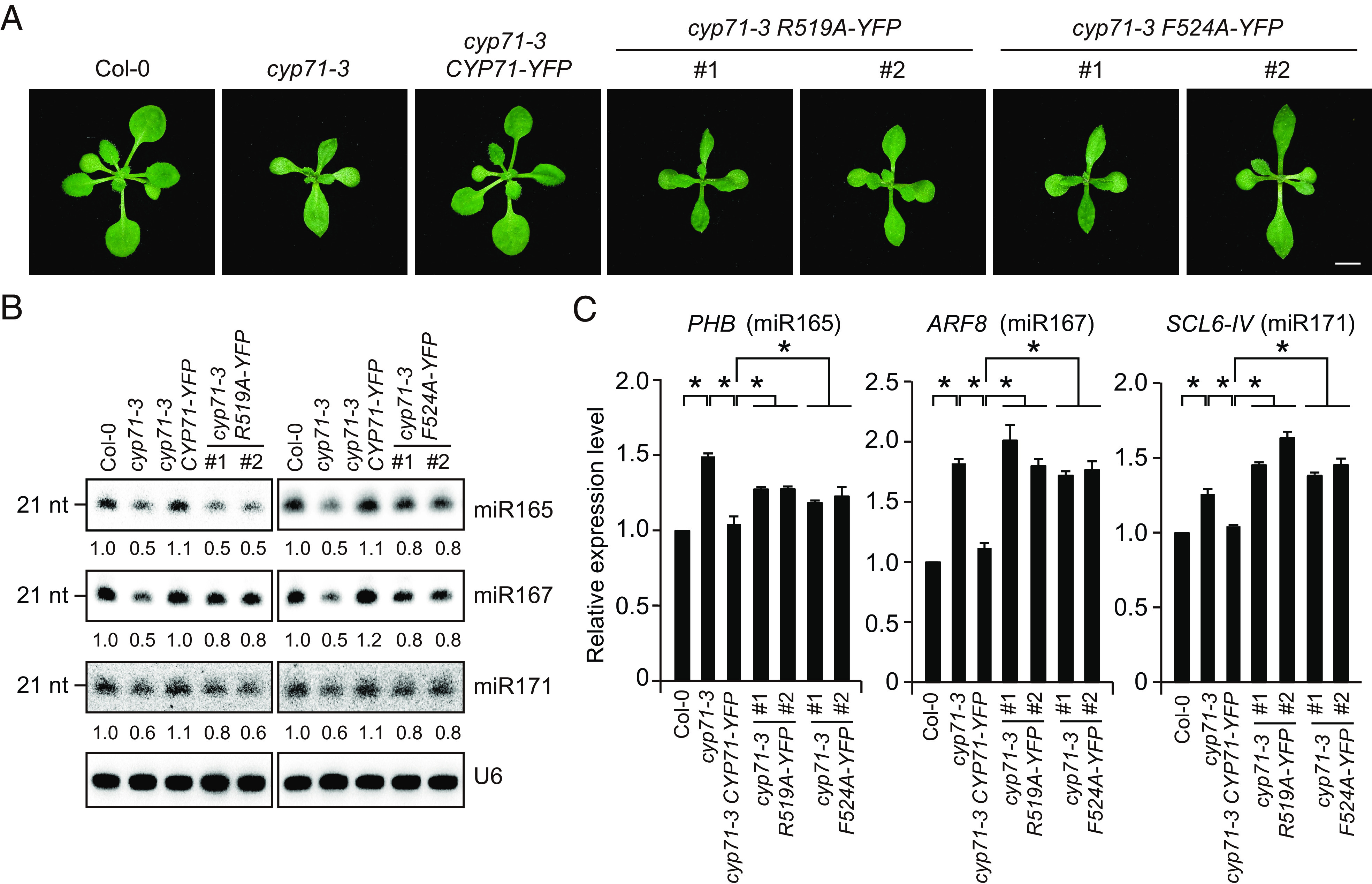
PPIase activity of CYP71 is important for miRNA production in vivo. (A) Phenotypes of 2-wk-old seedlings of Col-0, cyp71-3, and cyp71-3 complemented with CYP71-YFP, CYP71R519A-YFP (R519A-YFP), or CYP71F524A-YFP (F524A-YFP). The #1 and #2 indicate independent transgenic lines. (Scale bar, 10 mm.) (B) Northern blot results showing miRNA accumulation levels in Col-0, cyp71-3, and cyp71-3 complemented with CYP71-YFP, CYP71R519A-YFP (R519A-YFP), or CYP71F524A-YFP (F524A-YFP). U6 was probed as loading controls. The signals are quantified, and the relative intensities are shown. (C) qRT-PCR analysis of the expression levels of miRNA targets in Col-0, cyp71-3, and cyp71-3 complemented with CYP71-YFP, CYP71R519A-YFP (R519A-YFP), or CYP71F524A-YFP (F524A-YFP). Error bars represent SD (n = 3). Asterisks indicate significant differences (P < 0.05, t test).
Discussion
Efficient miRNA processing depends on SE phase separation and the resultant assembly of D-bodies. In this study, we uncover that CYP71, a cyclophilin protein that directly interacts with SE, promotes SE phase separation and D-body assembly to enhance Dicing complex activity and miRNA processing efficiency by using its PPIase activity. Our study reveals CYP71 as a regulatory component in the miRNA pathway and peptidyl-prolyl cis–trans isomerization as a mechanism for efficient miRNA biogenesis.
Multiple mechanisms that alter intermolecular contacts, including pH, temperature, posttranslational modifications, and association with proteins or RNA, are involved in the regulation of protein phase separation (34, 35). Some of these mechanisms induce protein conformational changes that alter surface properties of a protein, thereby modulating interactions between protein molecules. Thus far, it remains unknown what types of protein conformational changes have an effect on protein phase separation. We found that CYP71 promotes SE droplet formation in vitro and is required for D-body formation in vivo, suggesting that CYP71 is a positive regulator of SE phase separation and D-body assembly. Prolines are enriched in the intrinsically disordered region 1 (IDR1) of SE, and the PPIase activity of CYP71 is important for the role of CYP71 in increasing SE droplet formation, suggesting that CYP71 may induce peptidyl-prolyl cis–trans isomerization of SE to promote SE phase separation. Within a folded protein, a peptide bond tends to adopt trans conformation due to geometrical and thermodynamic parameters. The peptidyl-prolyl bond, as an exception, can adopt completely distinct cis and trans conformations and undergo interconversion between these two configurations (36, 37). Constrained by the cyclic 5-membered ring, intrinsic cis–trans isomerization of a peptidyl-prolyl bond is slow and becomes a rate-limiting step for protein folding and conformational transition (38). CYP71, which can speed up the reaction, promotes SE phase separation, suggesting that rapid peptidyl-prolyl cis–trans isomerization may be the key for efficient SE phase separation. Whether such regulation serves as a mechanism to ensure D-body formation when SE is deficient remains to be tested. Recently, it was found that prolines are enriched in IDRs and proteins that undergo phase separation are enriched in Peptidyl-Prolyl Isomerase A (PPIA or Cyclophilin A) substrates in mouse and human cells (39), suggesting that peptidyl-prolyl cis–trans isomerization could be a common mechanism that regulates protein phase separation.
Research efforts in the past two decades have identified a plethora of protein factors that regulate miRNA biogenesis. Interestingly, the number of D-bodies per cell is affected by mutations in multiple regulatory factors. Mutations in MODIFIER OF SNC1, 2 (MOS2) (40), ELONGATOR COMPLEX PROTEIN 2 (ELP2), ELP5 (10), MOS4-ASSOCIATED COMPLEX (MAC7) (41), PROTEIN PHOSPHATASE4 REGULATORY SUBUNIT 3A (PP4R3A) (42), Tho2/Hpr1 phenotype (THP1) (43), and REDUCTION IN BLEACHED VEIN AREA (RBV) (44) and knockdown of RNA helicase 6 (RH6), RH8, and RH12 (45) lead to a decrease in the number of D-bodies, whereas mutations in Negative on TATA less2 (NOT2) (46), PSR1-Interacting Protein 1 (PINP1) (47), and RNA debranching enzyme 1 (DBR1) (48) lead to an increase in the number of D-bodies. All of these mutations impair miRNA biogenesis, suggesting that the efficiency of miRNA processing is not simply proportional to the number of D-bodies. Another possibility is that D-bodies are misassembled in the mutants and deficient in miRNA processing. Among these protein factors, RH6, RH8, and RH12 were found to promote SE phase separation, possibly through altering the secondary structure of pri-miRNAs (45). We speculate that additional protein factors could be modulators of SE phase separation, considering the effects of their loss on D-body formation, plus their interactions with SE. Determining the mechanisms through which these factors regulate SE phase separation could be a shortcut to unravel the mechanisms that regulate protein phase separation.
We have previously shown that D-body formation promotes pri-/pre-mRNA processing (19). As pri-/pre-miRNA processing could be cotranscriptional and D-bodies may associate with chromatin (10, 11), D-body formation could have roles in cotranscriptional processing. CYP71 does not regulate the transcription of MIR genes. However, CYP71 can still have a cotranscriptional effect. As a protein acting at the chromatin level (23, 25), CYP71 may mediate the recruitment of Dicing complex components to MIR gene loci to promote cotranscriptional processing of pri-/pre-miRNAs. Future investigations are required to test this possibility.
Materials and Methods
Arabidopsis plants were grown under long photoperiod conditions (16 h light, 22 °C/8 h dark, 18 °C). Standard molecular biology and genetic methods were used for vector construction and for plant transformation. Detailed methods used in this study are listed in SI Appendix, Materials and Methods. Primers used in this study are listed in Dataset S4.
Supplementary Material
Appendix 01 (PDF)
Dataset S01 (XLSX)
Dataset S02 (XLSX)
Dataset S03 (XLSX)
Dataset S04 (XLSX)
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China to Y.L. (Grant no. 32070618) and to Y.Q. (Grant no. 31788103)
Author contributions
Y.Q. designed research; G.Z., J.N., Z.H., and D.X. performed research; G.Z., J.N., T.L., Y.L., and Y.Q. analyzed data; and G.Z., Y.L., and Y.Q. wrote the paper.
Competing interests
The authors declare no competing interest.
Footnotes
This article is a PNAS Direct Submission.
Contributor Information
Yan Li, Email: yyaayanli@mail.tsinghua.edu.cn.
Yijun Qi, Email: qiyijun@tsinghua.edu.cn.
Data, Materials, and Software Availability
The small RNA sequencing and RNA sequencing datasets generated in this study have been deposited in the National Center for Biotechnology Information Gene Expression Omnibus (http://www.ncbi.nlm.nih.gov/geo/) under accession numbers GSE207396 (49) and GSE235656 (50). All codes used for data analysis were uploaded to https://github.com/Chipeyown/cyp71_analysis (51).
Supporting Information
References
- 1.Song X., Li Y., Cao X., Qi Y., MicroRNAs and their regulatory roles in plant-environment interactions. Annu. Rev. Plant Biol. 70, 489–525 (2019). [DOI] [PubMed] [Google Scholar]
- 2.Xu L., et al. , An expression atlas of miRNAs in Arabidopsis thaliana. Sci. China Life Sci. 61, 178–189 (2018). [DOI] [PubMed] [Google Scholar]
- 3.Fang X., Qi Y., RNAi in plants: An argonaute-centered view. Plant Cell 28, 272–285 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu L., et al. , DNA methylation mediated by a microRNA pathway. Mol. Cell 38, 465–475 (2010). [DOI] [PubMed] [Google Scholar]
- 5.Huang T., et al. , Evolution of lmiRNAs and their targets from MITEs for rice adaptation. J. Integr. Plant Biol. 64, 2411–2424 (2022). [DOI] [PubMed] [Google Scholar]
- 6.Xie Z., et al. , Expression of Arabidopsis MIRNA genes. Plant Physiol. 138, 2145–2154 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee Y., et al. , MicroRNA genes are transcribed by RNA polymerase II. EMBO J. 23, 4051–4060 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moro B., et al. , Efficiency and precision of microRNA biogenesis modes in plants. Nucleic Acids Res. 46, 10709–10723 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu H. L., et al. , Bidirectional processing of pri-miRNAs with branched terminal loops by Arabidopsis Dicer-like1. Nat. Struct. Mol. Biol. 20, 1106–1115 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fang X., Cui Y., Li Y., Qi Y., Transcription and processing of primary microRNAs are coupled by Elongator complex in Arabidopsis. Nat. Plants 1, 15075 (2015). [DOI] [PubMed] [Google Scholar]
- 11.Gonzalo L., et al. , R-loops at microRNA encoding loci promote co-transcriptional processing of pri-miRNAs in plants. Nat. Plants 8, 402–418 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu Y., Jia T., Chen X., The “how” and “where” of plant microRNAs. New Phytol. 216, 1002–1017 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vazquez F., Gasciolli V., Crete P., Vaucheret H., The nuclear dsRNA binding protein HYL1 is required for microRNA accumulation and plant development, but not posttranscriptional transgene silencing. Curr. Biol. 14, 346–351 (2004). [DOI] [PubMed] [Google Scholar]
- 14.Han M. H., Goud S., Song L., Fedoroff N., The Arabidopsis double-stranded RNA-binding protein HYL1 plays a role in microRNA-mediated gene regulation. Proc. Natl. Acad. Sci. U.S.A. 101, 1093–1098 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lobbes D., Rallapalli G., Schmidt D. D., Martin C., Clarke J., SERRATE: A new player on the plant microRNA scene. EMBO Rep. 7, 1052–1058 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang L., Liu Z., Lu F., Dong A., Huang H., SERRATE is a novel nuclear regulator in primary microRNA processing in Arabidopsis. Plant J. 47, 841–850 (2006). [DOI] [PubMed] [Google Scholar]
- 17.Fang Y., Spector D. L., Identification of nuclear dicing bodies containing proteins for microRNA biogenesis in living Arabidopsis plants. Curr. Biol. 17, 818–823 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Song L., Han M. H., Lesicka J., Fedoroff N., Arabidopsis primary microRNA processing proteins HYL1 and DCL1 define a nuclear body distinct from the Cajal body. Proc. Natl. Acad. Sci. U.S.A. 104, 5437–5442 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xie D., et al. , Phase separation of SERRATE drives dicing body assembly and promotes miRNA processing in Arabidopsis. Nat. Cell Biol. 23, 32–39 (2021). [DOI] [PubMed] [Google Scholar]
- 20.Wang P., Heitman J., The cyclophilins. Genome Biol. 6, 226 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singh H., Kaur K., Singh M., Kaur G., Singh P., Plant cyclophilins: Multifaceted proteins with versatile roles. Front. Plant Sci. 11, 585212 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He Z., Li L., Luan S., Immunophilins and parvulins. Superfamily of peptidyl prolyl isomerases in Arabidopsis. Plant Physiol. 134, 1248–1267 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li H., et al. , A WD40 domain cyclophilin interacts with histone H3 and functions in gene repression and organogenesis in Arabidopsis. Plant Cell 19, 2403–2416 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lakhanpal S., Fan J. S., Luan S., Swaminathan K., Structural and functional analyses of the PPIase domain of Arabidopsis thaliana CYP71 reveal its catalytic activity toward histone H3. FEBS Lett. 595, 145–154 (2021). [DOI] [PubMed] [Google Scholar]
- 25.Li H., Luan S., The cyclophilin AtCYP71 interacts with CAF-1 and LHP1 and functions in multiple chromatin remodeling processes. Mol. Plant 4, 748–758 (2011). [DOI] [PubMed] [Google Scholar]
- 26.Wang W., et al. , An importin beta protein negatively regulates MicroRNA activity in Arabidopsis. Plant Cell 23, 3565–3576 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laubinger S., et al. , Dual roles of the nuclear cap-binding complex and SERRATE in pre-mRNA splicing and microRNA processing in Arabidopsis thaliana. Proc. Natl. Acad. Sci. U.S.A. 105, 8795–8800 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raczynska K. D., et al. , The SERRATE protein is involved in alternative splicing in Arabidopsis thaliana. Nucleic Acids Res. 42, 1224–1244 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ma Z. Y., et al. , Arabidopsis serrate coordinates histone methyltransferases ATXR5/6 and RNA Processing factor RDR6 to regulate transposon expression. Dev. Cell 45, 769–784 (2018). [DOI] [PubMed] [Google Scholar]
- 30.Speth C., et al. , Arabidopsis RNA processing factor SERRATE regulates the transcription of intronless genes. Elife 7, e37078 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takahashi N., Hayano T., Suzuki M., Peptidyl-prolyl cis-trans isomerase is the cyclosporin A-binding protein cyclophilin. Nature 337, 473–475 (1989). [DOI] [PubMed] [Google Scholar]
- 32.Fischer G., Wittmann-Liebold B., Lang K., Kiefhaber T., Schmid F. X., Cyclophilin and peptidyl-prolyl cis-trans isomerase are probably identical proteins. Nature 337, 476–478 (1989). [DOI] [PubMed] [Google Scholar]
- 33.Zydowsky L. D., et al. , Active site mutants of human cyclophilin A separate peptidyl-prolyl isomerase activity from cyclosporin A binding and calcineurin inhibition. Protein Sci. 1, 1092–1099 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bratek-Skicki A., Pancsa R., Meszaros B., Van Lindt J., Tompa P., A guide to regulation of the formation of biomolecular condensates. FEBS J. 287, 1924–1935 (2020). [DOI] [PubMed] [Google Scholar]
- 35.Snead W. T., Gladfelter A. S., The control centers of biomolecular phase separation: How membrane surfaces, PTMs, and active processes regulate condensation. Mol. Cell 76, 295–305 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pal D., Chakrabarti P., Cis peptide bonds in proteins: Residues involved, their conformations, interactions and locations. J. Mol. Biol. 294, 271–288 (1999). [DOI] [PubMed] [Google Scholar]
- 37.Stewart D. E., Sarkar A., Wampler J. E., Occurrence and role of cis peptide bonds in protein structures. J. Mol. Biol. 214, 253–260 (1990). [DOI] [PubMed] [Google Scholar]
- 38.Lu K. P., Finn G., Lee T. H., Nicholson L. K., Prolyl cis-trans isomerization as a molecular timer. Nat. Chem. Biol. 3, 619–629 (2007). [DOI] [PubMed] [Google Scholar]
- 39.Maneix L., et al. , Cyclophilin A regulates protein phase separation and mitigates hematopoietic stem cell aging. Exp. Hematol. 100, S41 (2021). [Google Scholar]
- 40.Wu X., et al. , A role for the RNA-binding protein MOS2 in microRNA maturation in Arabidopsis. Cell Res. 23, 645–657 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jia T., et al. , The Arabidopsis MOS4-associated complex promotes microRNA biogenesis and precursor messenger RNA splicing. Plant Cell 29, 2626–2643 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang S., et al. , The PROTEIN PHOSPHATASE4 complex promotes transcription and processing of primary microRNAs in Arabidopsis. Plant Cell 31, 486–501 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang B., et al. , Linking key steps of microRNA biogenesis by TREX-2 and the nuclear pore complex in Arabidopsis. Nat. Plants 6, 957–969 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liang C., et al. , Arabidopsis RBV is a conserved WD40 repeat protein that promotes microRNA biogenesis and ARGONAUTE1 loading. Nat. Commun. 13, 1217 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li Q., et al. , DEAD-box helicases modulate dicing body formation in Arabidopsis. Sci Adv 7, eabc6266 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang L., et al. , NOT2 proteins promote polymerase II-dependent transcription and interact with multiple MicroRNA biogenesis factors in Arabidopsis. Plant Cell 25, 715–727 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qiao Y., Shi J., Zhai Y., Hou Y., Ma W., Phytophthora effector targets a novel component of small RNA pathway in plants to promote infection. Proc. Natl. Acad. Sci. U.S.A. 112, 5850–5855 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li Y., Syed J., Sugiyama H., RNA-DNA triplex formation by long noncoding RNAs. Cell Chem. Biol. 23, 1325–1333 (2016). [DOI] [PubMed] [Google Scholar]
- 49.Zhao G., et al. , Peptidyl-prolyl isomerase Cyclophilin71 promotes SERRATE phase separation and miRNA processing in Arabidopsis. NCBI GEO database. https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE207396. Deposited 3 July 2022. [DOI] [PMC free article] [PubMed]
- 50.Zhao G., et al. , Peptidyl-prolyl isomerase Cyclophilin71 promotes SERRATE phase separation and miRNA processing in Arabidopsis. NCBI GEO database. https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE235657. Deposited 23 June 2023. [DOI] [PMC free article] [PubMed]
- 51.Zhao G., et al. , Peptidyl-prolyl isomerase Cyclophilin71 promotes SERRATE phase separation and miRNA processing in Arabidopsis. GitHub. https://github.com/Chipeyown/cyp71_analysis. Deposited 23 June 2023. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Dataset S01 (XLSX)
Dataset S02 (XLSX)
Dataset S03 (XLSX)
Dataset S04 (XLSX)
Data Availability Statement
The small RNA sequencing and RNA sequencing datasets generated in this study have been deposited in the National Center for Biotechnology Information Gene Expression Omnibus (http://www.ncbi.nlm.nih.gov/geo/) under accession numbers GSE207396 (49) and GSE235656 (50). All codes used for data analysis were uploaded to https://github.com/Chipeyown/cyp71_analysis (51).