Abstract
Mpox represents a persistent health concern with varying disease severity. Reinfections with mpox virus (MPXV) are rare, possibly indicating effective memory responses to MPXV or related poxviruses, notably vaccinia virus (VACV) from smallpox vaccination. We assessed cross-reactive and virus-specific CD4+ and CD8+ T cells in healthy individuals and mpox convalescent donors. Cross-reactive T cells were most frequently observed in healthy donors over 45 years. Notably, long-lived memory CD8+ T cells targeting conserved VACV/MPXV epitopes were identified in older individuals more than four decades after VACV exposure and exhibited stem-like characteristics, defined by T cell factor-1 (TCF-1) expression. In mpox convalescent donors, MPXV-reactive CD4+ and CD8+ T cells were more prevalent than in controls, demonstrating enhanced functionality and skewing toward effector phenotypes, which correlated with milder disease. Collectively, we report robust effector memory MPXV-specific T cell responses in mild mpox and long-lived TCF-1+ VACV/MPXV-specific CD8+ T cells decades after smallpox vaccination.
Keywords: viral immunity, T cells, infectious diseases, MPXV, VACV, monkeypox, mpox, vaccinia virus, long-lived memory T cells
Graphical abstract
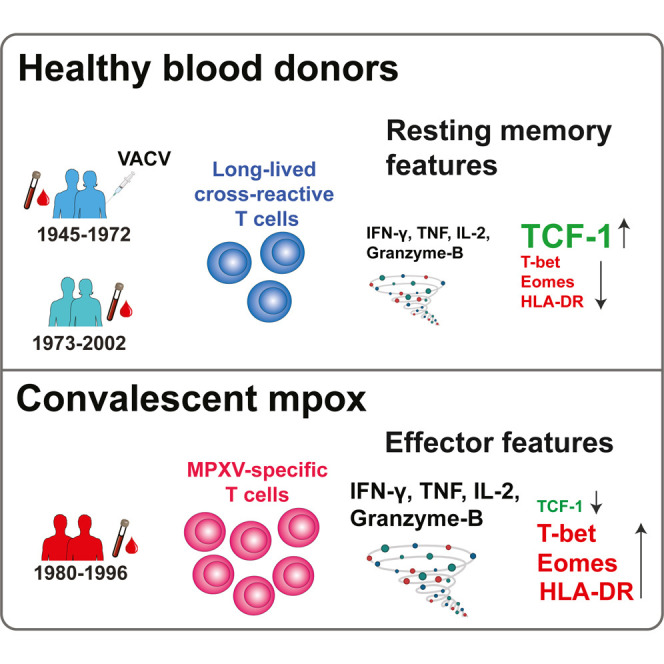
Adamo et al. demonstrate that MPXV-reactive T cells are present in many healthy blood donors—especially in older individuals. Smallpox-vaccinated individuals maintain very long-lived resting memory T cells that are cross-reactive to MPXV, whereas those recovering from mpox develop polyfunctional effector T cell responses associated with milder disease outcomes.
Introduction
In 2022, the unprecedented spread of mpox (previously monkeypox) worldwide led the WHO to declare mpox a global health emergency.1 Mpox is a zoonotic infection caused by the mpox virus (MPXV), an orthopoxvirus (OPXV) belonging to the Poxviridae family.2 Human-to-human transmission of mpox occurs upon contact with infected skin, bodily fluids, or contaminated items such as bedding.3 Symptoms of the infection include fever, myalgia, lymphadenopathies, skin and mucosal rash.3 , 4 Although disease presentation resembles smallpox, caused by the closely related variola virus (VARV), mpox has lower mortality.4 Once individuals clear the MPXV infection, re-infection is rare,5 probably due to the establishment of functional immunological memory. However, given that mpox has largely been confined to the African continent until the recent outbreak, the extent of pre-existing immunity in the general population of non-African countries is very limited.
A few studies have shown smallpox vaccination to provide at least partial protection from mpox,6 , 7 likely due to the high sequence homology between MPXV and the vaccinia virus (VACV) employed in smallpox vaccination.8 Notably, CD4+ and CD8+ T cells generated through smallpox vaccination boosted MPXV-reactive T cell responses in individuals who encountered MPXV.6 Indeed, VACV-specific memory T cells can cross-recognize MPXV antigens, as demonstrated by a recent study showing a strong increase in the ability to respond to MPXV-derived peptide pools following a booster of the smallpox vaccine.9 As global smallpox vaccination campaigns ended in the 1970s, and most individuals have not received smallpox boosters in the last few decades, it is unclear whether residual T cell reactivity from VACV exposure would translate into more robust T cell responses to MPXV in older adults and the elderly. To answer these questions, a more comprehensive assessment of cross-reactive T cell responses with targeted MPXV peptide pools across different age spans is needed. In addition, although durable cellular immune responses upon VACV infection are well documented,10 much less is known about T cell responses after MPXV infection. While the presence of human MPXV-specific T cells in mpox disease has been reported by a handful of studies,6 , 11 , 12 it remains unknown if T cells induce a robust and functional memory response after MPXV infection.
Results
Uninfected blood donors show cross-reactive memory responses to MPXV antigens
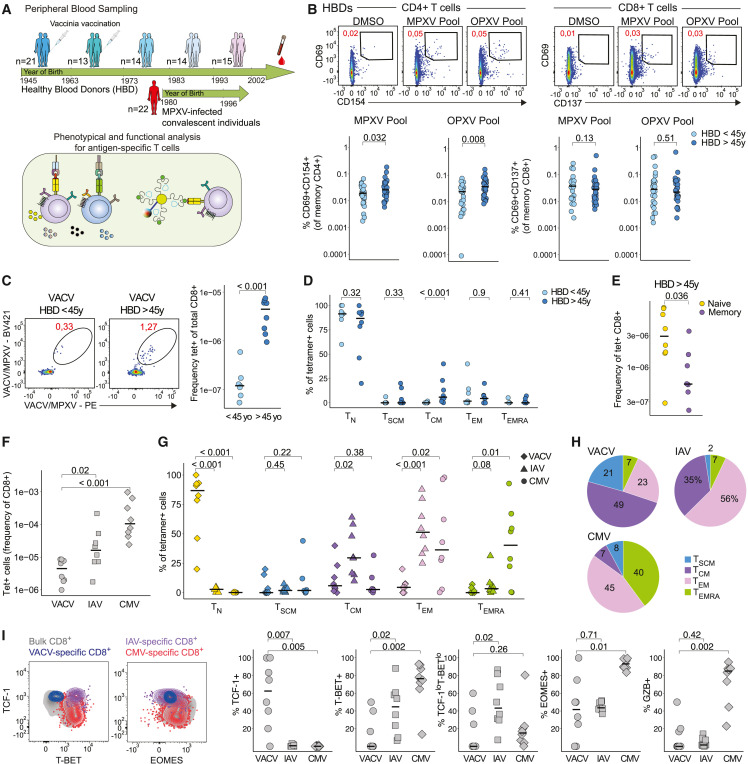
To examine the impact of cross-reactive MPXV memory T cell responses, we assessed CD4+ and CD8+ T cell reactivity to MPXV-derived (MPXV) and orthopox-derived (OPXV) peptide pools9 in healthy blood donors (HBDs) of different age groups, as well as individuals who recovered from mpox (hereafter convalescent donors) (Figures 1 A and S1A; Table S1). Activation-induced marker (AIM) assays were used to quantify MPXV/OPXV-reactive CD4+ and CD8+ T cells via the upregulation of CD69 and CD40L (CD154) and CD69 and 4-1BB (CD137), respectively, as previously described.13 , 14 , 15 We hypothesized that uninfected adult HBDs could have cross-reactive memory T cells to MPXV due to exposure to other poxviruses, such as VACV. Indeed, about 30% of HBDs, including younger individuals, showed positive CD4+ and CD8+ T cells responses to MPXV and OPXV pools (Figures S1B and S1C). While we observed no direct correlation between the magnitude of T cell responses and age (Figure S1B), individuals >45 years of age (i.e., born before 1976) showed significantly higher CD4+ T cell reactivity to both MPXV and OPXV pools (Figure 1B). These trends were not distinguished for CD8+ T cells (Figure 1B).
Figure 1.
VACV-induced T cell responses associate with cross-reactive responses to MPXV in HBDs.
(A) Schematic of study design.
(B) Representative plots showing CD69/CD154 and CD69/CD137 expression after peptide stimulation on memory CD4+ and CD8+ T cells (top) and quantification of net frequencies (background-subtracted using dimethyl sulfoxide [DMSO] control) (bottom).
(C) Tetramer staining of VACV/MPXV epitopes in HBDs of different age groups and quantification.
(D) Comparison of memory population frequency among tetramer+ CD8+ T cells in different age subgroups.
(E) Frequencies of naive and memory T cells within tetramer+ CD8+ T cells from HBDs.
(F) Frequencies of VACV+, IAV+, and CMV+ CD8+ T cells in HBDs.
(G) Comparison of naive and memory population frequency among tetramer+ CD8+ T cells.
(H) Subpopulation distribution of memory VACV+, IAV+, and CMV+ CD8+ T cells in HBDs.
(I) Representative plots showing transcription factors expression (left) and frequency of transcription factor and Granzyme B expression within VACV+, IAV+, and CMV+ CD8+ T cells in HBDs (right). In (B)–(G) and (I), Mann-Whitney test. See also Figure S1.
To investigate whether individuals exposed to VACV would maintain reactive CD8+ T cells more than four decades after primary exposure, we screened HBDs for three A∗02:01-restricted VACV/MPXV cross-reactive epitopes (CLTEYILWV, ILDDNLYKV, and KVDTFYYV), using MHC-I tetramers. Given the expected low frequency of VACV/MPXV+ cells, we combined tetramers from all three epitopes and used an enrichment strategy for tetramer-specific cells (Figure S1D), as previously described.16 , 17 We observed a higher frequency of VACV/MPXV+ memory CD8+ T cells in individuals >45 years of age as compared with younger individuals (<45 years of age) (Figure 1C). While the frequency of naive precursors (CD45RA+, CCR7+, and CD95−) was comparable between the two age groups, older individuals showed a higher proportion of CD45RA− CCR7+ central memory T (TCM) cells (Figure 1D). Collectively, these data demonstrate that individuals above the age of 45 maintain low levels of memory CD8+ T cells that cross-recognize MPXV epitopes (Figure 1E).
Differential effector potential of memory CD8+ T cells recognizing VACV/MPXV, IAV, and CMV
We next compared the features of VACV/MPXV-specific CD8+ T cells to other memory populations probed with MHC-I tetramers (see STAR Methods for epitopes) in HBDs. Compared with cytomegalovirus (CMV)- and influenza A virus (IAV)-specific T cells, VACV/MPXV-specific CD8+ T cells were maintained at lower frequencies (Figure 1F) and had a markedly distinct phenotype, mainly comprising naive precursor phenotypes (Figures 1G and S1E). Within the antigen-experienced compartment, VACV/MPXV-specific CD8+ T cells were predominantly TCM cells and T stem cell memory cells (TSCM), whereas IAV-specific and CMV-specific CD8+ T cells mostly displayed CD45RA−, CCR7− effector memory T (TEM) or CD45RA+, CCR7− effector memory T (TEMRA) cell phenotypes, respectively (Figures 1H and S1F). Both memory subsets are associated with heightened effector functional and reduced re-circulation and self-renewal capability.18 Accordingly, memory VACV/MPXV-specific CD8+ T cells showed differential expression of key transcription factors regulating T cell function, with high expression levels of T cell factor-1 (TCF-1) and low expression levels of T-bet (Figures 1I and S1G). CMV-specific T cells expressed the highest levels of T-bet, Eomes, and Granzyme B (GZB) relative to VACV/MPXV-specific and IAV-specific CD8+ T cells (Figures 1I and S1H). Collectively, these data suggest that VACV/MPXV-specific CD8+ T cells generated upon VACV exposure are maintained decades after vaccination as TCF-1+ memory cells.
Mpox patients generate robust T cell responses to MPXV antigens
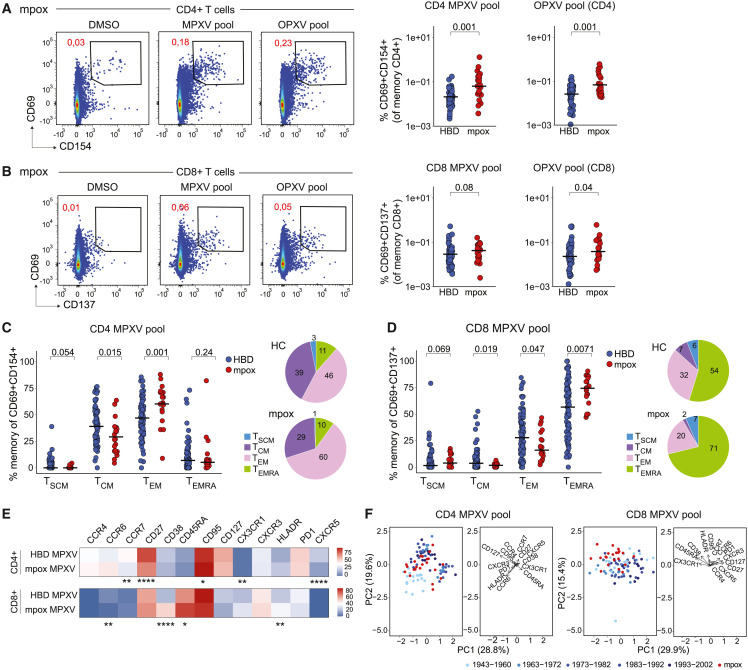
We next assessed CD4+ and CD8+ T cell responses post-MPXV infection, observing positive responses in 72% of convalescent donors for MPXV and 75% for OPXV pools (Figures S2A and S2B). Overall, mpox convalescent individuals had higher CD4+ T cell responses to MPXV, compared with HBDs (Figure 2 A), with no strong relationship to time after symptom onset (Figure S2C). Similar data were observed for the OPXV pool (Figures 2A and S2C) and were confirmed by comparing patients with HBDs of different age groups (Figure S2D). Notably, differences were less pronounced between convalescent donors and HBDs of the oldest age group (Figure S2D). OPXV-reactive CD8+ T cell responses were also significantly higher among convalescent donors than HBDs, while MPXV-reactive CD8+ T cell responses showed a modest non-significant increase in convalescent donors (Figure 2B). In total, 53% and 56% of previously infected patients showed positive CD8+ T cell responses to MPXV and OPXV pools, respectively (Figure S2B), with no clear relationship to time after symptom onset (Figure S2C). Reactivity was generally lower for CD8+ T cells, with no significant differences between convalescent donors and individual HBD age groups (Figure S2D). Post-mpox MPXV-reactive T cells displayed a higher effector-like memory phenotype. MPXV-reactive CD4+ T cells were predominantly TEM, with HBDs having higher TCM frequencies (Figure 2C). MPXV-reactive CD8+ T cells had higher TEMRA proportions, with reduced TCM and TEM frequencies, compared with HBDs (Figure 2D). Similar phenotypes were observed among OPXV-reactive T cells (Figures S2E and S2F). We did not observe strong dynamics in memory phenotypes within the evaluated time frame (36–143 days), except for a modest enrichment in CD4+ TEMRA cells (Figures S3A and S3B). Collectively, these data demonstrate robust inductions of T cell responses and predominance of TEM phenotypes after mpox.
Figure 2.
Magnitude of T cell responses in HBDs and mpox convalescent patients
(A) Representative flow plots showing CD69/CD154 expression after peptide stimulations, gated on memory CD4+ cells (left) and comparison of net frequencies of AIM+ CD4+ between mpox patients and HBDs (right).
(B) Representative flow plots showing CD69/CD137 expression on memory CD8+ T cells and quantification as in (A).
(C) Comparison of frequencies (left) and distribution (right) of CD4+ T cell memory subsets in MPXV-reactive cells of HBDs and mpox patients.
(D) Comparison of frequencies (left) and distribution (right) of CD8+ T cell memory subsets as in (C).
(E) Heatmap showing marker expression level on MPXV-reactive CD4+ and CD8+ T cells between HBDs and convalescent donors.
(F) PCA plot using the dataset in (E) to show the segregation of HBDs and convalescent donors and key markers associated with the segregation.
In (A)–(E), Mann-Whitney test. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, and ∗∗∗∗p < 0.0001 in (E). See also Figures S2 and S3.
MPXV-reactive T cells after mpox exhibit stronger activation and effector profiles
After examining the phenotypical features of AIM+ T cells, we observed differential expression of surface markers and chemokine receptors between convalescent donors and HBDs. Following mpox, MPXV-reactive CD4+ T cells had lower CCR7, CD27, and CXCR5 and higher CD95 expression (Figures 2E and S3C), consistent with skewing toward effector phenotypes. MPXV-reactive CD8+ T cells displayed increased CD45RA, CD38, and HLA-DR expression post-mpox (Figures 2E and S3D), with similar phenotypes in OPXV-reactive CD4+ and CD8+ T cells (Figures S3E-S3G). Interestingly, the polarization of MPXV-reactive CD4+ T cells was also affected by recent mpox. Convalescent donors exhibited lower CXCR5+ frequency and higher CCR6 and CCR4 co-expression than HBDs (Figures S3H and S3I ), with CCR6+CCR4+ frequency increasing and CXCR3+ frequency decreasing over time post-symptom onset (Figure S3J). When combining all parameters in a principal-component analysis (PCA), we found no clear separation between MPXV/OPXV-reactive CD4+ T cells from mpox convalescents and HBDs (Figures 2F and S3K), with HBDs separated by age. In contrast, MPXV/OPXV-reactive CD8+ T cells showed better PCA separation, driven by CD38, CD45RA, and CX3CR1 (Figures 2F and S3K), with less age effect. Overall, these data indicate that age and recent mpox affect memory profiles of MPXV/OPXV-reactive CD4+ and CD8+ T cells, respectively.
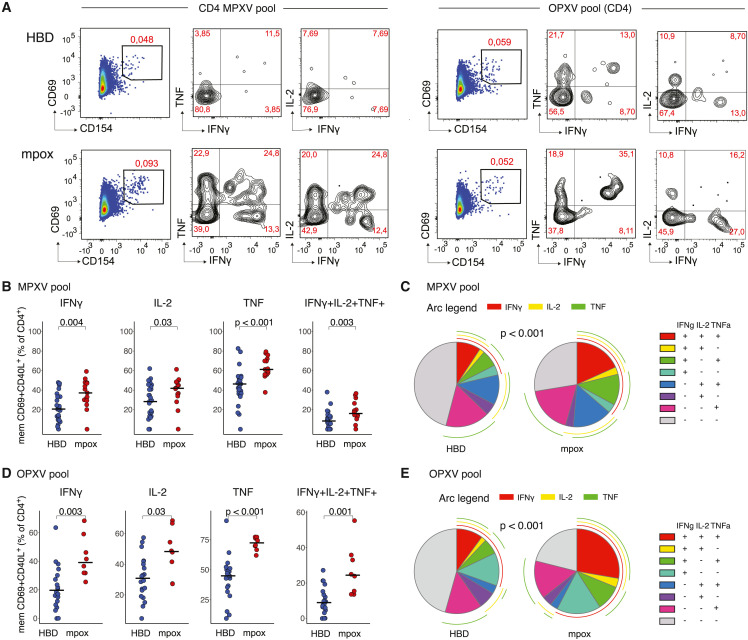
MPXV-specific CD4+ T cells display polyfunctionality upon mpox infection
To gain functional insights into T cell responses generated after mpox, we combined our AIM assay with assessing IFN-γ, IL-2, and TNF production (Figure 3 A). MPXV-reactive CD4+ T cells showed more frequent production of all measured cytokines (IFN-γ, IL-2, and TNF) in convalescent donors compared with HBDs (Figure 3B). Overall, CD4+ T cells from convalescent donors showed higher polyfunctionality, demonstrated as significantly increased co-expression patterns for all three cytokines (Figures 3B and 3C). OPXV-reactive CD4+ T cells showed a similar profile, with overall higher proportions of cytokine-secreting cells (Figure 3D) and markedly increased polyfunctionality after mpox, i.e., a high frequency of CD4+ T cells secreting all three cytokines (Figure 3E).
Figure 3.
Functional profile of AIM+ CD4+ T cells
(A) Representative flow plot for IFN-γ, IL-2, and TNF expression within the AIM+ CD4+ T cell population following stimulation with MPXV and OPXV peptide pools.
(B and C) Comparison between patterns of single expression and co-expression for IFN-γ, IL-2, and TNF after MPXV pool stimulation.
(D and E) Comparison between patterns of single expression and co-expression for IFN-γ, IL-2, and TNF after OPXV pool stimulation.
In (B) and (D), Mann-Whitney test. In (C) and (E), permutation test.
MPXV-specific memory CD8+ T cells after mpox exhibit increased T-bet and GZB expression
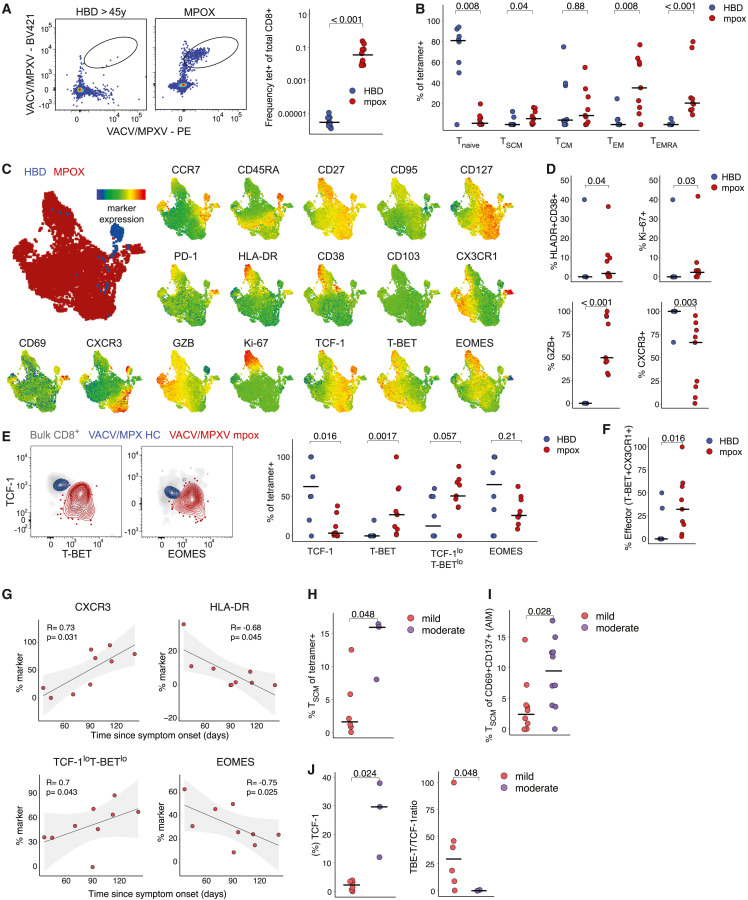
To investigate MPXV-specific CD8+ T cells after mpox infection directly ex vivo, we analyzed HLA-A∗02:01+ mpox convalescent individuals (n = 9) with VACV/MPXV cross-reactive MHC-I tetramers. Convalescent donors had significantly higher frequencies of MPXV/VACV-specific CD8+ T cells (Figure 4 A). Furthermore, they showed a markedly higher proportion of memory MPXV/VACV-specific CD8+ T cells (Figure S4A), compared with previously VACV-exposed HBDs. Overall, a higher proportion of VACV/MPXV-specific CD8+ T cells had TEM or TEMRA phenotypes after mpox, compared with VACV-exposed HBDs (Figures 4B and S4A).
Figure 4.
Phenotypical profile of VACV/MPXV tetramer+ CD8+ T cells in HBDs and mpox patients
(A) Representative flow plots showing VACV/MPXV tetramer staining on CD8+ T cells (left) and frequencies (right).
(B) Frequencies of naive and memory T cell subsets within tetramer+ CD8+ T cells in HBDs and convalescent donors.
(C) UMAP visualization of tetramer+ CD8+ T cells from HBDs and convalescent donors, and expression of individual markers colored by marker expression levels.
(D) Frequency of HLA-DR+CD38+, Ki-67+, Granzyme B+ CD8+ T cells, and CXCR3+ expression in HBDs vs. convalescent donors.
(E) Representative flow plots of transcription factors expression within VACV/MPXV+ CD8+ T cells in HBDs vs. convalescent donors (left) and quantification (right).
(F) Frequency of T-bet+CX3CR1+ terminally differentiated (effector) cells in HBDs vs. convalescent donors.
(G) Spearman correlation of marker expression with time after symptom onset.
(H and I) Frequency of MPXV-specific TSCM cells identified with tetramers (H) or AIM assay (I) after mild vs. moderate mpox.
(J) Frequency of TCF1+ cells and TCF1/T-bet ratio among tetramer+ cells after mild vs. moderate mpox.
In (A), (B), (D)–(F), and (H)–(J), Mann-Whitney test. In (G), Spearman rank correlation. See also Figure S4.
We next concatenated VACV/MPXV-specific CD8+ T cell data (n = 17 markers) from patients and HBDs and visualized cells using uniform manifold approximation and projection (UMAP) dimensionality reduction. HBD VACV/MPXV-specific CD8+ T cells exhibited a distinct phenotype from mpox convalescent donors (Figure 4C), primarily due to higher CD45RA, CCR7, and TCF-1 expression and lower GZB, CX3CR1, and T-bet levels. After limiting the comparison between HBDs and convalescent to memory VACV/MPXV-specific CD8+ T cell (TSCM, TCM, TEM, or TEMRA), we still observed marked differences between the two groups. VACV/MPXV-specific CD8+ T cells post-mpox displayed elevated signs of residual activation (CD38 and HLA-DR), proliferation (Ki-67), cytotoxicity (GZB), and lower expression intensity of CXCR3 (Figure 4D). We observed a notably higher expression of TCF-1 among HBDs, while T-bet expression was elevated in VACV/MPXV-specific CD8+ T cells following mpox. The TCF-1loT-betlo (Figure 4E) and T-bet+CX3CR1+ terminally differentiated (effector) (Figure 4F) phenotypes were more prevalent in convalescent donors. Examining the relationship between activation markers, transcription factors, and time since symptom onset, we found a predictable decrease in HLA-DR+ T cell frequency, and a progressive increase in CXCR3+ cells (Figure 4G). A tendency of decline in Ki-67 was also observed over time (Figure S4B). We also observed a progressive increase in the TCF-1loT-betlo phenotype and a decrease in Eomes expression, while TCF-1 and T-bet levels remained stable (Figure 4G and Figure S4C).
We finally categorized mpox convalescent donors based on disease severity (see STAR Methods). Convalescent donors were differentiated into mild mpox (local therapy) and moderate mpox (systemic analgesic or antibiotic therapy) groups. Following moderate mpox, we observed increased frequencies of tetramer+ TSCM cells (Figures 4H and S4D) and MPXV-reactive TSCM cells, identified by the AIM assay (Figures 4I, S4E, and S4F) in the absence of changes in total antigen-specific T cell frequencies (Figure S4G), and enrichment in CXCR3+ MPXV-reactive CD4+ T cells (Figure S4H). The enhanced stem-like state was not affected by the time since symptom onset (Figures S4I and S4J) and correlated with increased TCF-1 levels (Figure 4J) and a trend toward lower GZB and T-bet frequencies (Figures S4K and S4L) after moderate mpox. In conclusion, our findings indicate that memory VACV/MPXV-specific CD8+ T cells exhibit strong effector potential following MPXV infection, especially in mild cases.
Discussion
T cells are crucial for resolving viral infections and generating protective immunological memory. We here assessed cross-reactive and virus-specific T cell immunity to MPXV in HBDs and individuals recovering from mpox.
Memory T cells generated from a single infection can cross-react to antigens from unrelated pathogens13 , 19 , 20 and influence disease outcomes.21 This is particularly relevant for viral infections since emerging pathogenic viruses are often closely related to known viruses and can exhibit significant overlap in T cell epitopes. Individuals immunized with VACV acquired cross-reactive immunity to smallpox, which facilitated the eradication of the disease in 1980. Consequently, mass vaccination ceased in the 1970s, and immunity to VACV/VARV in the general population began to wane.10 Given the high homology between MPXV and VACV/VARV, waning VACV-induced immunity could be a contributing factor to the current mpox outbreak.4 As in other epidemics, we face the important task of identifying risk populations, i.e., individuals with a higher risk of infection or severe disease. Both immunological and non-immunological factors are likely contributors. According to epidemiological studies of the current outbreak, mpox transmission occurs mainly through close physical contact and seems to predominantly affect men who have sex with men (MSM).22 , 23 The median age of reported cases is relatively low (3722 and 3523 years), but whether this is partially due to stronger pre-existing immunity in older individuals remains unclear. In this study, we examined the prevalence of T cell cross-reactivity in unexposed HBDs of different ages and found that about one-third of HBDs had cross-reactive T cell responses to mpox antigens. Interestingly, cross-reactivity was also present in individuals born after 1976 who had not received smallpox vaccination but were likely exposed to other poxviruses such as molluscum contagiosum virus (MCV). These findings align with a previous study that showed MPXV reactivity in young individuals without exposure to mpox.9 Upon examining individuals of an age compatible with having received smallpox vaccination, we observed higher VACV/MPXV reactivity than among younger donors. In older blood donors, memory CD8+ T cells specific for conserved VACV/MPXV epitopes were maintained four decades after priming, albeit at low frequencies. VACV/MPXV-specific memory CD8+ T cells exhibited less of an effector profile, compared with CMV- and IAV-specific memory CD8+ T cells, including lower T-bet and GZB expression. These data could be indicative of memory T cell de-differentiation in the absence of antigen exposure, as described after yellow fever virus (YFV) vaccination.24 In patients who contracted mpox, we detected robust T cell responses that differed in phenotype from cross-reactive memory T cells detected in HBDs. In convalescent donors, we observed skewing toward effector phenotypes (TEM among CD4+ T cells and TEMRA among CD8+ T cells) and higher expression of T-bet and GZB. Interestingly, effector skewing and cytotoxic potential appeared to be higher in patients with mild mpox compared with those with moderate disease, potentially accounting for more prompt virus eradication. Larger studies are needed to confirm these findings.
In summary, we provide evidence of long-term maintenance of immunological memory decades after smallpox vaccination, which accounts for a small but detectable population of cross-reactive memory T cells to MPXV in older unexposed individuals. Furthermore, we describe the generation of robust polyfunctional TEM responses upon mpox infection, particularly in cases of mild disease, and demonstrate that T cells acquire high cytokine and cytotoxic capacity.
Limitations of the study
The current study has several limitations. First, the smallpox vaccination status of individual blood donors was unknown. The analysis was performed considering the age of the donors at the end of the smallpox vaccination program in Sweden.25 To compare virus-specific memory T cell populations, the analysis was carried out on HBDs aged 45 years or older. However, CMV- and IAV-specific memory T cell responses were detected in HBDs for which demographic information was incomplete, making it impossible to control for age. Due to the limited availability of patient samples, several CD8-directed MPXV peptide pools were pooled together for stimulations (see STAR Methods), which could lead to suboptimal stimulation and subsequent underestimation of the frequency of MPXV-reactive CD8+ T cell responses. Finally, the mpox patient cohort comprised young males only. Although the mpox outbreak was seen at a younger age, it precluded us from studying conserved VACV/MPXV-specific memory responses in age-matched individuals.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Anti-human CCR7 APC-Cy7 | BioLegend |
Cat# 353212; RRID:AB_10916390 |
| Anti-human CCR4 BB700 | BD Biosciences |
Cat# 566475; RRID:AB_2744302 |
| Anti-human CCR6 BUV737 | BD Biosciences |
Cat# 612780; RRID:AB_2870109 |
| Anti-human CXCR3 AF647 | BioLegend |
Cat# 353712; RRID:AB_10962948 |
| Anti-human CX3CR1 PE | BioLegend |
Cat# 341604; RRID:AB_1595456 |
| Anti-human CD40L BV421 | BioLegend |
Cat# 310824; RRID:AB_2562721 |
| Anti-human 4-1BB PE-Cy7 | BioLegend |
Cat# 309818; RRID:AB_2207741 |
| Anti-human CD4 BUV496 | BD Biosciences |
Cat# 612936; RRID:AB_2870220 |
| Anti-human CD14 BV510 | BioLegend |
Cat# 301842; RRID:AB_2561946 |
| Anti-human CD19 BV510 | BioLegend |
Cat# 302242; RRID:AB_2561668 |
| Anti-human CD45RA BV570 | BioLegend |
Cat# 304132; RRID:AB_2563813 |
| Anti-human CD69 BV650 | BioLegend |
Cat# 310934; RRID:AB_2563158 |
| Anti-human CD3 BUV805 | BD Biosciences |
Cat# 612895; RRID:AB_2870183 |
| Anti-human CD8 BUV395 | BD Biosciences |
Cat# 563795; RRID:AB_2722501 |
| Anti-human HLADR BUV615 | BD Biosciences |
Cat# 751142; RRID:AB_2875168 |
| Anti-human CD95 PEDazzle594 | BioLegend |
Cat# 305634; RRID:AB_2564221 |
| Anti-human PD1 BV711 | BioLegend |
Cat# 329928; RRID:AB_2562911 |
| Anti-human CD127 PE-Cy5 | BioLegend |
Cat# 351324; RRID:AB_10915554 |
| Anti-human CD27 BV786 | BioLegend |
Cat# 302832; RRID:AB_2562674 |
| Anti-human CD38 APC-R700 | BD Biosciences |
Cat# 564979; RRID:AB_2744373 |
| Anti-human Ki67 AF647 | BD Biosciences |
Cat# 558615; RRID:AB_647130 |
| Anti-human IL-2 PE-Dazzle594 | BioLegend |
Cat# 500344; RRID:AB_2564091 |
| Anti-human TNFa BV650 | BD Biosciences |
Cat# 563418; RRID:AB_2738194 |
| Anti-human CD69 BUV563 | BD Biosciences |
Cat# 748764; RRID:AB_2873167 |
| Anti-human IFN-γ PE | BioLegend |
Cat# 506507; RRID:AB_315440 |
| Anti-human TCF1 AF488 | CellSignaling | Cat# 6444S; RRID:AB_2199302 |
| Anti-human Granzyme B BB790 | BD Biosciences | N/A (custom) |
| Anti-human CX3CR1 BUV661 | BD Biosciences |
Cat# 750690; RRID:AB_2874813 |
| Anti-human CXCR3 PE-Cy5 | Biolegend | Cat# 353756; RRID:AB_2904375 |
| Anti-human PD1 BUV737 | BD Biosciences | Cat# 612791; RRID:AB_2870118 |
| Anti-human CD38 BUV496 | BD Biosciences | Cat# 612946; RRID:AB_287022 |
| Anti-human CD27 BV786 | BioLegend |
Cat# 302832; RRID:AB_2870225 |
| Anti-human CD69 BV750 | BD Biosciences | Cat# 747522; RRID: AB_2872182 |
| Anti-human HLADR BV650 | BD Biosciences |
Cat# 564231; RRID:AB_2738685 |
| Anti-human CD103 BV605 | BioLegend BV605 |
Cat# 350218; RRID:AB_2564283 |
| Anti-human CD127 BB630 | BD Biosciences | N/A (custom) |
| Anti-human CD4 PE Cy5.5 | Invitrogen | Cat# 35-0042-82; RRID:AB_11218300 |
| Anti-human TBET PE-Cy7 | Ebioscience | Cat# 25-5825-82; RRID:AB_11042699 |
| Anti-human KI67 AF700 | Biosciences | Cat# 561277; RRID:AB_10611571 |
| Anti-human EOMES EF660 | Ebioscience | Cat# 50-4877-42; RRID:AB_257422 |
| Biological samples | ||
| Healthy blood donor (HBD) PBMCs, cryopreserved | Karolinska Institutet | N/A |
| Mpox convalescent donor PBMCs, cryopreserved | Karolinska Institutet | N/A |
| Chemicals, peptides, and recombinant proteins | ||
| MPXV peptide pools | Grifoni et al.9 | Grifoni et al.9; Tables S1 and S4 |
| OPXV peptide pools | Grifoni et al.9 | Grifoni et al.9; Tables S1 and S4 |
| BD Golgi Stop (with Monensin) | BD Biosciences | Cat# 554724; RRID:AB_2869012 |
| Brefeldin A | BioLegend | Cat# 420601 |
| Brilliant Stain Buffer Plus | BD Biosciences | Cat# 566385; RRID:AB_2869761 |
| DNAse | BD Biosciences | Cat# 4716728001 |
| FoxP3/Transcription Factor Buffer Set | ThermoFisher Scientific | Cat# 00-5523-00 |
| LIVE/DEAD Fixable Aqua Dead Cell stain Kit | ThermoFisher Scientific | Cat# L34957 |
| Paraformaldehyde | Biotium | Cat# 22023 |
| RPMI-1640 without L-Glutamine | Cytiva | Cat# SH30096.01 |
| Fetal bovine serum | Sigma-Aldrich | Cat# F7524 |
| Penicillin-streptomycin | Cytiva | Cat# SV30010 |
| L-glutamine | Sigma-Aldrich | Cat# 59202C |
| Software and algorithms | ||
| FlowJo v 10.7.1 | FlowJo | RRID:SCR_008520 |
| R v 4.2.1 | R | RRID: SCR_001905 |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Marcus Buggert (marcus.buggert@ki.se).
Materials availability
HLA class I tetramers can be generated and shared on a collaborative basis.
Experimental model and subject details
Study design
This study aimed to characterize the responses of the cross-reactive and antigen-specific T-cell against the mpox virus in healthy donors and mpox infected individuals.
Human subjects and ethics
In this study, adult (> 18 years of age) healthy donors (n=105, n=38 females, n=48 males, and n=19 unknown) and convalescent patients (n=22, all males) with mpox virus infection were recruited between June and September of 2022 (Table S1). Among mpox convalescent donors, all individuals were males between the ages of 26 and 43. All individuals were MSM and reported sex as a likely source of infection; none was HIV positive. One out of n=22 individuals was prescribed an immune-modulating drug (adalimumab) for an unrelated diagnosis. All subjects presented with skin lesions, and 11 out of 22 additionally reported fever and/or fatigue. Three subjects had bacterial superinfections of mpox lesions, and six had sexually transmitted co-infections. Mpox severity was defined based on the therapy received: mpox disease that was treatable with local therapy was defined as mild mpox (n=11); mpox disease that required systemic analgesic or antibiotic therapy was defined as mpox (n=11); mpox disease requiring hospitalization was defined as severe mpox (n=0). The study was approved by the Swedish Ethical Review Authority (dnr 2022-04503-02, 2022-03195-02). PBMCs were isolated via standard density gradient centrifugation and cryopreserved in fetal bovine serum (FBS) containing 10% dimethyl sulfoxide (DMSO).
Method details
Peptides
The MPXV and OPXV peptide mega pools (MP) were shared from Alessandro Sette’s lab (La Jolla Institute for Immunology (LJI), USA). In brief, synthesized as crude material (TC Peptide Lab, San Diego, CA) and then individually resuspended in dimethyl sulfoxide (DMSO) at a concentration of 10–20 mg/ml. Aliquots of all peptides were pooled into megapools (MP) designated as OP-CD4-E, OP-CD8-E, MPX-CD4-P, MPX-CD8-P1, MPX-CD8-P2, MPX-CD8-P3, MPX-CD8-P4, and MPX-CD8-P5.9 Based on our experimental design, OP-CD4-E, and OP-CD8-E were pooled together as the OPXV MP; MPX-CD8-P1, MPX-CD8-P2, MPX-CD8-P3, MPX-CD8-P4, and MPX-CD8-P5 were pooled together as the MPXV CD8 MP for further peptides pool stimulation.
Tetramers
HLA class I monomers were generated as described previously.26 Biotinylated pMHCI monomers were conjugated by adding fluorochrome-conjugated streptavidin at a 4:1 molar ratio, respectively, to produce tetrameric pMHCI complexes. The following specificities were used in this study: CMV A∗0201 NLVPMVATV, IAV A∗0201 GILGFVFTL, VACV A∗0201 CLTEYILWV, VACV A∗0201 ILDDNLYKV, and VACV A∗0201 KVDTFYYV.
Activation-Induced Marker (AIM) assay
Cryopreserved PBMCs were thawed quickly, resuspended in complete medium in the presence of DNase I (10 U/ml; Sigma-Aldrich), and rested at 1-2×106 cells/well in 96-well U-bottom plates (Corning) for 3 hours at 37°C. For surface-stained analyses, the media was then supplemented with anti-CXCR5-BB515 (clone RF8B2; BD Biosciences) and unconjugated anti-CD40 (clone HB14; Miltenyi) followed 15 min later by the relevant peptide pool (0.5 μg/ml). Cells were then incubated at 37°C and 5% CO2 for 12 hours. Media was supplemented with relevant peptide pool (0.5 μg/ml) for intracellular-stained analyses. After 1 hour, the media was supplemented with CD107a (clone H4A3; Biolegend), brefeldin A (1 μg/ml; Sigma-Aldrich), and monensin (0.7 μg/ml; BD Biosciences). Cells were then incubated at 37°C and 5% CO2 for 9 hours. Negative control wells contained equivalent DMSO to the peptide pool. After stimulation, cells were washed in PBS supplemented with 2% FBS and 2 mM EDTA (FACS buffer). Cells were then stained according to the protocols detailed in Tables S2 and S3. Briefly, cells were first stained for viability, then chemokine receptors at 37°C, followed by surface markers at room temperature, fixed and permeabilized if required, and stained for intracellular markers at room temperature. Cells were finally fixed with 1% paraformaldehyde in PBS and acquired using a FACSymphony A5 (BD Biosciences). Data were analyzed in FlowJo (version 10).
Fold change was calculated by dividing the frequency of the AIM+ population divided by the equivalent population in the negative control (DMSO stimulation) sample. For analyses involving intracellular AIM+ populations, only samples with a minimum of 10 events in the target gate were included for analyses.
Tetramer staining
Cryopreserved PBMCs were thawed quickly, resuspended in complete medium in the presence of DNase I (10 U/ml; Sigma-Aldrich), and seeded at 2∗106 cells/well. Cells were first incubated with Dasatinib (Stemcell, catalog number 73082) at 50 μM final concentration in phosphate-buffered saline (PBS) for 10 minutes at room temperature. After incubation, PE-conjugated HLA class I tetramers were added to the cells and incubated for 20 minutes at room temperature. Cells were then washed and incubated with BV421-conjugated HLA class I tetramers for 20 minutes at room temperature. Cells were then stained according to the protocol detailed in Table S4. Briefly, cells were first stained for viability, then chemokine receptors at 37°C, followed by surface markers at room temperature, fixed and permeabilized, and stained for intracellular markers at room temperature. Cells were finally acquired using a FACSymphony A3 (BD Biosciences). Data were analyzed in FlowJo (version 10).
Tetramer enrichment
Cryopreserved PBMCs were thawed quickly and resuspended in complete medium in the presence of DNase I (10 U/ml; Sigma-Aldrich). 2∗106 cells were separated for tetramer staining of pre-enriched fractions and treated as described in the “tetramer staining” section. Remaining cells we re-suspended in PBS supplemented with 2% fetal bovine serum (FBS) and 2 μM EDTA (FACS buffer) at a concentration of approximately 10∗106 cells/ml. Cells were incubated with Dasatinib (Stemcell, catalog number 73082) at 50 μM final concentration for 10 minutes at room temperature. After incubation, PE-conjugated HLA class I tetramers were added to the cells and incubated for 1 hour at room temperature. Cells were then washed and incubated PE-conjugated Micro-beads (Miltenyi Biotec, catalog number 130-048-801) for 20 minutes at 4°C and then added to MS columns (Miltenyi Biotec, catalog number 130-122-727). After washing, columns were eluted, and PE-enriched fractions were seeded in a V-bottom plate and stained as described above.
Quantification and statistical analysis
Flow cytometry data were analyzed using FlowJo software version 10.7.1 (FlowJo LLC). Gating strategies are shown in Figure S1. Only responses assigned as positive based on these criteria were included in downstream analyses to limit the impact of background noise. For AIM analysis, stimulation indices were only included if the calculations were based on>10 cells in each marker+ population. Statistical analyses were performed using R (v.4.2.1). Significance between two unpaired groups was assessed using the Mann-Whitney test. Spearman correlations were used to establish relationships between variables. Permutation tests were used to evaluate statistical differences in polyfunctionality analyses.
Acknowledgments
We thank the Blood Center Skanstull and Venhälsan, Stockholm South General Hospital, and Oriana Ribeiro for their help in recruiting HBDs and MPXV-infected individuals. S.A. was supported by the Swiss National Science Foundation Postdoc Mobility Grant (P500PB_211069). Y.G. was supported by the Åke Wibergs Stiftelse, Magnus Bergvalls Stiftelse, and Karolinska Institutet (2202-01968). M.B. was supported by the Swedish Research Council (2018-02330, 2020-06121, and 2021-04779), the Knut and Alice Wallenberg Foundation (KAW 2021.0136), the European Research Council (101057129 and 101041484), Karolinska Institutet (2019-00969), the Swedish Society for Medical Research (CG-22-0009), the Swedish Cancer Society (22 2237 Pj), the Åke Wibergs Stiftelse (M20-0190), and the Jonas Söderquist Stiftelse. This project has been funded in part with Federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under contract no. 75N93019C00065 to A.S.
Author contributions
Conceptualization, S.A., Y.G., M.S., H.-G.L., P.B., and M.B.; sample collection, S.A., Y.G., T.S., A.M., J.W., E.S., V.W., F.F., C.-J.T., A.-M.E., P.B.., and M.B.; investigation, S.A., Y.G., J.W., and M.B.; formal analysis, S.A. and Y.G.; visualization, S.A., Y.G., and M.B.; resources, J.K.S., S.L.-L., D.A.P., A.S., A.G., and M.B.; funding acquisition, S.A., A.S., A.G., and M.B.; supervision, A.S., A.G., and M.B.; writing – original draft, S.A., Y.G., and M.B.
Declaration of interests
M.B. is a consultant for Oxford Immunotec, Mabtech, Bristol-Myers Squibb, and MSD.
Inclusion and diversity
We support inclusive, diverse, and equitable conduct of research.
Published: April 25, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.chom.2023.04.015.
Supplemental information
Data and code availability
-
•
Flow cytometry data are available from lead contact upon request.
-
•
All original code used to generate plots and graphs is available from lead contact upon request.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- 1.WHO WHO Director-General declares the ongoing monkeypox outbreak a Public Health Emergency of International Concern. 2022. https://www.who.int/europe/news/item/23-07-2022-who-director-general-declares-the-ongoing-monkeypox-outbreak-a-public-health-event-of-international-concern
- 2.McCollum A.M., Damon I.K. Human monkeypox. Clin. Infect. Dis. 2014;58:260–267. doi: 10.1093/cid/cit703. [DOI] [PubMed] [Google Scholar]
- 3.Gessain A., Nakoune E., Yazdanpanah Y. Monkeypox. N. Engl. J. Med. 2022;387:1783–1793. doi: 10.1056/NEJMra2208860. [DOI] [PubMed] [Google Scholar]
- 4.Lum F.M., Torres-Ruesta A., Tay M.Z., Lin R.T.P., Lye D.C., Rénia L., Ng L.F.P. Monkeypox: disease epidemiology, host immunity and clinical interventions. Nat. Rev. Immunol. 2022;22:597–613. doi: 10.1038/s41577-022-00775-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Golden J., Harryman L., Crofts M., Muir P., Donati M., Gillett S., Irish C., Bristol H. Case of apparent mpox reinfection. Sex. Transm. Infect. 2023 doi: 10.1136/sextrans-2022-055736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hammarlund E., Lewis M.W., Carter S.V., Amanna I., Hansen S.G., Strelow L.I., Wong S.W., Yoshihara P., Hanifin J.M., Slifka M.K. Multiple diagnostic techniques identify previously vaccinated individuals with protective immunity against monkeypox. Nat. Med. 2005;11:1005–1011. doi: 10.1038/nm1273. [DOI] [PubMed] [Google Scholar]
- 7.Karem K.L., Reynolds M., Hughes C., Braden Z., Nigam P., Crotty S., Glidewell J., Ahmed R., Amara R., Damon I.K. Monkeypox-induced immunity and failure of childhood smallpox vaccination to provide complete protection. Clin. Vaccine Immunol. 2007;14:1318–1327. doi: 10.1128/CVI.00148-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shchelkunov S.N., Totmenin A.V., Babkin I.V., Safronov P.F., Ryazankina O.I., Petrov N.A., Gutorov V.V., Uvarova E.A., Mikheev M.V., Sisler J.R., et al. Human monkeypox and smallpox viruses: genomic comparison. FEBS Lett. 2001;509:66–70. doi: 10.1016/S0014-5793(01)03144-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grifoni A., Zhang Y., Tarke A., Sidney J., Rubiro P., Reina-Campos M., Filaci G., Dan J.M., Scheuermann R.H., Sette A. Defining antigen targets to dissect vaccinia virus and monkeypox virus-specific T cell responses in humans. Cell Host Microbe. 2022;30:1662–1670.e4. doi: 10.1016/j.chom.2022.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hammarlund E., Lewis M.W., Hansen S.G., Strelow L.I., Nelson J.A., Sexton G.J., Hanifin J.M., Slifka M.K. Duration of antiviral immunity after smallpox vaccination. Nat. Med. 2003;9:1131–1137. doi: 10.1038/nm917. [DOI] [PubMed] [Google Scholar]
- 11.Song H., Sidney J., Wiseman R.W., Josleyn N., Cohen M., Blaney J.E., Jahrling P.B., Sette A. Characterizing monkeypox virus specific CD8+ T cell epitopes in rhesus macaques. Virology. 2013;447:181–186. doi: 10.1016/j.virol.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Agrati C., Cossarizza A., Mazzotta V., Grassi G., Casetti R., de Biasi S., Pinnetti C., Gili S., Mondi A., Cristofanelli F., et al. Immunological signature in human cases of monkeypox infection in 2022 outbreak: an observational study. Lancet Infect. Dis. 2023;23:320–330. doi: 10.1016/S1473-3099(22)00662-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Niessl J., Sekine T., Lange J., Konya V., Forkel M., Maric J., Rao A., Mazzurana L., Kokkinou E., Weigel W., et al. Identification of resident memory CD8+ T cells with functional specificity for SARS-CoV-2 in unexposed oropharyngeal lymphoid tissue. Sci. Immunol. 2021;6 doi: 10.1126/sciimmunol.abk0894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao Y., Cai C., Grifoni A., Müller T.R., Niessl J., Olofsson A., Humbert M., Hansson L., Österborg A., Bergman P., et al. Ancestral SARS-CoV-2-specific T cells cross-recognize the Omicron variant. Nat. Med. 2022;28:472–476. doi: 10.1038/s41591-022-01700-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao Y., Cai C., Wullimann D., Niessl J., Rivera-Ballesteros O., Chen P., Lange J., Cuapio A., Blennow O., Hansson L., et al. Immunodeficiency syndromes differentially impact the functional profile of SARS-CoV-2-specific T cells elicited by mRNA vaccination. Immunity. 2022;55:1732–1746.e5. doi: 10.1016/j.immuni.2022.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nguyen T.H.O., Rowntree L.C., Petersen J., Chua B.Y., Hensen L., Kedzierski L., van de Sandt C.E., Chaurasia P., Tan H.X., Habel J.R., et al. CD8+ T cells specific for an immunodominant SARS-CoV-2 nucleocapsid epitope display high naive precursor frequency and TCR promiscuity. Immunity. 2021;54:1066–1082.e5. doi: 10.1016/j.immuni.2021.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alanio C., Lemaitre F., Law H.K.W., Hasan M., Albert M.L. Enumeration of human antigen-specific naive CD8+ T cells reveals conserved precursor frequencies. Blood. 2010;115:3718–3725. doi: 10.1182/blood-2009-10-251124. [DOI] [PubMed] [Google Scholar]
- 18.Buggert M., Vella L.A., Nguyen S., Wu V.H., Chen Z., Sekine T., Perez-Potti A., Maldini C.R., Manne S., Darko S., et al. The identity of human tissue-emigrant CD8+ T cells. Cell. 2020;183:1946–1961.e15. doi: 10.1016/j.cell.2020.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koutsakos M., Illing P.T., Nguyen T.H.O., Mifsud N.A., Crawford J.C., Rizzetto S., Eltahla A.A., Clemens E.B., Sant S., Chua B.Y., et al. Human CD8 + T cell cross-reactivity across influenza A, B and C viruses. Nat. Immunol. 2019;20:613–625. doi: 10.1038/s41590-019-0320-6. [DOI] [PubMed] [Google Scholar]
- 20.Wedemeyer H., Mizukoshi E., Davis A.R., Bennink J.R., Rehermann B. Cross-reactivity between hepatitis C virus and influenza A virus determinant-specific cytotoxic T cells. J. Virol. 2001;75:11392–11400. doi: 10.1128/JVI.75.23.11392-11400.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kundu R., Narean J.S., Wang L., Fenn J., Pillay T., Fernandez N.D., Conibear E., Koycheva A., Davies M., Tolosa-Wright M., et al. Cross-reactive memory T cells associate with protection against SARS-CoV-2 infection in COVID-19 contacts. Nat. Commun. 2022;13 doi: 10.1038/s41467-021-27674-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tarín-Vicente E.J., Alemany A., Agud-Dios M., Ubals M., Suñer C., Antón A., Arando M., Arroyo-Andrés J., Calderón-Lozano L., Casañ C., et al. Clinical presentation and virological assessment of confirmed human monkeypox virus cases in Spain: a prospective observational cohort study. Lancet. 2022;400:661–669. doi: 10.1016/S0140-6736(22)01436-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martínez J.I., Montalbán E.G., Jiménez Bueno S., Martínez F.M., Juliá A.N., Díaz J.S., García Marín N., Deorador E.C., Forte A.N., García M.A., et al. Monkeypox outbreak predominantly affecting men who have sex with men, Madrid, Spain, 26 April to 16 June 2022. Euro Surv. 2022;27 doi: 10.2807/1560-7917.ES.2022.27.27.2200471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akondy R.S., Fitch M., Edupuganti S., Yang S., Kissick H.T., Li K.W., Youngblood B.A., Abdelsamed H.A., McGuire D.J., Cohen K.W., et al. Origin and differentiation of human memory CD8 T cells after vaccination. Nature. 2017;552:362–367. doi: 10.1038/nature24633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.The Public Health Agency of Sweden Previous Swedish vaccination programmes. https://www.folkhalsomyndigheten.se/the-public-health-agency-of-sweden/communicable-disease-control/vaccinations/previous-swedish-vaccination-programmes/
- 26.Price D.A., Brenchley J.M., Ruff L.E., Betts M.R., Hill B.J., Roederer M., Koup R.A., Migueles S.A., Gostick E., Wooldridge L., et al. Avidity for antigen shapes clonal dominance in CD8+ T cell populations specific for persistent DNA viruses. J. Exp. Med. 2005;202:1349–1361. doi: 10.1084/jem.20051357. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
Flow cytometry data are available from lead contact upon request.
-
•
All original code used to generate plots and graphs is available from lead contact upon request.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.