Abstract
The Escherichia coli proP P2 promoter, which directs the expression of an integral membrane transporter of proline, glycine betaine, and other osmoprotecting compounds, is induced upon entry into stationary phase to protect cells from osmotic shock. Transcription from the P2 promoter is completely dependent on RpoS (ς38) and Fis. Fis activates transcription by binding to a site centered at −41, which overlaps the promoter, where it makes a specific contact with the C-terminal domain of the α subunit of RNA polymerase (α-CTD). We show here that Fis and cyclic AMP (cAMP) receptor protein (CRP)-cAMP collaborate to activate transcription synergistically in vitro. Coactivation both in vivo and in vitro is dependent on CRP binding to a site centered at −121.5, but CRP without Fis provides little activation. The contribution by CRP requires the correct helical phasing of the CRP site and a functional activation region 1 on CRP. We provide evidence that coactivation is achieved by Fis and CRP independently contacting each of the two α-CTDs. Efficient transcription in vitro requires that both activators must be preincubated with the DNA prior to addition of RNA polymerase.
During the transition from rapid vegetative growth to stationary phase, a set of genes is induced which is involved in long-term survival under environmental stress. One such gene in Escherichia coli is proP, which encodes a transporter of proline, glycine betaine, and other osmoprotecting compounds (12, 23, 32). While the upstream P1 promoter of proP is transiently induced as part of a specific stress response to osmotic shock, the downstream P2 promoter is expressed for a brief period as cells are about to enter stationary phase, presumably as a safeguard from potential osmotic stress (39, 56).
Expression from the proP P2 promoter has an unusually high dependence both in vivo and in vitro on the Fis protein and the stationary-phase sigma factor, ς38, encoded by rpoS (57). Fis belongs to the general family of nucleoid-associated factors and is a global regulator of transcription, acting as a repressor at some promoters and an activator at others (13, 19, 21, 24, 35, 43, 48, 54, 55). Under rapid growth conditions, Fis is the most abundant transcriptional regulator in the cell (1, 3). However, Fis levels decline dramatically in late exponential phase and become undetectable in stationary phase. In contrast, ς38 levels do not begin to accumulate until late exponential phase (34). This results in a narrow window of P2 expression due to declining Fis levels and a rising ς38 population shortly before cells enter stationary phase.
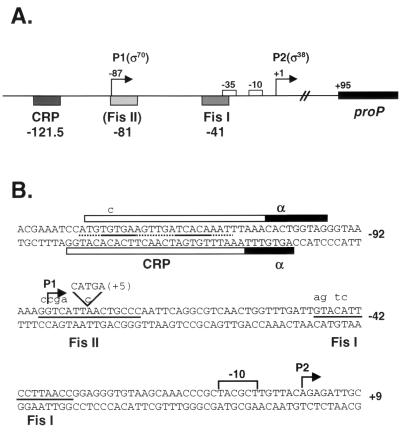
We have previously investigated transcriptional activation by Fis at the proP P2 promoter. As shown in Fig. 1A, there are two specific Fis binding sites, located at −41 (site I) and −81 (site II) nucleotides from the start of transcription (56). Activation at P2 is mediated by Fis at site I, which overlaps the −35 binding element for the sigma subunit of RNA polymerase, dictating that Fis is acting as a class II transcriptional activator (57). Fis binding to the weaker Site II does not significantly affect transcription (57). An essential activation patch on Fis has been localized to a four-amino-acid region spanning the loop between helices B and C adjacent to the DNA binding domain of one subunit of the Fis homodimer (7, 38). This region on the upstream subunit of Fis is believed to directly contact the C-terminal domain of the α subunit of RNA polymerase (α-CTD) (38).
FIG. 1.
The proP regulatory region. (A) Schematic of the proP regulatory region. The two Fis binding sites centered at −41 and −81 plus the CRP site centered at −121.5 relative to the start of transcription from P2 (+1) are depicted (39, 56, 57). Also shown are the start of the coding region and promoters P1 and P2. Expression from the P2 promoter is entirely dependent on Eς38, while the P1 promoter is transcribed most efficiently by Eς70. Fis site II binding has a slightly inhibitory effect on transcription from the P1 promoter in vitro, while CRP binding strongly inhibits P1 transcription. Fis binding at site I plus CRP binding at −121.5 coactivate transcription at P2. (B) Sequence of the proP regulatory region (15). The core recognition sequences for the Fis binding sites are depicted as solid lines between the top and bottom DNA strands. The CRP binding site is denoted by a dotted line between the top and bottom DNA strands, with solid lines at the nucleotides most important for CRP binding. The region on both the top and bottom strands protected from DNase I digestion by CRP is shown with an open bar. Regions protected from DNase I digestion by the addition of α-CTD peptide are shown with filled bars. Mutations that reduce protein binding to the CRP site, Fis site II, and Fis site I are depicted above the top DNA strand. The +5 bp insertion within Fis site II is also shown.
In addition to Fis activation, there is an upstream cyclic AMP (cAMP) receptor protein (CRP) binding site located at −121.5 relative to the start of P2 transcription (57). The CRP protein represses the P1 promoter under low osmotic growth conditions; however, the effect of CRP on P2 activation has not been reported (33, 58). In this report, we further investigate the regulation of proP by examining the effects of CRP on transcriptional activation of the P2 promoter. We find that while CRP alone is a very weak activator of P2 transcription, CRP and Fis coactivate transcription synergistically. The properties of CRP and Fis coactivation are explored.
MATERIALS AND METHODS
Plasmids.
Plasmid pRJ4069 was used as a template for most of the single-round in vitro transcription assays (57). It contains a segment of the proP sequence from +109 to −200 with respect to the P2 transcription initiation site upstream of the rrnB terminators (T1T2) in the vector pKK223-3 (Pharmacia). The DNA template with the mutation that prevents CRP binding (pRJ4070) was derived from pRJ4069 (Fig. 1) (58). pRJ1677 (+5 insertion in Fis site II) was created from pRJ4069 by a two-step PCR method using an oligonucleotide ranging from −66 to −100 nucleotides that contained a 5-bp insertion between nucleotides −81 and −82 as shown in Fig. 1. For multiple-round transcription reactions, the plasmids used were pRJ4039 (wild type), pRJ4045 (mutations in Fis site II), and pRJ4051 (mutations in Fis site I) (57). All of these plasmids are proP-104Δ1–lacZ fusion derivatives of the lacZ protein fusion vector pRS414 (50). The sequence changes in these mutations are as noted in Fig. 1 and have been shown previously to strongly reduce or abolish Fis or CRP binding to their respective sites (56, 58).
The in vivo β-galactosidase assays were performed using two sets of plasmids containing CRP. pZYCRP was the parent of the activation region 1 (AR1) mutations, which were obtained from R. Ebright, Rutgers University. The AR2 mutation (H19YK101E) was derived from pDCRP+, which was provided by S. Busby, University of Birmingham.
Proteins.
Fis wild-type and mutant proteins were purified as described elsewhere (7, 22, 45). CRP was obtained from J. Krakow, Hunter College. The core RNA polymerase enzyme was isolated by the protocol of Burgess and Jendrisak (9) and Lowe et al. (36) as previously described (57). The ς38 protein was overproduced from plasmid pETF and purified as described elsewhere (51). To purify the α-CTD, extracts containing overproduced levels of the α-CTD peptide (amino acids 245 to 329) were prepared from cells containing pEBT7-αCTD (20). DNA was removed by precipitation with polyethyleimine in the presence of 1 M NaCl, and the supernatant was subjected to a 50 to 80% ammonium sulfate fractionation. This material was loaded onto a phenyl-Sepharose (Pharmacia) column in 2.2 M ammonium sulfate–50 mM NaCl–20 mM Tris-HCl (pH 7.5)–10% glycerol–1 mM dithiothreitol–0.1 mM EDTA and washed extensively with the same buffer. Near-homogeneous preparations of the α-CTD peptide were obtained after elution with the same buffer minus ammonium sulfate.
In vitro transcription.
Single-round in vitro transcription reactions were performed as described elsewhere (38); 0.1 pmol of supercoiled pRJ4069 was used in all reactions unless otherwise noted. Reactions were typically carried out in 0.6 M potassium glutamate–0.1 mM cAMP with 1.6 pmol of Fis protein, 1.1 pmol of CRP, and 0.2 pmol of RNA polymerase (Eς38) in a 50-μl reaction volume. Multiple-round transcription reactions were performed under the same conditions, and transcripts were visualized by primer extension assays as previously described (57). Fold activation was determined by quantification of the P2 transcript on a phosphorimager, normalizing each lane to the amount of rna1 transcript.
Determination of proP transcription in vivo.
For β-galactosidase assays, CRP mutants were transformed into strains RJ7024 (MG1655 ΔlacX74 Δcrp zhe::Tn10 λRJ4065 F′ lacIqs A4 proAB+ fzz::Tn10dcam) and RJ7025 (MG1655 ΔlacX74 Δcrp zhe::Tn10 λRJ4066 F′ lacIqs A4 proAB+ fzz::Tn10dcam). Both λRJ4065 and λRJ4066 are recombinants of λRS45 with plasmids pRJ4065 and pRJ4066, respectively (50, 58). Plasmid pRJ4065 contains a proP-104Δ1–lacZ gene fusion where the P1 promoter is inactivated by a mutation at −12. Plasmid pRJ4066 is identical to pRJ4065 except that it also carries a mutation that greatly reduces CRP binding as depicted in Fig. 1. Each strain was grown overnight, subcultured in Luria broth supplemented with ampicillin, and grown at 37°C for 4 h, where the peak of proP P2 expression occurs for the crp+ strain and the AR1 and AR2 mutants. β-Galactosidase activity was measured as described elsewhere (40). Each strain was grown in parallel from at least two separate transformants and assayed in duplicate. Primer extension assays of total mRNA isolated from RJ4446 (crp+) and RJ4445 (Δcrp zhe::Tn10) (MG1655 ΔlacX74) were performed as previously described (58).
DNase I footprint assay.
DNase I footprinting assays were performed as previously described (8). A uniquely 5′-end 32P-labeled fragment from −190 to +103 (with respect to P2 transcriptional initiation) of proP was used; 1.1 pmol of CRP protein and 2.2 nmol of α-CTD were bound to the DNA fragment for 10 min under the same conditions used in the in vitro transcription reaction in a total volume of 20 μl. After binding, DNase I was added for 30 s at room temperature. The reaction was quenched, extracted with phenol-chloroform, and then subjected to ethanol precipitation and electrophoresis on a sequencing gel.
RESULTS
Fis and CRP activate transcription synergistically in vitro at proP P2.
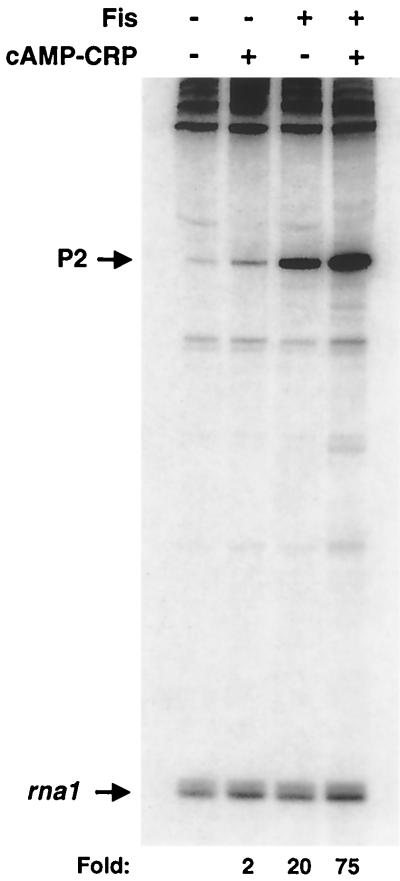
A high-affinity CRP site is centered −121.5 nucleotides from the start of transcription at the proP P2 promoter (33, 58). To test the effect of CRP on proP P2 regulation, single-round in vitro transcription reactions were performed with CRP-cAMP and RNA polymerase complexed with ς38 (Eς38). As shown in Fig. 2, addition of CRP-cAMP had only a small effect, stimulating transcription about twofold over the basal level (no added activator). Fis typically activated transcription 20- to 30-fold. However, when both Fis and CRP-cAMP were added to the in vitro transcription reaction, the level of activated transcription ranged from 75- to 125-fold. This level of activation is greater than the multiplicative effect of each activator alone. Transcripts from each lane were normalized to the levels of rna1 from the vector promoter, which were unaffected by the addition of saturating amounts of CRP-cAMP or Fis. CRP coactivation was dependent on the addition of cAMP, as expected (data not shown). These data show that Fis and CRP activate transcription synergistically at proP P2.
FIG. 2.
Coactivation of proP P2 transcription in vitro by Fis and CRP-cAMP. The denaturing polyacrylamide gel displays the products of single-round in vitro transcription of supercoiled pRJ4069 in the presence of Fis, CRP-cAMP, or both activators. Locations of transcripts generated by the proP P2 and rna1 promoters are indicated by arrows; fold activation over the basal level (no activator added) is shown at the bottom.
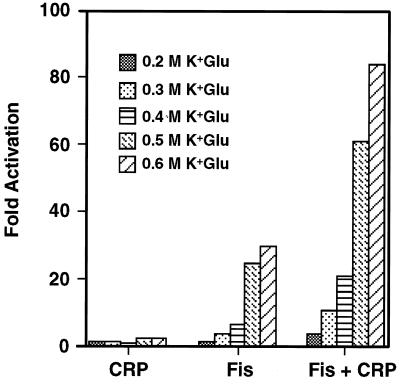
The conditions that promote maximal coactivation by Fis and CRP in vitro were examined. Coactivation was strongly stimulated by a supercoiled template, as was observed with Fis alone (57) (data not shown). Figure 3 shows the effects on in vitro transcription of different concentrations of potassium glutamate in the presence of Fis and CRP, both as solitary activators and in combination. The weak activation by CRP-cAMP alone was constant at different concentrations of potassium glutamate, while the most efficient coactivation by CRP-cAMP plus Fis occurs at 0.6 M. Activation by Fis alone was greatly stimulated by the high concentrations of potassium glutamate, as was observed previously (57). Generally, the conditions which best promote activated transcription by Fis alone are the most favorable for coactivation with CRP.
FIG. 3.
Effect of potassium glutamate concentration on coactivation of proP P2 in vitro. The bar graph shows fold activation over the basal level (no added activator) from in vitro transcription reactions performed with CRP, Fis, and Fis plus CRP in the presence of 0.2 to 0.6 M potassium glutamate (K+Glu).
Coactivation requires the CRP site at −121.5 and Fis site I but not Fis site II.
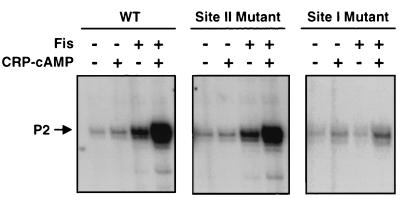
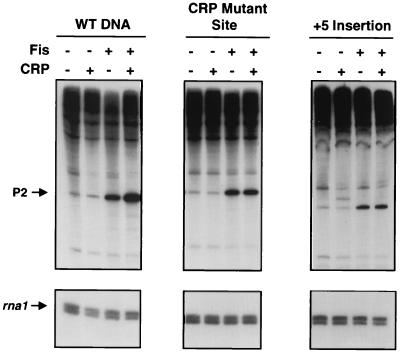
Fis site II, located at −81, has previously been shown to have little affect on Fis-dependent transcription at proP P2 (57). Since Fis site II is positioned between the essential Fis binding site I at −41 and the CRP binding site at −121.5, it seemed possible that it could influence coactivation with CRP. To test this hypothesis, transcription reactions on the wild-type proP P2 promoter and a template containing a mutation that prevents Fis from binding at site II (56) were compared (Fig. 4). The site II mutant template was able to support efficient synergistic activation with Fis and CRP, indicating that Fis binding to site II is not important for coactivation. As expected, Fis binding to site I was essential for coactivation of proP P2 (Fig. 4). We have shown previously that the site I mutation virtually eliminates binding whereas the site II mutation abolishes binding but creates a weak binding site centered 7 bp upstream of site II (56). The site II mutant was shown to have no effect on Fis activation (57). Likewise, when the CRP site was mutated to prevent binding (58), little coactivation was obtained in the presence of Fis (Fig. 5). Therefore, synergistic activation of proP P2 requires CRP binding at −121.5 and Fis binding at site I but not site II.
FIG. 4.
Coactivation of proP P2 transcription from DNA templates with Fis binding site mutations. Gels show primer extension products generated after multiple-round in vitro transcription reactions performed in the presence of Fis and/or CRP-cAMP. The DNA templates were wild type (WT), and plasmids that contained mutations that reduce Fis binding to site II and site I, as indicated. Transcripts originating from proP P2 are indicated with an arrow.
FIG. 5.
Coactivation of proP P2 transcription from mutant DNA templates with a CRP binding site mutation and change in spacing between the CRP site and the P2 promoter. Single-round in vitro transcription reactions were performed in the presence of Fis and/or CRP-cAMP. Panels, from left to right, show transcripts obtained from a wild-type (WT) DNA template, from a template with a mutation that greatly reduces CRP binding, and from a template with a +5 bp insertion at Fis site II between the CRP site and the P2 promoter. Locations of the transcripts generated from the proP P2 and rna1 promoters are indicated with arrows.
Coactivation is dependent on the phasing between CRP and Fis at site I.
To determine if the relative helical orientation between Fis and CRP was important for activation, a 5-bp insertion was created between nucleotides −81 and −82 in the Fis site II binding site which would shift the orientation of CRP by a half helical turn of DNA. As shown in Fig. 5, this change in the helical position of the CRP dimer abolished any contribution by CRP on proP P2 activation. As expected, Fis still activated transcription on this template in both the presence and absence of CRP. Thus, coactivation by Fis and CRP is absolutely dependent on the helical phasing between CRP and Fis bound at site I within the promoter.
CRP coactivates proP P2 in vivo.
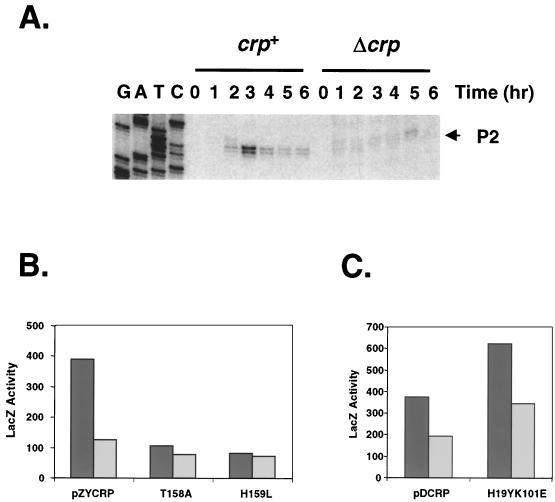
Transcriptional activation by CRP in vivo was also examined. Figure 6A shows proP P2 transcripts visualized by primer extension of total mRNA isolated from wild-type and Δcrp cells. A sharp peak of P2 transcript at 3 h after subculture, typical of proP P2 transcription, was seen only in the wild-type cells, suggesting that CRP is important for optimal P2 expression. However, the growth defect inherent to the Δcrp strain raised the possibility that the CRP effect on P2 transcription might be amplified in this assay. For example, Fis and/or RpoS levels may be altered in the Δcrp relative to the crp+ cells. Therefore, β-galactosidase assays were also performed using two different proP-lacZ fusions carried on a λ prophage as reporters. Both reporters contain a P1(−12) mutation that abolishes transcriptional initiation from the P1 promoter of proP (39, 56). One construct has an otherwise wild-type proP promoter region, while the other carries a mutation that strongly reduces CRP binding to the promoter in vitro as well as in vivo (Fig. 1) (58). With these constructs, the effect of CRP on proP P2 transcription can be evaluated without using cells lacking CRP. Figure 6B shows that in the presence of wild-type CRP (pZYCRP+) and a functional CRP binding site, transcription at proP P2 was stimulated 2.5-fold in vivo when the cells were grown in Luria broth. The CRP site mutant had 126 U of β-galactosidase, while the natural promoter produced 391 U. Up to a sevenfold increase in β-galactosidase activity by CRP binding has been measured in M9 glycerol medium, although the overall level of expression was considerably lower (80 U of β-galactosidase). The stimulation by CRP in vivo was completely dependent on the presence of Fis; cells lacking Fis have <5 U of β-galactosidase (data not shown).
FIG. 6.
Effect of CRP on proP P2 transcription in vivo. (A) Primer extension assays were performed with 10 μg of mRNA isolated from RJ4446 (crp+) and RJ4445 (Δcrp) at 1-h intervals after subculture of an overnight culture into Luria broth. The sequencing ladder generated from the same primer used in the primer extension assays is shown on the left; the proP P2 transcript is indicated with the arrow. (B) β-Galactosidase assays of strains containing crp+ and AR1 crp mutants (T158A and H159L) supplied on pZYCRP in a Δcrp background carrying one of two ProP-LacZ reporters. The dark bars depict β-galactosidase activities (Miller units) obtained from a proP P2-lacZ reporter that contains the wild-type CRP binding site; the gray bars represent activities from a strain with the same reporter except for a mutant CRP binding site. The peak activities are given, which was 4 h after subculture for each strain. In all cases, standard deviations were less than 10%. proP P1 activity is abolished in these reporters because of a mutation at −12 within its promoter. (C) β-Galactosidase assays of the CRP AR2 (H19YK101E) mutant together with the parent crp+ plasmid, pDCRP, performed as for panel B.
CRP activates proP P2 through AR1.
Two regions on the CRP protein, AR1 and AR2, have been found to be critical for transcriptional activation at other promoters (10). Depending on the position of the CRP binding site, either AR1 or both regions are required for efficient activation. CRP mutants containing substitutions in both AR1 and AR2 were tested for the ability to activate transcription in vivo at proP P2 by measuring β-galactosidase activity generated by the reporter constructs described above. Wild-type and mutant crp genes were supplied on a plasmid in a Δcrp strain. Figure 6B shows that the CRP mutants T158A and H159L (52), which contain mutations in AR1, had similar levels of activity regardless of the presence of the CRP binding site, indicating that they were unable to stimulate P2 transcriptional activation. The transcriptional activation was reduced to 106 and 82 U, respectively, from the 391 U of β-galactosidase produced by the wild-type control. Immunoblotting of cell extracts indicated that the CRP AR1 mutants did not alter the temporal expression of Fis (data not shown). In contrast to the AR1 mutants, CRP containing the substitutions H19Y K101E (47) located in AR2 had no detectable effect on P2 transcription (Fig. 6C). The twofold stimulation of transcription in the presence of a functional CRP binding site was maintained in the AR2-CRP mutant, although overall transcription in both reporters was elevated. The reason for these higher levels of transcription with the AR2 mutant is not known. Thus, AR1, but not AR2, is required for CRP activation of proP P2.
The α-CTD of RNA polymerase binds to the DNA adjacent to CRP.
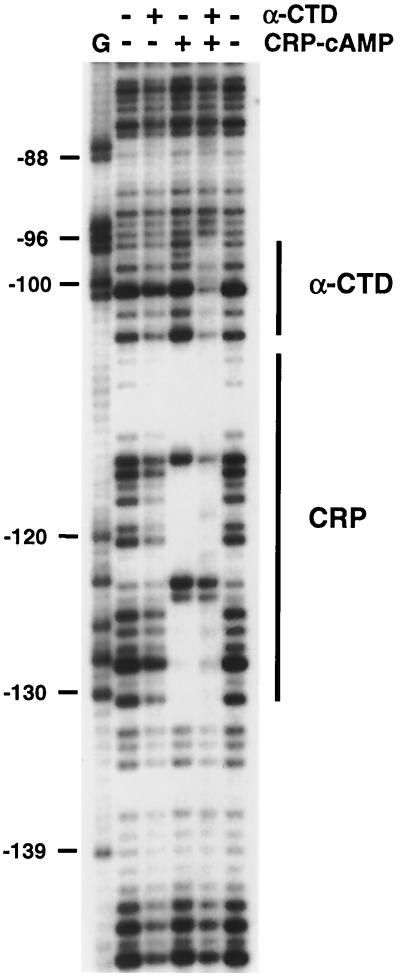
Due to the remote position of the CRP binding site at proP, CRP is most likely contacting Eς38 through the α-CTD of polymerase, which is attached to the rest of polymerase through a flexible linker (6, 29). As shown above, AR1 of CRP, a region that has been shown to contact α-CTD at other promoters (10), is necessary for CRP activation of P2. To determine if CRP is contacting the α-CTD at proP, we mapped the binding position on the proP promoter of a truncated α subunit containing only the last 85 amino acids comprising the C-terminal domain (20) by DNase I footprinting. The α-CTD peptide was used for these experiments since Eς38 binds poorly to a linearized proP P2 template, consistent with the supercoiling requirement for transcription (57). The α-CTD peptide caused a modest reduction in DNase I cleavages throughout the lanes. However, in the presence of CRP, specific protection of DNase I cleavage was observed immediately adjacent to the downstream side of CRP at concentrations of α-CTD used to detect binding at the rrnB P1 promoter containing a strong UP element (6). This protection extends the CRP protected region at least 9 bp on the top strand (Fig. 7) and 7 bp on the bottom strand, as indicated in Fig. 1. The precise boundaries at the CRP and α-CTD junctions are not possible to determine because of the lack of DNase I reactivity within this A/T-rich segment. Since the α-CTD preferentially binds to A/T-rich regions in the minor groove (18), the binding site for the α-CTD may overlap the highly A/T-rich segment present at the downstream end of the CRP protected region. No CRP-dependent protection by the α-CTD was observed upstream of the CRP site. This footprinting data is consistent with CRP stimulating transcription by the promoter-proximal subunit of CRP directly contacting the α-CTD.
FIG. 7.
DNase I footprint of DNA complexes with CRP and α-CTD. CRP protein (1.1 pmol) and/or α-CTD (2.2 nmol) were bound to a 5′-end 32P-labeled proP fragment (top strand) for 10 min prior to DNase I cleavage, as indicated at the top. Lane G refers to the G chemical sequencing ladder; numbers to the left are in relation to the proP P2 initiation site. Bars designate the regions protected by CRP-cAMP and α-CTD in the presence of CRP-cAMP.
Coactivation by CRP and mutant Fis proteins.
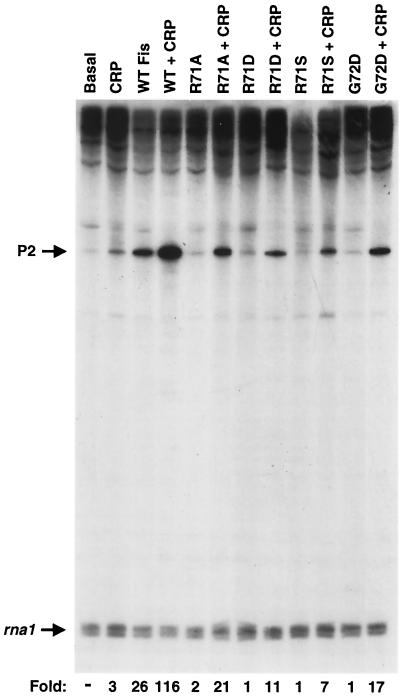
We have previously shown that a small region on one subunit of Fis, the B-C loop, is required for activation of proP P2 by Fis alone. The important amino acids within this region include residues Gln 68, Arg 71, Gly 72, and Gln 74. Arginine 71 is believed to directly contact RNA polymerase because this residue strongly affects cooperative binding with Eς38, and most substitutions at Arg 71 strongly reduce transcriptional activation (38). To determine the effect of this region on coactivation with CRP, in vitro transcription reactions were performed with CRP together with Fis mutants that by themselves are strongly defective in P2 activation. A gel of in vitro transcription reactions showing coactivation by CRP and representative Fis mutants containing substitutions at residue 71 and 72 is shown in Fig. 8. These Fis mutants promoted little to no activated transcription. However, when these mutants were combined with CRP, transcript levels were considerably increased. Coactivation ranged up to 30-fold, depending on the Fis mutant, even though these mutants only weakly potentiated P2 transcription on their own. The full level of activation that is seen with CRP and wild-type Fis is not achieved; however, it is evident that the transcriptionally impaired Fis mutants are still competent for coactivation with CRP.
FIG. 8.
Coactivation of proP P2 by Fis mutants and CRP. Single-round in vitro transcription reactions were performed with CRP and Fis mutants containing substitutions in the B-C loop region that is required for P2 activation by Fis. Basal indicates that no activators were added. Locations of the transcripts from the P2 and rna1 promoters are indicated with arrows; fold activation over the basal level for each reaction is given at the bottom. WT, wild type.
The order of addition of activators is important for synergistic activation at proP P2.
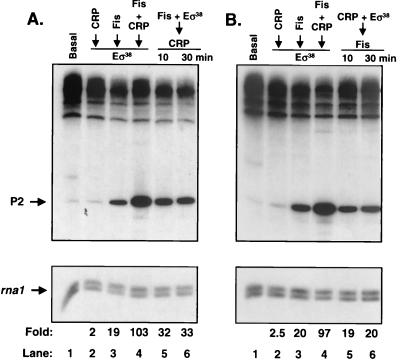
In the previous transcription reactions, Fis and CRP were allowed to bind to the DNA prior to Eς38 addition. To determine whether this order of addition was important for efficient activation, we incubated one activator plus Eς38 with the plasmid template prior to addition of the second activator. Single-round transcription was then initiated by the addition of nucleoside triphosphates (NTPs) and heparin. In Fig. 9A, Fis plus Eς38 were incubated with the DNA for 15 min followed by the addition of CRP-cAMP. This resulted in a 32-fold activation of transcription, which was somewhat greater than the level for Fis alone but considerably less than the 100-fold activation obtained by preincubating both activators with DNA prior to Eς38 addition. Incubation for a longer period (30 min) prior to elongation did not improve the efficiency of coactivation. In Fig. 9B, CRP-cAMP plus Eς38 were incubated with the template for 15 min before addition of Fis. Under these conditions, the degree of activation was similar to that obtained with Fis alone (20-fold). Again, longer incubations prior to elongation did not improve the efficiency of coactivation. We conclude that efficient coactivation by Fis and CRP requires that both activators be bound to the DNA prior to Eς38.
FIG. 9.
Effect of the order of addition of activators on single-round proP P2 transcription. (A) Basal (lane 1) indicates that the reaction was performed with no activators. Reactions in lanes 2 to 4 were performed under standard conditions (e.g., Fig. 2). Lanes 5 and 6 show transcripts produced when Fis and Eς38 were incubated with the DNA template for 15 min at 37°C prior to addition of CRP. After a further 10 or 30 min of incubation, transcription was initiated by the addition of NTPs and heparin. (B) In vitro transcription reactions as in panel A except that in lanes 5 and 6 CRP and Eς38 were incubated with the DNA template for 15 min prior to Fis addition. After an additional 10 or 30 min, transcription was initiated with NTPs and heparin. Locations of transcripts generated from the P2 and rna1 promoters are indicated by arrows; fold activation over the basal level for each reaction is given at the bottom.
DISCUSSION
Previously it has been shown that proP P2 expression is completely dependent on Fis and the stationary-phase sigma factor, ς38 (39, 56, 57). The 20- to 30-fold activation by Fis is absolutely dependent on the α-CTD of RNA polymerase that contacts the B-C loop region of one of the subunits of the Fis homodimer (7, 38). In this report, we show that the CRP protein acts synergistically with Fis to mediate even higher levels of transcription. Binding of CRP at a site centered 121.5 bp upstream of the P2 promoter gives little (<2-fold) activation by itself. In combination with Fis binding at site I, which overlaps the promoter, CRP mediates a 75- to 125-fold stimulation of transcription at proP P2.
While transcriptional synergy by multiple activators is often found in eukaryotic organisms, there have been relatively few reports of synergy in prokaryotes (26, 46). Examples of synergistic activation include the ansB promoters: in E. coli the coactivation is dependent on both CRP and FNR, and in Salmonella enterica serovar Typhimurium coactivation is dependent on two CRP dimers (49). Synergy has been clearly demonstrated in artificial bacterial promoters between CRP bound upstream of the promoter and either another CRP or lambda cI protein bound to a site overlapping the −35 element (11, 30, 31). Transcriptional activation at proP P2 is the first example of synergy involving the Fis protein, though the reports of Fis as a coregulator of transcription appear to be increasing (14, 16, 25, 28, 37, 54). To our knowledge, a direct role in activation by CRP binding this far upstream of the promoter has not been previously reported.
Coactivation by CRP at proP P2.
To provide evidence that CRP binding at −121.5 directly contacts Eς38 at proP P2, CRP mutants containing substitutions at residues that disrupt polymerase-CRP interactions at either class I or class II promoters were tested. The CRP AR1 mutants were found to strongly reduce activation, while the AR2 mutants had no effect on activation of transcription at proP P2. This is not surprising because CRP positioned upstream of the promoter usually does not mediate transcriptional activation through AR2. These results for CRP activation at proP through AR1 are similar to those found where CRP is acting as an upstream activator alone or in conjunction with either another molecule of CRP, FNR, or the λ cI protein bound in an analogous position to Fis site I at proP P2 (11, 30, 31). However, 121 bp upstream of the transcriptional start site is an unusually large distance for direct activation by CRP.
Because AR1 of CRP has been shown to stimulate transcription at other promoters by contacting the α-CTD of RNA polymerase, it is highly likely that CRP is contacting the α-CTD of Eς38 at proP P2 (17, 27). In support of this, DNase I footprint analysis with purified α-CTD revealed binding of α-CTD to the DNA just downstream of the CRP binding site. This specific association of the α-CTD to the DNA is dependent on CRP binding, suggesting that the CRP protein, together with the sequence of the DNA on the downstream side, is directing the positioning of the α-CTD. Murakami et al. measured the position of both α-CTDs of RNA polymerase at promoters containing two CRP binding sites by affinity cleavage of α-CTD-EDTA · Fe conjugants (41). They found that the α-CTD was unable to contact the DNA when the binding site was at −113.5, even when another CRP dimer was bound at −41.5. Similar results have also been obtained by hydroxyl radical footprinting of CRP-RNA polymerase complexes (5). These findings differ from what we observe at proP P2, where the α-CTD contacts the CRP protein bound even further upstream at −121.5. While the localization of α-CTD by DNase I footprinting performed here is with the α-CTD not tethered to RNA polymerase, in contrast to the previously mentioned experiments, CRP bound at −121.5 nonetheless functions to activate transcription of proP P2 in a manner dependent on AR1. In addition to the sequence of the intervening DNA, an obvious difference between these situations is that at proP the Fis protein is bound at −41 instead of a second CRP molecule. However, it is not expected that Fis would induce a greater degree of DNA bending or a significantly different trajectory of the DNA from CRP bound at the same position (38, 45).
Coactivation by Fis at proP P2.
An essential activation region on Fis has previously been localized to the B-C loop region (38). In transcription reactions performed in vitro with Fis as the sole activator, B-C loop mutants stimulate little, if any, activated transcription. However, together with CRP, these mutants are able to synergistically activate transcription. Therefore, with CRP bound at the promoter, the dependence on the B-C loop of Fis appears to be alleviated. A competent B-C loop region is necessary to achieve the full level of transcription seen with CRP and wild-type Fis, implying that the B-C loop is still playing a role in coactivation. It is possible that CRP may stabilize the polymerase sufficiently to partially overcome a weakened interaction by the Fis B-C loop mutants with the α-CTD. In addition, it is possible, although we consider it unlikely, that DNA bending promoted by the B-C loop Fis mutants is sufficient to stimulate transcription when CRP is present.
An alternative explanation for coactivation between CRP and the Fis B-C loop mutants is that a second determinant other than the B-C loop region on the Fis dimer mediates the residual coactivation with CRP. Without a competent B-C loop region, this potential second transcriptional activation region is unable to stimulate significant transcription when Fis is the solitary activator (38). This postulated second patch has two possible targets. One is CRP protein bound at −121.5, but we have not observed any cooperative binding between Fis and CRP at proP that could support such a model. Another explanation is that a second patch on Fis could contact polymerase in a manner different from the previously discovered contact between the B-C loop of Fis and α-CTD of RNA polymerase. Because Fis binding site I overlaps the −35 sigma subunit recognition element, a Fis-ς38 protein-protein contact is possible. Other transcriptional activators such as CRP, FNR, and the Mu Mor protein that bind to an analogous position have been shown to make multiple contacts with RNA polymerase (2, 4, 44, 53). Most of these activators contact the α-CTD through their upstream side and also make a contact on the downstream side with either the sigma subunit or the N-terminal domain of the alpha subunit. An α-CTD-independent stimulation of rrnB transcription has been noted by Bokal et al. (7). Muskhelishvili et al. have also reported cooperative DNA binding between Fis and ς70 at the tyrT promoter (42).
A model of synergistic activation at proP P2.
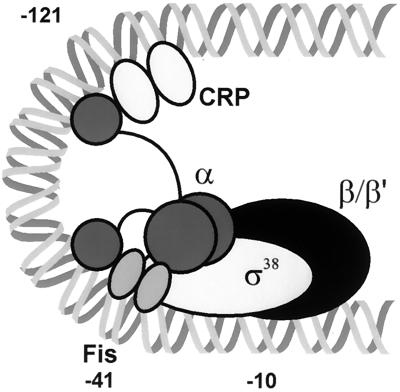
In our model for synergistic activation of proP P2, Fis minimally contacts one α-CTD domain, and the second α-CTD domain is bound to the CRP subunit oriented proximal to the promoter (Fig. 10). The lack of coactivation by CRP on a DNA template with a 5-bp insertion is consistent with the requirement for looping of the intervening DNA. One notable aspect of the coactivation seen at proP is that it is most efficient when both activators are bound to the DNA prior to Eς38. This observation implies that once a complex is formed between the first activator and Eς38, the second activator is no longer fully capable of stimulating transcription. This could be due to the formation of one of a variety of specific protein-DNA architectures which limit accessibility to the second activator or, in the case of CRP alone, the targeting of Eς38 to a nonproductive location. As mentioned previously, an alternative model is that Fis and CRP bound to their respective sites must first interact. In order to achieve maximal coactivation, this interaction may be required to colocalize the two activators close to the promoter via DNA looping prior to the binding of Eς38.
FIG. 10.
Model of coactivation of proP P2 by Fis and CRP. The DNA is represented as a double helix. CRP is bound at −121.5 with one α-CTD bound to the DNA on the downstream side of CRP. Fis activates transcription when bound at site I centered at −41, overlapping the −35 DNA recognition element for ς38. The second α-CTD is believed to contact the upstream subunit of the Fis homodimer (38).
ACKNOWLEDGMENTS
We thank J. Krakow for the gift of CRP protein, R. Ebright and S. Busby for CRP plasmids, and Leah Corselli for purification of the α-CTD. We also thank Ann Hochschild for valuable discussions and Richard Gourse for Fis plasmids and critical reading of the manuscript.
This work was supported by National Institutes of Health grant GM38509 to R.C.J.
REFERENCES
- 1.Ali Azam T, Iwata A, Nishimura A, Ueda S, Ishihama A. Growth phase-dependent variation in protein composition of the Escherichia coli nucleoid. J Bacteriol. 1999;181:6361–6370. doi: 10.1128/jb.181.20.6361-6370.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Artsimovitch I, Murakami K, Ishihama A, Howe M M. Transcription activation by the bacteriophage Mu Mor protein requires the C-terminal regions of both alpha and sigma70 subunits of Escherichia coli RNA polymerase. J Biol Chem. 1996;271:32343–32348. doi: 10.1074/jbc.271.50.32343. [DOI] [PubMed] [Google Scholar]
- 3.Ball C A, Osuna R, Ferguson K C, Johnson R C. Dramatic changes in Fis levels upon nutrient upshift in Escherichia coli. J Bacteriol. 1992;174:8043–8056. doi: 10.1128/jb.174.24.8043-8056.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bell A, Busby S. Location and orientation of an activating region in the Escherichia coli transcription factor, FNR. Mol Microbiol. 1994;11:383–390. doi: 10.1111/j.1365-2958.1994.tb00318.x. [DOI] [PubMed] [Google Scholar]
- 5.Belyaeva T A, Rhodius V A, Webster C L, Busby S J. Transcription activation at promoters carrying tandem DNA sites for the Escherichia coli cyclic AMP receptor protein: organization of the RNA polymerase alpha subunits. J Mol Biol. 1998;277:789–804. doi: 10.1006/jmbi.1998.1666. [DOI] [PubMed] [Google Scholar]
- 6.Blatter E E, Ross W, Tang H, Gourse R L, Ebright R H. Domain organization of RNA polymerase alpha subunit: C-terminal 85 amino acids constitute a domain capable of dimerization and DNA binding. Cell. 1994;78:889–896. doi: 10.1016/s0092-8674(94)90682-3. [DOI] [PubMed] [Google Scholar]
- 7.Bokal A J, Ross W, Gaal T, Johnson R C, Gourse R L. Molecular anatomy of a transcription activation patch: FIS-RNA polymerase interactions at the Escherichia coli rrnB P1 promoter. EMBO J. 1997;16:154–162. doi: 10.1093/emboj/16.1.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bruist M F, Glasgow A C, Johnson R C, Simon M I. Fis binding to the recombinational enhancer of the Hin DNA inversion system. Genes Dev. 1987;1:762–772. doi: 10.1101/gad.1.8.762. [DOI] [PubMed] [Google Scholar]
- 9.Burgess R R, Jendrisak J J. A procedure for the rapid, large-scale purification of Escherichia coli DNA-dependent RNA polymerase involving Polymin P precipitation and DNA-cellulose chromatography. Biochemistry. 1975;14:4634–4638. doi: 10.1021/bi00692a011. [DOI] [PubMed] [Google Scholar]
- 10.Busby S, Ebright R H. Transcription activation by catabolite activator protein (CAP) J Mol Biol. 1999;293:199–213. doi: 10.1006/jmbi.1999.3161. [DOI] [PubMed] [Google Scholar]
- 11.Busby S, West D, Lawes M, Webster C, Ishihama A, Kolb A. Transcription activation by the Escherichia coli cyclic AMP receptor protein. Receptors bound in tandem at promoters can interact synergistically. J Mol Biol. 1994;241:341–352. doi: 10.1006/jmbi.1994.1511. [DOI] [PubMed] [Google Scholar]
- 12.Cairney J, Booth I R, Higgins C F. Salmonella typhimurium proP gene encodes a transport system for the osmoprotectant betaine. J Bacteriol. 1985;164:1218–1223. doi: 10.1128/jb.164.3.1218-1223.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Champagne N, Lapointe J. Influence of FIS on the transcription from closely spaced and non-overlapping divergent promoters for an aminoacyl-tRNA synthetase gene (gltX) and a tRNA operon (valU) in Escherichia coli. Mol Microbiol. 1998;27:1141–1156. doi: 10.1046/j.1365-2958.1998.00745.x. [DOI] [PubMed] [Google Scholar]
- 14.Claret L, Rouviere-Yaniv J. Regulation of HU alpha and HU beta by CRP and FIS in Escherichia coli. J Mol Biol. 1996;263:126–139. doi: 10.1006/jmbi.1996.0564. [DOI] [PubMed] [Google Scholar]
- 15.Culham D E, Lasby B, Marangoni A G, Milner J L, Steer B A, van Nues R W, Wood J M. Isolation and sequencing of Escherichia coli gene proP reveals unusual structural features of the osmoregulatory proline/betaine transporter, ProP. J Mol Biol. 1993;229:268–276. doi: 10.1006/jmbi.1993.1030. [DOI] [PubMed] [Google Scholar]
- 16.Davies I J, Drabble W T. Stringent and growth-rate-dependent control of the gua operon of Escherichia coli K-12. Microbiology. 1996;142:2429–2437. doi: 10.1099/00221287-142-9-2429. [DOI] [PubMed] [Google Scholar]
- 17.Ebright R H, Busby S. The Escherichia coli RNA polymerase alpha subunit: structure and function. Curr Opin Genet Dev. 1995;5:197–203. doi: 10.1016/0959-437x(95)80008-5. [DOI] [PubMed] [Google Scholar]
- 18.Estrem S T, Ross W, Gaal T, Chen Z W, Niu W, Ebright R H, Gourse R L. Bacterial promoter architecture: subsite structure of UP elements and interactions with the carboxy-terminal domain of the RNA polymerase alpha subunit. Genes Dev. 1999;13:2134–2147. doi: 10.1101/gad.13.16.2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Falconi M, Brandi A, La Teana A, Gualerzi C O, Pon C L. Antagonistic involvement of FIS and H-NS proteins in the transcriptional control of hns expression. Mol Microbiol. 1996;19:965–975. doi: 10.1046/j.1365-2958.1996.436961.x. [DOI] [PubMed] [Google Scholar]
- 20.Gaal T, Ross W, Blatter E E, Tang H, Jia X, Krishnan V V, Assa-Munt N, Ebright R H, Gourse R L. DNA-binding determinants of the alpha subunit of RNA polymerase: novel DNA-binding domain architecture. Genes Dev. 1996;10:16–26. doi: 10.1101/gad.10.1.16. [DOI] [PubMed] [Google Scholar]
- 21.González-Gil G, Bringmann P, Kahmann R. FIS is a regulator of metabolism in Escherichia coli. Mol Microbiol. 1996;22:21–29. doi: 10.1111/j.1365-2958.1996.tb02652.x. [DOI] [PubMed] [Google Scholar]
- 22.Gosink K K, Gaal T, Bokal A J, Gourse R L. A positive control mutant of the transcription activator protein FIS. J Bacteriol. 1996;178:5182–5187. doi: 10.1128/jb.178.17.5182-5187.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gowrishankar J. proP-mediated proline transport also plays a role in Escherichia coli osmoregulation. J Bacteriol. 1986;166:331–333. doi: 10.1128/jb.166.1.331-333.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Green J, Anjum M F, Guest J R. The ndh-binding protein (Nbp) regulates the ndh gene of Escherichia coli in response to growth phase and is identical to Fis. Mol Microbiol. 1996;20:1043–1055. doi: 10.1111/j.1365-2958.1996.tb02545.x. [DOI] [PubMed] [Google Scholar]
- 25.Green J, Anjum M F, Guest J R. Regulation of the ndh gene of Escherichia coli by integration host factor and a novel regulator, Arr. Microbiology. 1997;143:2865–2875. doi: 10.1099/00221287-143-9-2865. [DOI] [PubMed] [Google Scholar]
- 26.Hochschild A, Joung J K. Synergistic activation of transcription in E. coli. Nucleic Acids Mol Biol. 1997;11:101–114. [Google Scholar]
- 27.Igarashi K, Ishihama A. Bipartite functional map of the E. coli RNA polymerase alpha subunit: involvement of the C-terminal region in transcription activation by cAMP-CRP. Cell. 1991;65:1015–1022. doi: 10.1016/0092-8674(91)90553-b. [DOI] [PubMed] [Google Scholar]
- 28.Jacobson B A, Fuchs J A. Multiple cis-acting sites positively regulate Escherichia coli nrd expression. Mol Microbiol. 1998;28:1315–1322. doi: 10.1046/j.1365-2958.1998.00897.x. [DOI] [PubMed] [Google Scholar]
- 29.Jeon Y H, Yamazaki T, Otomo T, Ishihama A, Kyogoku Y. Flexible linker in the RNA polymerase alpha subunit facilitates the independent motion of the C-terminal activator contact domain. J Mol Biol. 1997;267:953–962. doi: 10.1006/jmbi.1997.0902. [DOI] [PubMed] [Google Scholar]
- 30.Joung J K, Koepp D M, Hochschild A. Synergistic activation of transcription by bacteriophage lambda cI protein and E. coli cAMP receptor protein. Science. 1994;265:1863–1866. doi: 10.1126/science.8091212. [DOI] [PubMed] [Google Scholar]
- 31.Joung J K, Le L U, Hochschild A. Synergistic activation of transcription by Escherichia coli cAMP receptor protein. Proc Natl Acad Sci USA. 1993;90:3083–3087. doi: 10.1073/pnas.90.7.3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jovanovich S B, Martinell M, Record M T, Jr, Burgess R R. Rapid response to osmotic upshift by osmoregulated genes in Escherichia coli and Salmonella typhimurium. J Bacteriol. 1988;170:534–539. doi: 10.1128/jb.170.2.534-539.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Landis L, Xu J, Johnson R C. The cAMP receptor protein CRP can function as an osmoregulator of transcription in Escherichia coli. Genes Dev. 1999;13:3081–3091. doi: 10.1101/gad.13.23.3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lange R, Hengge-Aronis R. The cellular concentration of the sigma S subunit of RNA polymerase in Escherichia coli is controlled at the levels of transcription, translation, and protein stability. Genes Dev. 1994;8:1600–1612. doi: 10.1101/gad.8.13.1600. [DOI] [PubMed] [Google Scholar]
- 35.Lazarus L R, Travers A A. The Escherichia coli FIS protein is not required for the activation of tyrT transcription on entry into exponential growth. EMBO J. 1993;12:2483–2494. doi: 10.1002/j.1460-2075.1993.tb05903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lowe P A, Hager D A, Burgess R R. Purification and properties of the sigma subunit of Escherichia coli DNA-dependent RNA polymerase. Biochemistry. 1979;18:1344–1352. doi: 10.1021/bi00574a034. [DOI] [PubMed] [Google Scholar]
- 37.Martin R G, Rosner J L. Fis, an accessorial factor for transcriptional activation of the mar (multiple antibiotic resistance) promoter of Escherichia coli in the presence of the activator MarA, SoxS, or Rob. J Bacteriol. 1997;179:7410–7419. doi: 10.1128/jb.179.23.7410-7419.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McLeod S M, Xu J, Cramton S E, Gaal T, Gourse R L, Johnson R C. Localization of amino acids required for Fis to function as a class II transcriptional activator at the RpoS-dependent proP P2 promoter. J Mol Biol. 1999;294:333–346. doi: 10.1006/jmbi.1999.3262. [DOI] [PubMed] [Google Scholar]
- 39.Mellies J, Wise A, Villarejo M. Two different Escherichia coli proP promoters respond to osmotic and growth phase signals. J Bacteriol. 1995;177:144–151. doi: 10.1128/jb.177.1.144-151.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miller J H. A short course in bacterial genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Press; 1992. [Google Scholar]
- 41.Murakami K, Owens J T, Belyaeva T A, Meares C F, Busby S J, Ishihama A. Positioning of two alpha subunit carboxy-terminal domains of RNA polymerase at promoters by two transcription factors. Proc Natl Acad Sci USA. 1997;94:11274–11278. doi: 10.1073/pnas.94.21.11274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Muskhelishvili G, Travers A A, Heumann H, Kahmann R. FIS and RNA polymerase holoenzyme form a specific nucleoprotein complex at a stable RNA promoter. EMBO J. 1995;14:1446–1452. doi: 10.1002/j.1460-2075.1995.tb07131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nilsson L, Vanet A, Vijgenboom E, Bosch L. The role of FIS in trans activation of stable RNA operons of E. coli. EMBO J. 1990;9:727–734. doi: 10.1002/j.1460-2075.1990.tb08166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Niu W, Kim Y, Tau G, Heyduk T, Ebright R H. Transcription activation at class II CAP-dependent promoters: two interactions between CAP and RNA polymerase. Cell. 1996;87:1123–1134. doi: 10.1016/s0092-8674(00)81806-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pan C Q, Finkel S E, Cramton S E, Feng J A, Sigman D S, Johnson R C. Variable structures of Fis-DNA complexes determined by flanking DNA-protein contacts. J Mol Biol. 1996;264:675–695. doi: 10.1006/jmbi.1996.0669. [DOI] [PubMed] [Google Scholar]
- 46.Rhodius V A, Busby S J. Positive activation of gene expression. Curr Opin Microbiol. 1998;1:152–159. doi: 10.1016/s1369-5274(98)80005-2. [DOI] [PubMed] [Google Scholar]
- 47.Rhodius V A, West D M, Webster C L, Busby S J W, Savery N J. Transcription activation at class II CRP-dependent promoters: the role of different activating regions. Nucleic Acids Res. 1997;25:326–332. doi: 10.1093/nar/25.2.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ross W, Thompson J F, Newlands J T, Gourse R L. E. coli Fis protein activates ribosomal RNA transcription in vitro and in vivo. EMBO J. 1990;9:3733–4372. doi: 10.1002/j.1460-2075.1990.tb07586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scott S, Busby S, Beacham I. Transcriptional co-activation at the ansB promoters: involvement of the activating regions of CRP and FNR when bound in tandem. Mol Microbiol. 1995;18:521–531. doi: 10.1111/j.1365-2958.1995.mmi_18030521.x. [DOI] [PubMed] [Google Scholar]
- 50.Simons R W, Houman F, Kleckner N. Improved single and multicopy lac-based cloning vectors for protein and operon fusions. Gene. 1987;53:85–96. doi: 10.1016/0378-1119(87)90095-3. [DOI] [PubMed] [Google Scholar]
- 51.Tanaka K, Kusano S, Fujita N, Ishihama A, Takahashi H. Promoter determinants for Escherichia coli RNA polymerase holoenzyme containing sigma 38 (the rpoS gene product) Nucleic Acids Res. 1995;23:827–834. doi: 10.1093/nar/23.5.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tang H, Severinov K, Goldfarb A, Fenyo D, Chait B, Ebright R H. Location, structure, and function of the target of a transcriptional activator protein. Genes Dev. 1994;8:3058–3067. doi: 10.1101/gad.8.24.3058. [DOI] [PubMed] [Google Scholar]
- 53.Williams S M, Savery N J, Busby S J, Wing H J. Transcription activation at class I FNR-dependent promoters: identification of the activating surface of FNR and the corresponding contact site in the C-terminal domain of the RNA polymerase alpha subunit. Nucleic Acids Res. 1997;25:4028–4034. doi: 10.1093/nar/25.20.4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu H, Tyson K L, Cole J A, Busby S J. Regulation of transcription initiation at the Escherichia coli nir operon promoter: a new mechanism to account for co-dependence on two transcription factors. Mol Microbiol. 1998;27:493–505. doi: 10.1046/j.1365-2958.1998.00699.x. [DOI] [PubMed] [Google Scholar]
- 55.Xu J, Johnson R C. Identification of genes negatively regulated by Fis: Fis and RpoS comodulate growth-phase-dependent gene expression in Escherichia coli. J Bacteriol. 1995;177:938–947. doi: 10.1128/jb.177.4.938-947.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xu J, Johnson R C. Fis activates the RpoS-dependent stationary-phase expression of proP in Escherichia coli. J Bacteriol. 1995;177:5222–5231. doi: 10.1128/jb.177.18.5222-5231.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xu J, Johnson R C. Activation of RpoS-dependent proP P2 transcription by the Fis protein in vitro. J Mol Biol. 1997;270:346–359. doi: 10.1006/jmbi.1997.1133. [DOI] [PubMed] [Google Scholar]
- 58.Xu J, Johnson R C. Cyclic AMP receptor protein functions as a repressor of the osmotically inducible promoter proP P1 in Escherichia coli. J Bacteriol. 1997;179:2410–2417. doi: 10.1128/jb.179.7.2410-2417.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]