Abstract
Previous observations that the adenovirus type 5 (Ad5) E4orf6 and E4orf3 gene products have redundant effects in viral lytic infection together with the recent findings that E4orf6 possesses transforming potential prompted us to investigate the effect of E4orf3 expression on the transformation of primary rat cells in combination with adenovirus E1 oncogene products. Our results demonstrate for the first time that E4orf3 can cooperate with adenovirus E1A and E1A plus E1B proteins to transform primary baby rat kidney cells, acting synergistically with E4orf6 in the presence of E1B gene products. Transformed rat cells expressing E4orf3 exhibit morphological alterations, higher growth rates and saturation densities, and increased tumorigenicity compared with transformants expressing E1 proteins only. Consistent with previous results for adenovirus-infected cells, the E4orf3 protein is immunologically restricted to discrete nuclear structures known as PML oncogenic domains (PODs) in transformed rat cells. As opposed to E4orf6, the ability of E4orf3 to promote oncogenic cell growth is probably not linked to a modulation of p53 functions and stability. Instead, our results indicate that the transforming activities of E4orf3 are due to combinatorial effects that involve the binding to the adenovirus 55-kDa E1B protein and the colocalization with PODs independent from interactions with the PML gene product. These data fit well with a model in which the reorganization of PODs may trigger a cascade of processes that cause uncontrolled cell proliferation and neoplastic growth. In sum, our results provide strong evidence for the idea that interactions with PODs by viral proteins are linked to oncogenic transformation.
The transforming potential of human adenoviruses has been traditionally ascribed to the E1A and E1B transcription units encoded within early region 1 (E1). It has been well established that E1A and E1B gene products are both necessary and sufficient to initiate and promote complete cell transformation by virtue of their ability to interact with and manipulate the functions of several growth-regulatory proteins that control cell cycle progression and programmed cell death (reviewed by Nevins and Vogt [39]). However, over the past 5 years it has become apparent that human adenoviruses encode additional gene products with transforming and oncogenic potential that map outside of E1, within early region 4 (E4). As shown first for group D adenovirus type 9 (Ad9), E4 open reading frame 1 (E4orf1) promotes focus formation of CREF cells in vitro (50) and is required for mammary tumor formation in female rats (22, 23). Recent studies with group C Ad5 demonstrated that the 34-kDa gene product of E4orf6 can cooperate with Ad5 E1A and E1A plus E1B proteins to completely transform primary baby rat kidney (BRK) cells in tissue culture (33, 37). It appears that E4orf6 contributes to oncogenic transformation by modulating the function and stability of the tumor suppressor protein p53 (33, 37, 38) at a transcriptional level (8) and, in combination with the E1A and E1B proteins, at a posttranslational level (14, 41, 46).
The E4 region is located between map units 91 and 100 of the viral genome and encodes at least six different proteins which express an apparently disparate set of functions. Adenovirus mutants which lack E4 display complex phenotypes, including defects in viral gene expression, viral DNA replication, accumulation of late mRNAs, synthesis of late viral proteins, and virus-induced host cell shutoff (reviewed in reference 27). Phenotypic analyses of different E4 mutants revealed that most of these defects can be assigned to the E4orf6 protein and the 11-kDa gene product encoded by E4orf3. Both viral proteins are located in the nucleus (16, 43) and exhibit redundant functions at the level of RNA processing and viral DNA replication (reviewed by Leppard [28]). At the posttranscriptional level, E4orf3 and E4orf6 promote viral gene expression by facilitating the cytoplasmic accumulation of viral transcripts (4, 20, 49). These activities are likely due to the ability of both proteins to maintain the stability of late mRNAs (42) prior to translocation through the nuclear pores and might be directly linked to the observation that both proteins affect the relative frequency of splicing of alternatively spliced transcripts derived from the major late promoter (reviewed by Imperiale et al. [21]).
In addition, the E4orf3 and E4orf6 gene products have been shown to play an important role in adenovirus DNA replication. In the absence of both proteins viral DNA replication is substantially impaired (4, 16, 20), and these mutants produce heterogeneous populations of large concatemeric viral DNAs (48). Because these aberrant intermediates are not produced in the presence of either E4orf3 or E4orf6, it has been suggested that both proteins are essential for normal viral DNA synthesis and, hence, responsible for directly or indirectly preventing concatemer accumulation (48). The molecular mechanism by which both proteins affect viral DNA replication is unknown but may be linked to the ability of E4orf3 and, perhaps to a lesser extent, E4orf6 to initiate a program of nuclear reorganization that favors this process (10). The E4orf3 protein colocalizes and reorganizes discrete nuclear structures (6, 10), known as ND10 or PML oncogenic domains (PODs) (2, 11). Accumulating evidence indicates that PODs are macromolecular multiprotein complexes present in all cultured cell lines that may be active in various aspects of cellular gene expression, cell cycle control, and regulation of DNA damage (reviewed in references 9 and 47). In adenovirus-infected cells, E4orf3-induced POD reorganization is linked to efficient viral DNA replication, suggesting that DNA processing is one potential function of POD-associated proteins (10). A number of cellular factors have been found to colocalize with PODs (9); interestingly, the integrity of the POD structure is disrupted in lymphocytes from patients with acute promyelocytic leukemia (APL) by a translocation that fuses the retinoic acid receptor α (RARα) to PML (2, 25, 40). The disruption by PML-RARα is associated with cellular transformation and can be reversed by treatment with retinoic acid (15). Because viral oncoproteins such as adenovirus E1A, the 55-kDa E1B protein (E1B-55kDa), and simian virus 40 (SV40) large T antigen have been found in close association with these subnuclear domains, it has been hypothesized that PODs represent a general target in oncogenic processes (6, 10).
At present there is no evidence that the E4orf3-induced POD reorganization plays a role in the adenovirus-mediated transformation process. However, given the recent finding that E4orf6 possesses transforming potential (33, 37) together with the demonstration that E4orf3 and E4orf6 encode redundant activities in lytic viral infection, it seemed reasonable to examine the effect of E4orf3 expression on adenovirus E1A/E1B-induced transformation. This report demonstrates for the first time that the Ad5 E4orf3 protein has transforming potential. We show that the E4orf3 protein promotes focus formation of primary rat epithelial cells in cooperation with adenovirus E1A and E1A plus E1B oncoproteins. Established cell lines expressing E4orf3 exhibited typical hallmarks of oncogenically transformed cells, including morphological alterations, enhanced growth rates, growth to higher saturation densities, and increased tumorigenicity in nude mice. Interestingly, protein analyses revealed that E4orf3, like E4orf6, can bind to the adenovirus E1B-55kDa in transformed rat cells. However, E4orf3, unlike E4orf6, did not induce a reduction in p53 steady-state levels. Thus, this activity is a unique property of the E4orf6 gene product. In transformed rat cells, the E4orf3 protein colocalized with PODs and induced changes in POD morphology. Based on the findings that POD-associated factors play an important role in cell growth regulation and the observation that POD reorganization is linked to neoplastic growth in APL, our results suggest that E4orf3 may promote oncogenic transformation by altering the distribution of cellular factors sequestered in PODs. Together with the demonstration that viral oncoproteins from other DNA tumor viruses colocalize with PODs, our data provide further support for the view that these subnuclear domains may represent targets in viral oncogenesis.
MATERIALS AND METHODS
Plasmids used in this study.
pAd5 XhoI-C (30) contains the leftmost 15.5% of the Ad5 genome, including the E1A and E1B genes with their endogenous promoters. Plasmid pAd5 dl338XhoI-C is a derivative of pAd5XhoI-C that contains a deletion in the E1B-55kDa coding regions (30). Plasmids pCMV-E4orf3 and pSVHA-E4orf3 express wild-type and influenza virus hemagglutinin (HA) epitope-tagged Ad5 E4orf3 under the control of the cytomegalovirus (CMV) immediate-early promoter and the SV40 promoter/enhancer, respectively. These expression plasmids were constructed by PCR amplification of the E4orf3 coding sequence from pXbaC (16) with primers E4orf3fw (5′-CAGGGATCCGTCATGATTCGCTGCTTGAGGC-3′) and E4orf3rev (5′-CGCGGGATCCGTCGACTTATTCCAAAAGATTATCC-3′) containing BamHI restriction sites. To generate pCMV-E4orf3, the PCR product was cloned into the BamHI site of pcDNA3 (InVitrogen). For pSVHA-E4orf3, the same PCR fragment was first inserted into the BamHI site of pAS2 (19). From this plasmid (pAS-E4orf3), an EcoRI fragment containing the HA epitope-tagged E4orf3 coding sequence was isolated and cloned into plasmid vector pSVK3 (Pharmacia) to generate pSVHA-E4orf3. The sequence of each plasmid was confirmed by DNA sequencing. Plasmids pCMV-E4orf6 and pCMV-E1A express the Ad5 E4orf6 and Ad5 E1A proteins, respectively, from the CMV immediate-early promoter and have been described previously (8, 36). To generate pCMV-PML, an EcoRI fragment corresponding to the human full-length PML cDNA was isolated from pSG5-PML and inserted into the EcoRI site of pcDNA3 (InVitrogen). pSG5-PML was kindly provided by Anne Dejean.
Transformation assays and cell lines.
Sprague-Dawley rats were randomly bred at the university’s animal facilities under standardized conditions. Primary cultures of BRK cells were prepared from 6- to 7-day-old rats as described previously (37) and grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal calf serum (FCS). For transformation assays, subconfluent cells were transfected 2 days postplating by the calcium phosphate procedure (13) with salmon sperm carrier (Boehringer) and plasmid DNA as described elsewhere (37). To avoid effects of promoter competition, the total amount of CMV or SV40 promoter was held constant by inclusion of empty pcDNA3 or pSVK3 vector plasmid. Three to four weeks after transfection, cultures were stained with crystal violet (1% in 25% methanol) and dense foci of morphologically transformed cells were counted. Representative plates were scanned and cropped by using Adobe Photoshop. Alternatively, foci were pooled or cloned and subsequently expanded into permanent cell lines.
The transformed BRK cell lines AB7, AB16, ABS1, and ABS6 have been described previously (38). AB7 and AB16 cells express the Ad5 E1 region; ABS1 and ABS6 cells express different levels of the Ad5 E4orf6 protein in addition to the Ad5 E1A and E1B genes. ABS16, ABT29, ABST4, and ABST5 are G418-selected cell lines of polyclonal origin derived from transfections of primary BRK cells with pAd5 XhoI-C and pCMV-E4orf6 (ABS16), pCMV-E4orf3 (ABT29), or both E4 plasmids (ABST4 and ABST5). Cell lines ABT9 and ABT10 were derived from transfections with pAd5 XhoI-C plus pSVHA-E4orf3 and originated from single transformed BRK cell foci. ABT27 cells were established from foci obtained from transfection of pSVHA-E4orf3, pcDNA3, and pAd5 dl338XhoI-C. All BRK cell lines were maintained in DMEM with 10% FCS.
Growth studies were performed exactly as described previously (38). Cell morphology was examined and photographed on Scotch Chrome 640T films with a camera-mounted Olympus AX70 microscope using phase contrast.
Antibodies.
Unless otherwise noted, all mouse monoclonal antibodies (MAbs) used in this study were obtained from supernatants of hybridoma cell cultures grown in RPMI supplemented with 10% FCS. RSA3 recognizes the amino terminus of the Ad5 E4orf6 protein (32), 2A6 (44) is specific for E1B-55kDa, M73 (17) is directed against E1A proteins and PAb421 (18) is specific for p53 from different species. The anti-PML MAb 5E10 was generously provided by Luitzen de Jong, and the p53-specific rabbit polyclonal antibody CM-1 was kindly provided by David Lane. Anti-HA mouse MAb 12CA5 and anti-E1B-19kDa rat MAb 1G11 were obtained from Boehringer and Oncogene Research, respectively. The mouse MAb AC-15 is specific for β-actin and was collected from ascites fluid (Sigma).
The anti-Ad5 E4orf3 rat MAb 6A11 was raised against a glutathione S-transferase E4orf3 fusion protein produced in Escherichia coli and will be described elsewhere.
Immunoprecipitation and immunoblotting.
For analysis of proteins by immunoprecipitation and immunoblotting assays, cells were lysed on ice in radioimmunoprecipitation assay buffer (50 mM Tris-chloride [pH 8.0], 150 mM NaCl, 0.1% sodium dodecyl sulfate [SDS], 1% Nonidet P-40 [NP-40], 0.5% sodium deoxycholate, 1 mM phenylmethylsulfonyl fluoride, 0.3 μM aprotinin, 1 μM leupeptin, 1 μM pepstatin) or NP-40 lysis buffer (50 mM Tris-chloride [pH 8.0], 150 mM NaCl, 1% NP-40, 1 mM phenylmethylsulfonyl fluoride, 0.3 μM aprotinin, 1 μM leupeptin, 1 μM pepstatin). After normalization for protein concentration, whole-cell extracts were subjected to Western blotting or immunoprecipitation. For immunoprecipitations, protein A- or protein G-Sepharose (Sigma) was incubated with 100 μl of the appropriate hybridoma supernatant. Immune complexes were resuspended in SDS-sample buffer, separated on SDS–10 to 15% polyacrylamide gels, and blotted onto nitrocellulose membranes (Schleicher & Schüll). The filters were blocked in phosphate-buffered saline (PBS) containing 5% nonfat dry milk for at least 1 h and then overnight in PBS containing the appropriate antibodies. The proteins were visualized by a secondary antibody linked to horseradish peroxidase (Amersham) followed by enhanced chemiluminescence (NOWA; Energene). Autoradiograms were scanned and cropped by using Adobe Photoshop, and figures were prepared by using Macromedia FreeHand software on an Apple Macintosh computer.
Indirect immunofluorescence and confocal laser scan microscopy.
For indirect immunofluorescence studies, cells were grown on coverslips to subconfluent densities. They were fixed with methanol for 20 min at −20°C and reacted with undiluted hybridoma supernatants and appropriate fluorescein isothiocyanate (FITC)- or Cy3-conjugated secondary antibodies (Dianova) at a concentration of 7.5 μg/ml as described elsewhere (38). Samples were analyzed and photographed on Scotch Chrome 640T films with a camera-mounted Olympus AX70 microscope. For double-label studies, a TCS-NT confocal laser scanning microscope (Leica) was used. Excitation wavelengths of 488 nm (FITC) and 568 nm (Cy3) were selected from an argon-krypton laser. Each fluorochrome was independently selected; pseudocolor images of both signals were generated and superimposed.
Tumorigenicity in nude mice.
Analyses of tumor growth in NMRI(nu/nu) mice were exactly as described previously (38). Briefly, transformed cells were harvested by scraping into PBS. Nude mice were injected subcutaneously with 106 cells in serum-free DMEM, and tumor growth was recorded weekly with an electronic caliper.
RESULTS
The Ad5 E4orf3 protein can cooperate with E1A to stably transform primary BRK cells.
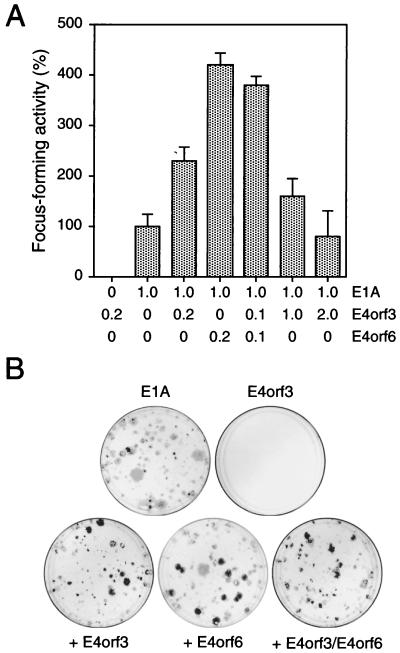
To explore the possibility that E4orf3 has transforming potential, we transfected primary BRK cells with plasmids expressing the E1A gene (pCMV-E1A) in combination with E4orf3 (pCMV-E4orf3), E4orf6 (pCMV-E4orf6), and E4orf3 plus E4orf6 (Fig. 1A). The E4orf3 protein alone was unable to induce the formation of transformed colonies, while transfection of BRK cells with DNA encoding E1A alone resulted in the appearance of a few, mostly abortive foci (Fig. 1B). In contrast, coexpression of E1A with E4orf3 resulted in a two- to threefold increase in the number of dense foci, which usually could be established into permanent cell lines. Thus, E4orf3, like E4orf6, can cooperate with E1A to stably transform primary rat cells, although on average, E4orf6 appeared to promote E1A-induced focus formation more efficiently than E4orf3. No further increase in the transformation frequency was obtained when increasing amounts of pCMV-E4orf3 were included in the transformation mixture or when both E4 plasmids were simultaneously introduced with E1A into BRK cells (Fig. 1A). Curiously, protein analyses revealed that all established cell lines derived from these experiments did not express the viral gene products (data not shown). Consistent with our previous work on transformed cells generated by E1A and E4orf6 (37), subsequent PCR analyses confirmed that all of these cell lines did not contain the viral E4orf3 gene (data not shown). This result strongly suggests that expression of E1A with E4orf3 or E4orf6 is sufficient for transformation but is not required to maintain the transformed cell phenotype.
FIG. 1.
E4orf3 cooperates with E1A to promote focus formation. (A) Primary BRK cells were transfected with the indicated amounts of plasmids (micrograms of DNA), and morphologically transformed colonies were scored 4 weeks after transfection. Focus-forming activity is represented as a percentage of pCMV-E1A activity. The mean and standard deviation are presented for three independent experiments. The average number of foci for pCMV-E1A was 5. (B) Representative crystal violet-stained plates showing foci from transfections with plasmids encoding Ad5 E1A, E4orf3, and E4orf6.
E4orf3 and E4orf6 can synergistically promote focus formation in cooperation with E1A and E1B.
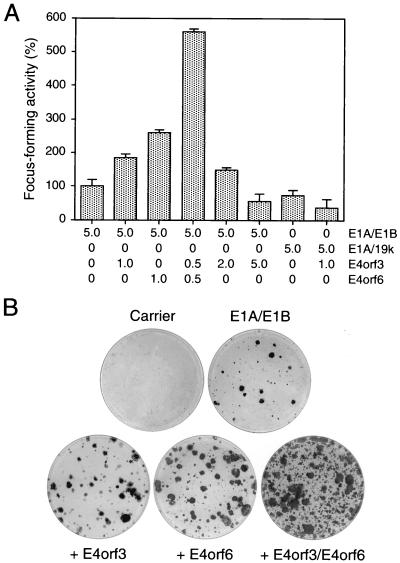
Expression of E4orf6 also enhances transformation by E1A and E1B proteins (33, 37). More importantly, these foci develop much more rapidly, contain more cells, and differ significantly from the E1A/E1B-transformed ones (33, 38). Similar to E4orf6, cotransfection of pCMV-E4orf3 with a plasmid expressing the E1A and E1B gene products (pAd5 XhoI-C) substantially increased the frequency of focus formation over transfection with pAd5 XhoI-C alone (Fig. 2A). Identical results were obtained when an epitope-tagged E4orf3 protein expressed from the SV40 promoter (pSVHA-E4orf3) was used in these assays (data not shown). Many of these foci grew rapidly and to a high density, closely resembling the transformed colonies produced by E1A/E1B and E4orf6 (Fig. 2B). The total number of transformed cells was further greatly increased when both E4 plasmids were simultaneously cotransfected with pAd5 XhoI-C, suggesting that both E4 proteins can synergistically promote focus formation in the presence of the E1B gene products. However, like E4orf6 (33, 37), the E4orf3 protein did not cooperate with E1A and E1B-19kDa (pAd5 dl338XhoI-C) in the same transformation assays (Fig. 2A). Rather, the E4orf3 protein reduced the number of foci produced by E1A and E1B-19kDa. We also noticed that as in the experiments using pCMV-E1A (Fig. 1A), a two- to fivefold increase of pCMV-E4orf3 resulted in a reduction of transformed foci (Fig. 2A). Interestingly, this negative effect was reproducibly not observed in cultures transfected with pAd5 XhoI-C and increasing amounts of pCMV-E4orf6 (data not shown). The decrease in focus-forming activity suggests that high levels of E4orf3 may cause cytotoxic effects, but the reason for the apparent difference between both E4 proteins in these assays is unclear.
FIG. 2.
Focus formation by Ad5 E1, E4orf3, and E4orf6 plasmids. (A) Primary BRK cells were transfected with the indicated amounts of plasmids (micrograms of DNA), and plates were stained 21 days after transfection with crystal violet. Focus-forming activity is represented as a percentage of pAd5 XhoI-C activity. The mean and standard deviation are presented for four independent experiments. The average number of foci for pAd5 XhoI-C was 68. E1A/19k denotes plasmid pAd5 dl338XhoI-C, which encodes E1A and E1B-19kDa proteins. (B) Plates were stained with crystal violet; a representative plate of each transfection with pAd5 XhoI-C is shown.
Together with the results presented above, these experiments demonstrate that E4orf3 can cooperate with E1A and E1A/E1B proteins to transform primary rat cells, and they show that there is cooperation between E4orf3 and E4orf6 in the presence of E1B proteins.
The E4orf3 protein does not antagonize E1A-induced metabolic stabilization of p53 in transformed rat cells.
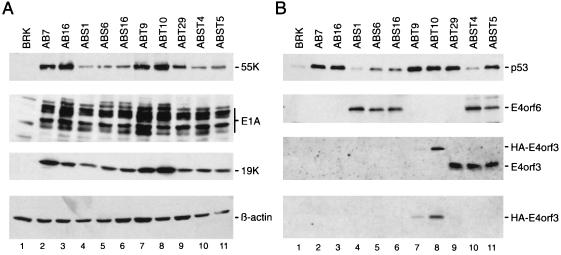
Adenovirus E1-transformed cells contain high levels of p53 due to the metabolic stabilization of this protein induced by E1A (31). The stability of p53 is further increased in the presence of the E1B gene products, although this effect may not require the binding of p53 to the large E1B protein (52). Recent studies demonstrated that the E4orf6 protein antagonizes this process. Expression of E4orf6 in E1-transformed rat cells induces a dramatic decrease of p53 steady-state levels (33, 37, 38), most likely by reducing the half-life of the tumor suppressor protein (33). Therefore, it was of interest to determine whether E4orf3 expression has similar effects on p53 levels. Following selection with G418, several different pools of foci from cultures transfected with pAd5 XhoI-C and pCMV-E4orf3 (ABT cells) and pAd5 XhoI-C with pCMV-E4orf6 plus pCMV-E4orf3 (ABST cells) were generated. These cells were compared with respect to expression of viral and cellular proteins to the previously described AB cells (expressing E1A and E1B) and ABS cells (expressing E1A, E1B, and E4orf6 proteins) (38). The levels of E1A and E1B proteins varied among the transformed cell lines (Fig. 3A). Significantly, ABS and ABST cells contained substantially lower levels of E1B-55kDa compared with AB and ABT cells. The molecular basis for this effect is unknown but may be related to the expression of the E4orf6 protein in these cell lines. In ABT and ABST cells, expression of E4orf3 was easily detectable; ABT9 cells (Fig. 3B, lane 7) contained significantly lower levels of the adenovirus protein compared with ABT10, ABT29, and both ABST cell lines (Fig. 3B, lanes 8 to 11). As expected, the p53 steady-state levels were considerably decreased in all ABS cells (Fig. 3A, lanes 4 to 6) relative to AB cells, where the p53 protein accumulated to high levels in the absence of the E4orf6 gene product (Fig. 3B, lanes 2 and 3). In contrast, no reduction of p53 was observed in the E4orf3-expressing ABT cell lines (Fig. 3B, lanes 7 to 9), which contained p53 protein in amounts comparable to those in both AB cells. Thus, the E4orf3 protein, unlike E4orf6, does not induce a decrease of p53 steady-state levels in E1A/E1B-transformed rat cells.
FIG. 3.
Protein analysis of selected AB, ABS, ABT, and ABST cells. (A) The same amount of whole-cell extract was separated on SDS–10% polyacrylamide gels and transferred to a nitrocellulose filter by Western blotting. The same filter was then subsequently probed with anti-E1B-55kDa MAb 2A6, anti-E1A antibody M73, and anti-E1B-19kDa antibody 1G11 followed by enhanced chemiluminescence. Quantitative loading of proteins was determined by probing the filter with the anti-β-actin antibody AC-15. (B) Equal amounts of total cell extracts were subjected to immunoprecipitation using anti-p53 MAb PAb421, anti-E4orf6 MAb RSA3, anti-E4orf3 MAb 6A11, or anti-HA antibody 12CA5. The precipitates were resolved on 10 to 15% protein gels and transferred to nitrocellulose membranes. The p53 and E4orf6 proteins were visualized with antibodies CM-1 and RSA3, respectively, followed by enhanced chemiluminescence. Native E4orf3 and HA-tagged E4orf3 were detected with MAb 6A11 and the HA-specific antibody 12CA5, respectively.
Interestingly, ABST5 cells exhibited higher levels of the p53 protein than ABS and ABST4 cells (Fig. 3B, lanes 4 to 6 and 10). These cell lines expressed comparable levels of both E1A and E1B proteins, excluding the possibility that different levels of these viral gene products contributed to this effect. Instead, this difference may be linked to the slightly lower E4orf6 levels in ABST5 cells, since we found that the reduction of p53 inversely correlates with E4orf6 expression (38). From these results, we conclude that E4orf3 does not interfere with the E4orf6-induced destabilization of p53 in transformed rat cells.
E4orf3 colocalizes with the PML nuclear structure in transformed rat cells.
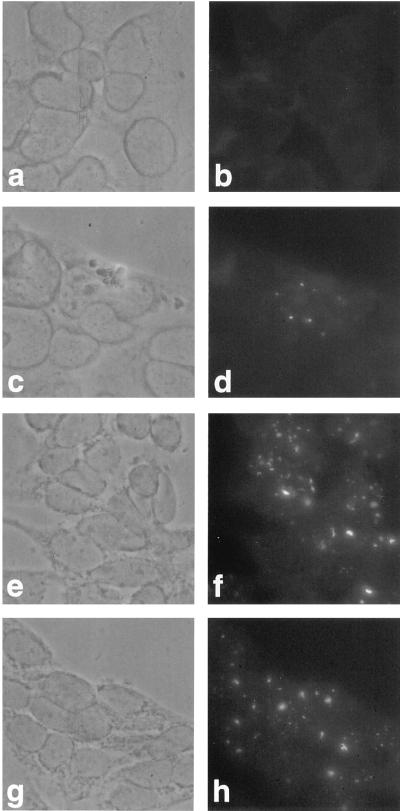
Adenovirus infection causes a drastic redistribution of PODs (6, 10). Analyses of adenovirus mutants and transient transfections demonstrated that the E4orf3 gene product alone is sufficient to induce this reorganization and that the viral protein colocalizes with the PML protein in these structures. We therefore tested whether expression of the E4orf3 protein affects the integrity of the POD structure in transformed rat cells. By conventional fluorescence microscopy, all three ABT cell lines, but not AB7 cells, incubated with the anti-E4orf3 MAb 6A11 exhibited a punctate and nuclear fluorescence characteristic of the POD (Fig. 4), which was also apparent in ABST cells (data not shown). Double-labeling experiments and confocal laser scanning microscopy confirmed that essentially all of the E4orf3 protein localized with PML in the same intranuclear structures (Fig. 5). It appeared, however, that not all of the PML-containing bodies were associated with E4orf3, most likely due to an excess of PML or PODs. Remarkably, in some structures where PML colocalized with the E4orf3 protein (Fig. 5c), the PML punctate bodies were changed into slightly elongated, less tightly stained structures, indicating that E4orf3 can induce a relocalization of the PML protein in transformed rat cells, although these alterations were less pronounced than those induced by E4orf3 in adenovirus-infected and transiently transfected human cells (6, 10).
FIG. 4.
Indirect immunofluorescence of AB7 and ABT cells. AB7 and ABT cells were probed with the anti-E4orf3 MAb 6A11 followed by FITC-conjugated sheep anti-rat antibodies. Phase-contrast images show AB7 (a), ABT9 (c), ABT10 (e), and ABT29 (g) cells and AB7 (b), ABT9 (d), ABT10 (f), and ABT29 (h) cells probed with MAb 6A11. Magnification, ×300.
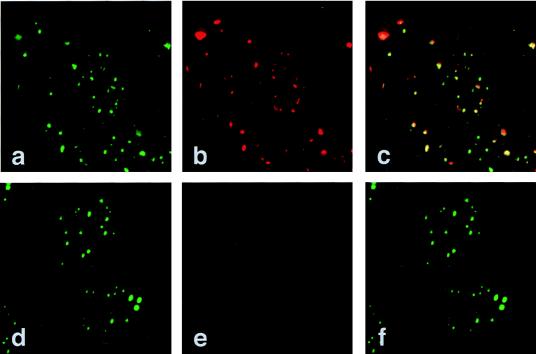
FIG. 5.
E4orf3 colocalizes with the PML nuclear structure. ABT10 and AB7 cells were double labeled in situ with anti-E4orf3 MAb 6A11 and MAb 5E10 specific for the PML protein. These were detected with Cy3- and FITC-conjugated secondary antibodies, respectively. Anti-PML (green; a and d) and anti-E4orf3 (red; b) staining patterns are shown. No signal was detected with MAb 6A11 in AB7 cells (e), demonstrating that the antibody is specific for the E4orf3 protein. An overlay of these two patterns is shown in panels c and f. In panel c, the pattern is mostly yellow, indicating that the two antigens colocalize. Magnification, ×7,616.
The E4orf3 protein can bind to E1B-55kDa in transformed rat cells.
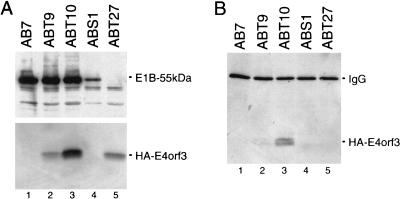
The transforming potential of E4orf6 correlates with the ability of the adenovirus protein to bind to and modulate the function and stability of p53 (37). In addition, the results from the colocalization studies suggested that E4orf3 might physically interact with the PML protein. To address these questions, we analyzed whether E4orf3 can bind to p53 and/or PML in ABT and ABST cells. Using combined immunoprecipitation-immunoblotting assays, we failed to coprecipitate E4orf3 with antibodies directed against p53 (PAb421) or PML (5E10) (data not shown). Instead, we found that a substantial amount of E4orf3 present in ABT9 and ABT10 cells coprecipitated with E1B-55kDa (Fig. 6B, lanes 2 and 3), whereas no E4orf3 was detected in precipitates from ABT27 cells, which do not express the large E1B protein (Fig. 6A, lane 5). The reduced amount of coprecipitated E4orf3 in ABT9 cells is most likely due to the lower expression levels of the E4 protein in these cells (Fig. 6A, lane 2). The significance of these observations is not known, but they may hint at the possibility that the redundant functions of E4orf3 and E4orf6 are linked, at least in part, to complex formation with E1B-55kDa.
FIG. 6.
E4orf3 can bind to E1B-55kDa in transformed rat cells. (A) Expression levels of E1B-55kDa and HA-E4orf3. Total-cell extracts were prepared from the indicated cell lines. Immunoprecipitations were performed with antibodies to E1B-55kDa (2A6) and HA-E4orf3 (12CA5), respectively, and both proteins were detected in immunoprecipitates by Western blot assays using the same antibodies. (B) The same total-cell extracts were subjected to immunoprecipitation using anti-E1B-55kDa MAb 2A6. The precipitates were resolved on a 15% protein gel and transferred to a nitrocellulose membrane. The HA-tagged E4orf3 proteins were visualized with MAb 12CA5 followed by enhanced chemiluminescence. The cell line ABT27 (lane 5) was established from foci obtained after transfection of pSVHA-E4orf3 and pAd5 dl338XhoI-C. IgG, immunoglobulin G.
The E4orf3 protein does not interfere with PML-mediated suppression of E1A/E1B-induced focus formation.
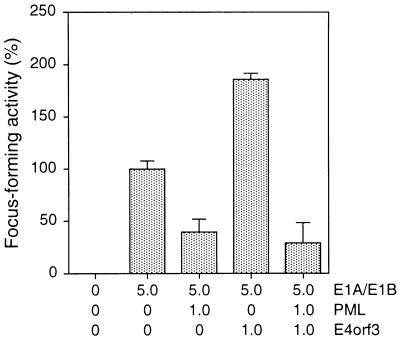
The observation that the POD structure is consistently disrupted in lymphocytes from patients with APL, together with the demonstration that viral oncoproteins are found associated with these subnuclear domains, has led to the suggestion that PODs represent a target in oncogenic processes (6, 9). In addition, accumulating evidence indicates that the POD-associated protein PML is a growth suppressor (34) that can inhibit oncogenic transformation (29), possibly by modulating cell cycle progression (35) and programmed cell death (3). These findings prompted us to investigate the effect of PML expression on E1A/E1B-mediated transformation of primary BRK cells (Fig. 7). Cotransfection of a plasmid encoding human wild-type PML (pCMV-PML) with pAd5 XhoI-C reduced the number of foci by over 60% compared with pAd5 XhoI-C alone. Hence, this result demonstrates for the first time that PML can efficiently suppress Ad5 E1A/E1B-mediated focus formation of primary rat cells. In light of the fact that E4orf3 colocalizes with the PML protein in transformed rat cells (Fig. 5), we also examined whether simultaneous expression of E4orf3 overcomes the inhibitory effect of PML. While E4orf3 alone promoted focus formation in combination with E1A and E1B, no increase in the number of foci was observed when E4orf3 was expressed in combination with PML and E1A plus E1B proteins. Thus, E4orf3 does not interfere with PML-mediated suppression of E1A/E1B-induced focus formation. These data, together with the results from coimmunoprecipitation assays, indicate that E4orf3 enhances focus formation by mechanisms that are independent from interactions with PML.
FIG. 7.
Focus formation by Ad5 E1, PML, and E4orf3 plasmids. Primary BRK cells were transfected with the indicated amounts of plasmids (micrograms of DNA), and dense foci were scored 3 weeks after transfection. Focus-forming activity is represented as a percentage of pAd5 XhoI-C activity. The mean and standard deviation are presented for three independent experiments. The average number of foci for pAd5 XhoI-C was 48.
E4orf3 expression in transformed cells induces morphological alterations and enhanced growth rates.
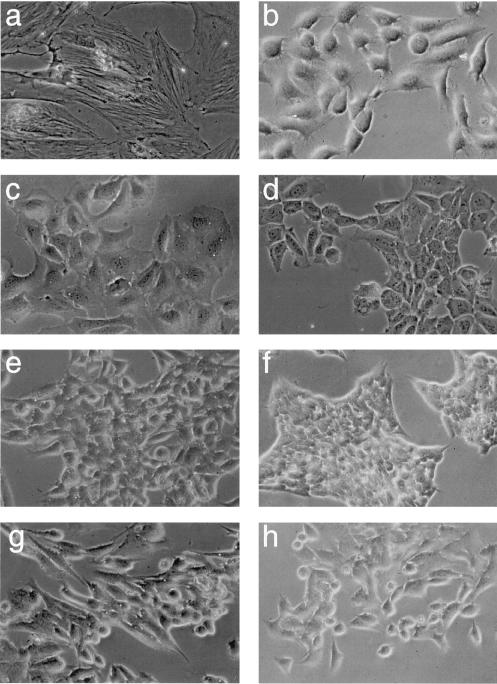
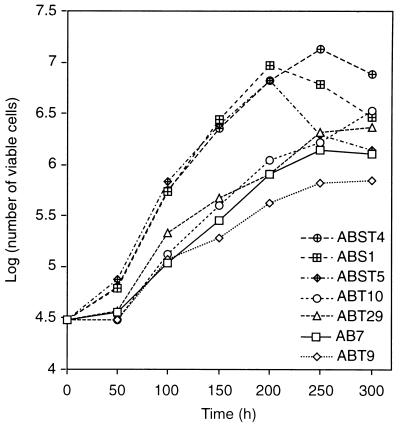
In our previous studies, we have demonstrated that transformed BRK cells stably expressing E4orf6 display additional properties commonly associated with a high grade of oncogenic transformation, including morphological alterations, markedly enhanced growth rates, and growth to higher saturation densities (38). To reveal the effect of E4orf3 expression on the transformed cell phenotype, we compared the morphology and growth rates of E4orf3 expressing cells with those of AB and ABS cells. Subtle differences existed in the morphology of the cell lines depending on whether they expressed the E4orf3, E4orf6, or both E4 proteins. Representative samples of these morphological differences are presented in Fig. 8. ABT cells differed from AB cells in that they were smaller, although these E4orf3-dependent alterations were less pronounced compared with those induced by E4orf6 in ABS1 cells. The E4orf3-expressing cell lines, and in particular ABT29 cells (Fig. 8e), tended to grow in colonies, which resembled the morphology of ABS1 cells (Fig. 8f), while ABST cells displayed an intermediate phenotype which seemed to reflect the contributions of both E4 proteins. Expression of the E4orf3 protein also affected the growth rates of these cells. Compared with AB7 cells, ABT10 and ABT29 cells expressing high levels of E4orf3 started to divide at higher growth rates at 4 days after plating and reached 2.6- and 1.8-times-higher saturation densities, respectively when this experiment was ended at 12 days after plating (Fig. 9). This positive effect was greatly enhanced when E4orf6 was coexpressed with E4orf3 in ABST cells. Clearly, ABST4 cells had the capacity to grow to a higher density than ABS1 cells, indicating a combinatorial promoting effect of both E4 proteins. Shortly after they reached confluency, these cells, like ABS1 and ABST5 cells, rapidly acidified the media and detached from the tissue culture plates as a result of cell death. The difference in growth rates between ABST4 and ABST5 cells might directly reflect the different levels of p53 which inversely correlate with E4orf6 expression levels in these cell lines (Fig. 3B). In support of this view, ABS1 cells expressing low levels of p53 divided at significantly higher rates than ABST5 cells containing higher amounts of p53. In sum, these findings demonstrated that the E4orf3 protein is capable of producing morphological alterations associated with the transformed cell phenotype, allowing the cells to grow more rapidly and to higher densities.
FIG. 8.
Morphology of selected cell lines at subconfluency. Cells derived from foci of plasmid DNA-transfected BRK cells were plated on coverslips and photographed. (a) BRK cells; (b) AB7 cells; (c to e) ABT9, ABT10, and ABT29 cells; (f) ABS1 cells; and (g and h) ABST4 and ABST5 cells. Magnification, ×100 (phase-contrast microscopy used in all panels).
FIG. 9.
Growth curves for AB7, ABT, ABS1, and ABST cells. A total of 3 × 104 cells was plated on six-well dishes in culture medium, and viable cells were counted every 48 h as described in the text. The log10 mean of viable cells for duplicate dishes is shown.
E4orf3 expression increases the tumorigenicity of transformed BRK cells in nude mice.
Because E4orf3 appeared to affect the growth properties of E1-transformed BRK cells in vitro, these cells were tested together with AB7 and ABS1 cell lines for tumorigenicity in nude mice (Table 1). During a 56-day observation period, all of the animals receiving 106 ABS1, ABST4, or ABST5 cells developed visually apparent, rapidly growing tumors as early as 7 days after injection whereas no tumors were induced by AB7 cells during the observation period. In contrast, four of six and three of six animals given the same number of ABT10 and ABT29 cells, respectively, produced tumors 7 days after injection. Two solid tumors were also observed with ABT9 cells but only after an extended delay of 41 days. Consistent with our data from the in vitro experiments, tumors induced by ABST4 cells grew more rapidly than those produced by ABS1 or ABST5 cells and reached sizes of approximately 120 mm2 at 28 days after injection (Table 1). Significantly smaller tumors were obtained with ABT cells after the same incubation period. Apparently, the ability of ABST4 and ABS1 cells to induce rapid tumor growth in nude mice correlated with lower p53 steady-state levels, and was dependent on the expression of the E4orf6 protein. Taken together, these in vivo studies demonstrated that E4orf3 expression increases the tumorigenicity of E1-transformed BRK cells.
TABLE 1.
Tumorigenicity in nude micea
| Cell line | No. of mice with palp-able tumors/total no. of animals | Latencyb | Mean tumor areac (mm2) ± SD |
|---|---|---|---|
| AB7 | 0 | ||
| ABT9 | 2 | 41 | 24d ± 12 |
| ABT10 | 4 | 7 | 4 ± 2 |
| ABT29 | 3 | 7 | 4 ± 2 |
| ABS1 | 6 | 7 | 95 ± 53 |
| ABST4 | 6 | 7 | 115 ± 54 |
| ABST5 | 6 | 7 | 21 ± 3 |
NMRI(nu/nu) mice (six per group) with a mean body weight of 20 g were given subcutaneous injections of 106 cells and monitored for tumor formation for 56 days. At this time, the animals were killed.
Day postinfection when palpable tumors were first detected.
Calculated 26 days after inoculation as the product of two perpendicular diameters, one measured across the greatest width of the tumor.
Measured 56 days after inoculation.
DISCUSSION
Over the past 5 years, it has been shown that a number of viral proteins target PODs and trigger a change in POD morphology (9). Based on the observation that adenovirus replication is linked to the dynamic properties of PODs, it has been postulated that these intranuclear domains may play an important role in the progression of infection by those viruses which can induce POD dissociation (9, 10). It has been hypothesized that the virus-induced reorganization of PODs may accelerate the release of cellular factors that facilitate a number of processes required for efficient viral replication. Accumulating evidence indicates that these proteins regulate various aspects of cellular gene expression, cell cycle control, apoptosis, and inhibition of DNA damage (9, 47). Apparently, the distribution of these factors must be tightly regulated during normal cell growth. Given the importance of PODs, we find it intriguing that E4orf3 colocalizes with these intranuclear structures in transformed rat cells (Fig. 4 and 5). Analogous to the PML-RARα fusion protein, the observed relocalization of the PML protein in these cells suggests that the E4 protein may facilitate the distribution of POD-associated factors. Accordingly, E4orf3 may trigger a cascade of regulatory processes that could potentially cause uncontrolled cell growth and thus enhance transformation and neoplastic growth in cooperation with adenovirus E1 oncogenes.
At present, it is unclear whether the observed redundancy between E4orf3 and E4orf6 during viral lytic infection is linked to their transforming potential. Earlier work on adenovirus-infected cells has shown that E4orf6 can also induce a reorganization of the PODs, although E4orf3 seems to be more effective in this process (6, 10). Thus, it might be possible that both E4 proteins contribute to transformation through similar mechanisms that affect the properties of the POD structure. Conversely, our current studies do not support the idea that E4orf3 promotes transformation by modulating the function and stability of p53. In fact, the inability of E4orf3 to reduce p53 steady-state levels in transformed rat cells (Fig. 3B) and in adenovirus-infected cells (41a) functionally distinguishes the E4orf3 protein from the E4orf6 gene product. In conclusion, these results imply that E4orf3 may promote focus formation by a reorganization of PODs, while E4orf6 enhances transformation through combinatorial effects that modulate p53 tumor suppressor functions and stability (8, 33, 37) as well as properties of the POD structure. Such a model would account for our observation that E4orf6 is more effective in enhancing transformation frequency (Fig. 1 and 2) and promoting neoplastic growth of transformed rat cells in cooperation with E1 oncogenes than is E4orf3 (Fig. 8 and Table 1). Obviously, a more detailed analysis of the intranuclear localization of E4orf6 in ABS and ABST cells is required to substantiate this hypothesis. The differences in growth properties and tumorigenicity between transformed rat cells expressing E4orf3 or E4orf6 correlate with the different p53 steady-state levels in these cells (Fig. 3B). These results provide further support for the view that E4orf6 dramatically enhances the intrinsic ability of E1-transformed rat cells to grow in a neoplastic state by actively reducing the p53 levels (38).
A striking observation from this study is that all established cell lines derived from transfections with pCMV-E1A and pCMV-E4orf3 or pSVHA-E4orf3 do not retain the viral E4orf3 cDNA. This result parallels the findings from our previous work on the transforming potential of the E4orf6 protein (37). The absence of the viral DNA templates in these transformants implies that both E4 gene products can transform primary cells with E1A by a “hit-and-run” mechanism. It is, therefore, tempting to speculate that transient expression of E4orf3 or E4orf6 with E1A proteins induces the accumulation of mutations in cellular genes that promote cell growth in culture. This hypothesis, however, has yet to be experimentally demonstrated. Interestingly, recent work has revealed that human cytomegalovirus (HCMV) immediate-early proteins IE1 and IE2 mediate a hit-and-run transformation of primary BRK cells in cooperation with E1A proteins (45). Significantly, the same viral proteins colocalize with and reorganize PML-associated nuclear bodies (1, 51). Although clearly speculative, these findings, along with the results presented in this report, hint at the possibility that E4orf3 and E4orf6 contribute to a hit-and-run transformation through their ability to target PODs. This model is intriguing because it has been proposed that DNA processing and/or inhibition of DNA damage is one potential function of cellular proteins associated with the PODs (7, 9, 10).
It is important to note that the ability of E4orf3 to promote focus formation in combination with E1 gene products is strictly dependent on the amounts of E4orf3 expression plasmids used in the transformation mixtures. We consistently find that higher concentrations of pCMV-E4orf3 or pSVHA-E4orf3 reduce the number of foci in a concentration-dependent manner, while lower amounts of these plasmids significantly increase the transformation frequency over E1 proteins alone (Fig. 1 and 2). This negative effect is also observed in transient transfection experiments with different human cell lines (36a). These observations suggests that E4orf3 may cause cytotoxic effects which result in a selection against cells expressing high levels of the adenovirus proteins. The molecular basis for these findings is unclear but may be also linked to the ability of E4orf3 to colocalize with PODs and to induce a relocalization of the PML protein. Recent studies have shown that increased expression of PML leads to decreased cell growth (26, 35). In addition, PML has a proapoptotic activity (3), and altered localization of PML is associated with growth suppression (34) and cellular senescence (24). Moreover, the PML-RARα fusion protein, which dramatically affects the distribution of POD-associated factors, has been recently found to induce cell death in different cell lines (12). Consequently, it may be possible that high levels of E4orf3 more efficiently induce the distribution of PML or other POD-associated proteins which may cause negative effects on cell growth under these conditions.
The enhancement on transformation frequency observed with low amounts of E4orf3-expressing plasmids is reminiscent of the previous notion that E4 proteins are very potent modulators of cell growth providing functions that may be more catalytic in nature rather than stoichiometric (5). In this context, it is possible that extremely low levels of E4orf3 are sufficient to start a cascade of events that cause uncontrolled cell growth. However, based on the observation that high levels of E4orf3 are deleterious to cell survival, it is conceivable that these activities must be tightly regulated by other viral proteins that counteract these negative effects. In fact, our studies suggest that E4orf3 functions are modulated in the presence of the E1B gene products. As opposed to the experiments using E1A alone, a fivefold increase of plasmid pCMV-E4orf3 (1 μg) increased the number of foci (Fig. 2A) rather than causing negative effects on transformation frequency (Fig. 1A). Also, in these assays both E4 proteins synergistically promoted focus formation (Fig. 2A), and more important, all established cell lines consistently expressed the viral proteins (Fig. 3). Clearly, ABT cells differed in the morphology from AB cells, exhibited higher growth rates and saturation densities (Fig. 9), and induced tumors more frequently than transformed cells expressing E1A and E1B proteins only (Table 1). This indicates that E4orf3 has growth-promoting activities under these conditions. The negative effect on transformation with E1A and E1B-19kDa (Fig. 2) further suggests that the large E1B protein promotes the positive effects on cell growth induced by the E4orf3 protein. Because we found that E4orf3, like E4orf6, can interact with E1B-55kDa in transformed rat cells (Fig. 6), it is possible that E1B-55kDa modulates the activity of the E4orf3 protein by binding to it. As an alternative, it is possible that E1B-55kDa directly or indirectly inhibits the activity of one or more POD-associated factors that are released by the E4orf3 protein. The latter possibility is intriguing because the colocalization of E1B-55kDa with PODs in virus-infected cells suggests that the E1B protein can interact with components of the POD (6, 10).
In this report, we also show for the first time that overexpression of PML efficiently suppresses transformation by adenovirus E1 gene products (Fig. 7). This result extents the previous observation that PML suppresses oncogenic transformation of NIH 3T3 cells by activated neu (29) and provides further support for the idea that PML is an important growth suppressor which may function similar to p53 (26, 34, 35). In addition, this result strongly indicates that modulation of POD-associated factors, such as PML, by adenovirus proteins plays a role in viral transformation. Our data suggest that E4orf3 does not antagonize the growth suppressing activity of PML, which is consistent with the observations that E4orf3 and PML do not physically interact in transformed rat cells and in vitro (10). Hence, E4orf3 may contribute to transformation through mechanisms that are independent from interactions with PML, although it has been shown that overexpression of PML blocks the E4orf3-induced reorganization of PODs (10). Our findings, however, predict that PML activity must be modulated by other adenovirus proteins to promote transformation. Because E1A, E1B-55kDa, and E4orf6 proteins have been found to colocalize with PODs, at least in virus-infected cells (6, 10), these viral proteins are prime candidates for such activities. Nevertheless, the ability of E4orf3 to colocalize with the PML structure suggests that other cellular POD-associated proteins deserve special attention with regard to the transforming activity of the E4orf3 protein. The identification of these cellular factors associated with E4orf3 will clarify pathways modulated by this protein and, perhaps, reveal new principles of viral transformation relevant to human neoplasms.
ACKNOWLEDGMENTS
We thank Georg Kuhn for helpful assistance with the confocal laser scan microscope, and we thank Franz Wiesenmeyer and Oskar Baumann for animal work.
This work was supported by the Deutsche Forschungsgemeinschaft (grant Do 343/4-1) and by the Fonds der Chemischen Industrie.
REFERENCES
- 1.Ahn J-H, Hayward G S. The major immediate-early proteins IE1 and IE2 of human cytomegalovirus colocalize with and disrupt PML-associated nuclear bodies at very early times in infected permissive cells. J Virol. 1997;71:4599–4613. doi: 10.1128/jvi.71.6.4599-4613.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ascoli C A, Maul G G. Identification of a novel nuclear domain. J Cell Biol. 1991;112:785–795. doi: 10.1083/jcb.112.5.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borden K L B, Campbell Dwyer E J, Salvato M S. The promyelocytic leukemia protein PML has a pro-apoptotic activity mediated through its RING domain. FEBS Lett. 1997;418:30–34. doi: 10.1016/s0014-5793(97)01344-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bridge E, Ketner G. Redundant control of adenovirus late gene expression by early region 4. J Virol. 1989;63:631–638. doi: 10.1128/jvi.63.2.631-638.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brough D E, Hsu H, Kulesa V A, Lee G M, Cantolupo L J, Lizonova A, Kovesdi I. Activation of transgene expression by early region 4 is responsible for a high level of persistent transgene expression form adenovirus vectors in vivo. J Virol. 1997;71:9206–9213. doi: 10.1128/jvi.71.12.9206-9213.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carvalho T, Seeler J S, Ohman K, Jordan P, Pettersson U, Akusjarvi G, Carmo Fonseca M, Dejean A. Targeting of adenovirus E1A and E4-ORF3 proteins to nuclear matrix-associated PML bodies. J Cell Biol. 1995;131:45–56. doi: 10.1083/jcb.131.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang J Y, Li L, Fan Y H, Mu Z M, Zhang W W, Chang K S. Cell-cycle regulation of DNA-damage-induced expression of the suppressor gene PML. Biochem Biophys Res Commun. 1997;240:640–646. doi: 10.1006/bbrc.1997.7692. [DOI] [PubMed] [Google Scholar]
- 8.Dobner T, Horikoshi N, Rubenwolf S, Shenk T. Blockage by adenovirus E4orf6 of transcriptional activation by the p53 tumor suppressor. Science. 1996;272:1470–1473. doi: 10.1126/science.272.5267.1470. [DOI] [PubMed] [Google Scholar]
- 9.Doucas V, Evans R M. The PML nuclear compartment and cancer. Biochim Biophys Acta. 1996;1288:M25–M29. doi: 10.1016/s0304-419x(96)00028-5. [DOI] [PubMed] [Google Scholar]
- 10.Doucas V, Ishov A M, Romo A, Juguilon H, Weitzman M D, Evans R M, Maul G G. Adenovirus replication is coupled with the dynamic properties of the PML nuclear structure. Genes Dev. 1996;10:196–207. doi: 10.1101/gad.10.2.196. [DOI] [PubMed] [Google Scholar]
- 11.Dyck J A, Maul G G, Miller W J, Chen J D, Kakizuka A, Evans R M. A novel macromolecular structure is a target of the promyelocyte-retinoic acid receptor oncoprotein. Cell. 1994;78:799–811. doi: 10.1016/0092-8674(94)90340-9. [DOI] [PubMed] [Google Scholar]
- 12.Ferrucci P F, Grignani F, Pearson M, Fagioli M, Nicoletti I, Pelicci P G. Cell death induction by the acute promyelocytic leukemia-specific PML/RARalpha fusion protein. Proc Natl Acad Sci USA. 1997;94:10901–10906. doi: 10.1073/pnas.94.20.10901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Graham F L, van der Eb A J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973;52:456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- 14.Grand R J, Grant M L, Gallimore P H. Enhanced expression of p53 in human cells infected with mutant adenoviruses. Virology. 1994;203:229–240. doi: 10.1006/viro.1994.1480. [DOI] [PubMed] [Google Scholar]
- 15.Grignani F, Fagioli M, Alcalay M, Longo L, Pandolfi P P, Donti E, Biondi A, Lo Coco F, Grignani F, Pelicci P G. Acute promyelocytic leukemia: from genetics to treatment. Blood. 1994;83:10–25. [PubMed] [Google Scholar]
- 16.Halbert D N, Cutt J R, Shenk T. Adenovirus early region 4 encodes functions required for efficient DNA replication, late gene expression, and host cell shutoff. J Virol. 1985;56:250–257. doi: 10.1128/jvi.56.1.250-257.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harlow E, Franza B R, Jr, Schley C. Monoclonal antibodies specific for adenovirus early region 1A proteins: extensive heterogeneity in early region 1A products. J Virol. 1985;55:533–546. doi: 10.1128/jvi.55.3.533-546.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harlow E, Pim D C, Crawford L V. Complex of simian virus 40 large-T antigen and host 53,000-molecular-weight protein in monkey cells. J Virol. 1981;37:564–573. doi: 10.1128/jvi.37.2.564-573.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harper J W, Adami G R, Wei N, Keyomarsi K, Elledge S J. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993;75:805–816. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- 20.Huang M M, Hearing P. Adenovirus early region 4 encodes two gene products with redundant effects in lytic infection. J Virol. 1989;63:2605–2615. doi: 10.1128/jvi.63.6.2605-2615.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Imperiale M J, Akusjärvi G, Leppard K N. Post-transcriptional control of adenovirus gene expression. Curr Top Microbiol Immunol. 1995;199:139–171. doi: 10.1007/978-3-642-79499-5_6. [DOI] [PubMed] [Google Scholar]
- 22.Javier R, Raska K J, Shenk T. Requirement for the adenovirus type 9 E4 region in production of mammary tumors. Science. 1992;257:1267–1271. doi: 10.1126/science.1519063. [DOI] [PubMed] [Google Scholar]
- 23.Javier R T. Adenovirus type 9 E4 open reading frame 1 encodes a transforming protein required for the production of mammary tumors in rats. J Virol. 1994;68:3917–3924. doi: 10.1128/jvi.68.6.3917-3924.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang W-Q, Ringertz N. Altered distribution of the promyelocytic leukemia-associated protein is associated with cellular senescence. Cell Growth Differ. 1997;8:513–522. [PubMed] [Google Scholar]
- 25.Kastner P, Perez A, Lutz Y, Rochette Egly C, Gaub M P, Durand B, Lanotte M, Berger R, Chambon P. Structure, localization and transcriptional properties of two classes of retinoic acid receptor alpha fusion proteins in acute promyelocytic leukemia (APL): structural similarities with a new family of oncoproteins. EMBO J. 1992;11:629–642. doi: 10.1002/j.1460-2075.1992.tb05095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Le X F, Vallian S, Mu Z H, Hung M C, Chang K S. Recombinant PML adenovirus suppresses growth arrest and tumorigenicity of human breast cancer cells by inducing G1 cell cycle arrest and apoptosis. Oncogene. 1998;16:1839–1849. doi: 10.1038/sj.onc.1201705. [DOI] [PubMed] [Google Scholar]
- 27.Leppard K N. E4 gene function in adenovirus, adenovirus vector and adeno-associated virus infections. J Gen Virol. 1997;78:2131–2138. doi: 10.1099/0022-1317-78-9-2131. [DOI] [PubMed] [Google Scholar]
- 28.Leppard K N. Regulated RNA processing and RNA transport during adenovirus infection. Semin Virol. 1998;8:301–307. [Google Scholar]
- 29.Liu J-H, Mu Z-M, Chang K-S. PML suppresses oncogenic transformation of NIH/3T3 cells by activated neu. J Exp Med. 1995;181:1965–1973. doi: 10.1084/jem.181.6.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Logan J, Pilder S, Shenk T. Functional analysis of adenovirus type 5 early region 1B. Cancer Cells. 1984;2:527–532. [Google Scholar]
- 31.Lowe S W, Ruley H E. Stabilization of the p53 tumor suppressor is induced by adenovirus 5 E1A and accompanies apoptosis. Genes Dev. 1993;7:535–545. doi: 10.1101/gad.7.4.535. [DOI] [PubMed] [Google Scholar]
- 32.Marton M J, Baim S B, Ornelles D A, Shenk T. The adenovirus E4 17-kilodalton protein complexes with the cellular transcription factor E2F, altering its DNA-binding properties and stimulating E1A-independent accumulation of E2 mRNA. J Virol. 1990;64:2345–2359. doi: 10.1128/jvi.64.5.2345-2359.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moore M, Horikoshi N, Shenk T. Oncogenic potential of the adenovirus E4orf6 protein. Proc Natl Acad Sci USA. 1996;93:11295–11301. doi: 10.1073/pnas.93.21.11295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mu Z-M, Chin K-V, Liu J-H, Lozano G, Chang K-S. PML, a growth suppressor disrupted in acute promyelocytic leukemia. Mol Cell Biol. 1994;14:6858–6867. doi: 10.1128/mcb.14.10.6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mu Z-M, Le X-F, Vallian S, Glassman A B, Chang K-S. Stable overexpression of PML alters regulation of cell cycle progression in HeLa cells. Carcinogenesis. 1997;18:2063–2069. doi: 10.1093/carcin/18.11.2063. [DOI] [PubMed] [Google Scholar]
- 36.Neill S D, Hemstrom C, Virtanen A, Nevins J R. An adenovirus E4 gene product trans-activates E2 transcription and stimulates stable E2F binding through a direct association with E2F. Proc Natl Acad Sci USA. 1990;87:2008–2012. doi: 10.1073/pnas.87.5.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36a.Nevels, M. Unpublished observation.
- 37.Nevels M, Rubenwolf S, Spruss T, Wolf H, Dobner T. The adenovirus E4orf6 protein can promote E1A/E1B-induced focus formation by interfering with p53 tumor suppressor function. Proc Natl Acad Sci USA. 1997;94:1206–1211. doi: 10.1073/pnas.94.4.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nevels, M., T. Spruss, H. Wolf, and T. Dobner. The adenovirus E4orf6 protein contributes to malignant transformation by antagonizing E1A-induced accumulation of the tumor suppressor protein p53. Oncogene, in press. [DOI] [PubMed]
- 39.Nevins J R, Vogt P K. Cell transformation by viruses. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Vol. 1. New York, N.Y: Lippincott-Raven; 1996. pp. 301–343. [Google Scholar]
- 40.Pandolfi P P, Alcalay M, Fagioli M, Zangrilli D, Mencarelli A, Diverio D, Biondi A, Lo Coco F, Rambaldi A, Grignani F, et al. Genomic variability and alternative splicing generate multiple PML/RAR alpha transcripts that encode aberrant PML proteins and PML/RAR alpha isoforms in acute promyelocytic leukemia. EMBO J. 1992;11:1397–1407. doi: 10.1002/j.1460-2075.1992.tb05185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Querido E, Marcellus R, Lai A, Rachel C, Teodoro J G, Ketner G, Branton P E. Regulation of p53 levels by the E1B 55-kilodalton protein and E4orf6 in adenovirus-infected cells. J Virol. 1997;71:3788–3798. doi: 10.1128/jvi.71.5.3788-3798.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41a.Rubenwolf, S. Unpublished data.
- 42.Sandler A B, Ketner G. Adenovirus early region 4 is essential for normal stability of late nuclear RNAs. J Virol. 1989;63:624–630. doi: 10.1128/jvi.63.2.624-630.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sarnow P, Hearing P, Anderson C W, Reich N, Levine A J. Identification and characterization of an immunologically conserved adenovirus early region 11,000 Mr protein and its association with the nuclear matrix. J Mol Biol. 1982;162:565–583. doi: 10.1016/0022-2836(82)90389-8. [DOI] [PubMed] [Google Scholar]
- 44.Sarnow P, Sullivan C A, Levine A J. A monoclonal antibody detecting the adenovirus type 5-E1b-58Kd tumor antigen: characterization of the E1B-58Kd tumor antigen in adenovirus-infected and -transformed cells. Virology. 1982;120:510–517. doi: 10.1016/0042-6822(82)90054-x. [DOI] [PubMed] [Google Scholar]
- 45.Shen Y, Zhu H, Shenk T. Human cytomegalovirus IE1 and IE2 proteins are mutagenic and mediate “hit-and-run” oncogenic transformation in cooperation with the adenovirus E1A proteins. Proc Natl Acad Sci USA. 1997;94:3341–3345. doi: 10.1073/pnas.94.7.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Steegenga W T, Riteco N, Jochemsen A G, Fallaux F J, Bos J L. The large E1B protein together with the E4orf6 protein target p53 for active degradation in adenovirus infected cells. Oncogene. 1998;16:349–357. doi: 10.1038/sj.onc.1201540. [DOI] [PubMed] [Google Scholar]
- 47.Sternsdorf T, Grotzinger T, Jensen K, Will H. Nuclear dots: actors on many stages. Immunobiology. 1997;198:307–331. doi: 10.1016/S0171-2985(97)80051-4. [DOI] [PubMed] [Google Scholar]
- 48.Weiden M D, Ginsberg H S. Deletion of the E4 region of the genome produces adenovirus DNA concatemers. Proc Natl Acad Sci USA. 1994;91:153–157. doi: 10.1073/pnas.91.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weinberg D H, Ketner G. Adenoviral early region 4 is required for efficient viral DNA replication and for late gene expression. J Virol. 1986;57:833–838. doi: 10.1128/jvi.57.3.833-838.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weiss R S, McArthur M J, Javier R T. Human adenovirus type 9 E4 open reading frame 1 encodes a cytoplasmic transforming protein capable of increasing the oncogenicity of CREF cells. J Virol. 1996;70:862–872. doi: 10.1128/jvi.70.2.862-872.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wilkinson G W G, Kelly C, Sinclair J H, Rickards C. Disruption of PML-associated nuclear bodies mediated by the human cytomegalovirus major immediate early gene product. J Gen Virol. 1998;79:1233–1245. doi: 10.1099/0022-1317-79-5-1233. [DOI] [PubMed] [Google Scholar]
- 52.Zantema A, Schrier P I, Davis O A, van L T, Vaessen R T, van der Eb A J. Adenovirus serotype determines association and localization of the large E1B tumor antigen with cellular tumor antigen p53 in transformed cells. Mol Cell Biol. 1985;5:3084–3091. doi: 10.1128/mcb.5.11.3084. [DOI] [PMC free article] [PubMed] [Google Scholar]