Abstract
The co-chaperone Bcl2-associated athanogene-3 (BAG3) maintains cellular protein quality control through regulation of heat shock protein 70 (HSP70). Cancer cells manipulate BAG3-HSP70-regulated pathways for tumor initiation and proliferation, which has led to the development of promising small molecule therapies, such as JG-98, that inhibit the BAG3-HSP70 interaction and mitigate tumor growth. However, it is not known how these broad therapies impact cardiomyocytes, where the BAG3-HSP70 complex is a key regulator of protein turnover and contractility. Here we show that JG-98 exposure is toxic in neonatal rat ventricular myocytes (NRVMs). Using immunofluorescence microscopy to assess cell death, we found that apoptosis increased in NRVMs treated with JG-98 doses as low as 10 nM. JG-98 treatment also reduced autophagy flux and altered expression of BAG3 and several binding partners involved in BAG3-dependent autophagy, including SYNPO2 and HSPB8. We next assessed protein half-life with disruption of the BAG3-HSP70 complex by treating with JG-98 in the presence of cycloheximide and found that BAG3, HSPB5, and HSPB8 half-lives were reduced, indicating complex formation with HSP70 is important for their stability. Next, we assessed sarcomere structure using super-resolution microscopy and found that disrupting the interaction with HSP70 lead to sarcomere structural disintegration. To determine if the effects of JG-98 could be mitigated by pharmacological autophagy induction, we co-treated NRVMs with rapamycin, which partially reduced the extent of apoptosis and sarcomere disarray. Finally, we investigated whether the effects of JG-98 extended to skeletal myocytes using C2C12 myotubes and found again increased apoptosis and reduced autophagic flux. Together, our data suggest that non-specific targeting of the BAG3-HSP70 complex to treat cancer may be detrimental for cardiac and skeletal myocytes.
Keywords: BAG3, HSP70, JG-98, cardiomyocyte, cytotoxicity, cancer therapy, cardio-oncology
1. INTRODUCTION
The co-chaperone Bcl2-associated athanogene-3 (BAG3) helps to maintain cell survival through regulation of protein quality control (PQC). The involvement of BAG3 in PQC stems from its association with heat shock protein 70 (HSP70), for which BAG3 is a nucleotide exchange factor, regulating client handling and connection with autophagy (Stürner & Behl, 2017). However, increased activity of BAG3-HSP70 in cancer is associated with tumor grade, metastasis, and chemotherapy resistance (De Marco et al., 2020). A considerable effort has therefore been made to develop therapies that inhibit BAG3 and its modulation of HSP70 activity (De Marco et al., 2018; Zhu et al., 2012). The small molecule JG-98, which binds an allosteric pocket in the nucleotide binding domain of HSP70 and disrupts the interaction with BAG3, displays potent anti-cancer effects (Li et al., 2015; Yaglom et al., 2018). Importantly, the toxic effects of JG-98 also appear to be cancer cell-specific as healthy fibroblasts were viable with similar treatment (Li et al., 2015). However, while these studies highlight therapeutic promise for targeting BAG3-HSP70 in cancer, little is known regarding the impact of JG-98 on other tissues, such as the heart, where the BAG3-HSP70 interaction is crucial for function (Fang et al., 2017; Hishiya et al., 2010).
The highest expression of BAG3 in healthy tissues is in striated muscle, which experiences constant stress from the mechanical strain of muscle contraction. Previous work in skeletal myocytes found that the BAG3-HSP70 complex was essential for maintaining the sarcomere – the fundamental contractile unit in muscle cells – through chaperone-assisted selective autophagy (CASA) (Arndt et al., 2010; Ulbricht et al., 2015). In this process, BAG3 and HSP70 form a complex with small heat shock proteins (HSPBs) and synaptopodin-2 to target misfolded proteins to autophagy (Ulbricht & Höhfeld, 2013). We have recently shown that CASA extends to cardiac muscle and is essential for maintaining cardiomyocyte contractile function (Martin et al., 2021). Given the significance of BAG3-HSP70 for PQC and contractile function, it is necessary to determine whether disrupting this complex to treat cancer with compounds like JG-98 will have detrimental off-target effects in cardiomyocytes.
In this study, we show exposure of neonatal rat ventricular myocytes (NRVMs) to JG-98 has severe consequences. Disrupting the BAG3-HSP70 complex with JG-98 caused apoptosis, reduced autophagy flux, and altered expression and protein stability of BAG3 and its binding partners. JG-98 also caused sarcomere structural disintegration and impaired stress-responsive sarcomere maintenance. The negative impact of JG-98 on cell viability and sarcomere structure could be partially rescued by co-treatment of NRVMs with the autophagy inducing compound rapamycin. Finally, using C2C12 myotubes we show the toxic effects of JG-98 may extend to skeletal muscle. Our data support previous findings that the BAG3-HSP70 complex is fundamental for cardiomyocytes and suggest that targeting this interaction to treat cancer will have negative consequences in the heart.
2. MATERIALS & METHODS
2.1 –. NRVM isolation and culture
Hearts were isolated from one-day-old Sprague-Dawley rats and placed in Krebs-Henseleit Buffer (KHB). Tissue was digested in KHB containing collagenase type II (Worthington, Lot #: 49A19003) and 0.05% trypsin at 37 °C for 15 minutes. The solution was then added to isolation medium (DMEM, 4.5 g/L glucose, +L-glutamine, -pyruvate, 10% FBS, 1X ITS-X, 100 U/mL penicillin-streptomycin) to stop the digestion reaction. This was repeated five times. The isolated cells were resuspended in fresh isolation medium and added to a cell culture dish for 90 minutes to remove fibroblasts. The dish was tapped to dislodge myocytes, which were collected, resuspended in maintenance medium (DMEM, 4.5 g/L glucose, +L-glutamine, -pyruvate, 18.5% M199 medium, 5% horse serum, 1% FBS, 1X ITS-X, 100 U/mL penicillin-streptomycin, 10 μM BrdU to prevent proliferation of contaminating fibroblasts) and plated at ~80% confluency.
2.2 –. C2C12 culture and differentiation
C2C12 myoblasts were grown to ~100% confluency in growth medium (DMEM, 4.5 g/L glucose, +L-glutamine, -pyruvate, 10% FBS). The media was then switched to differentiation media (DMEM, 2% horse serum), which was changed every 24 hours. Experiments were performed on days 5–7 post-differentiation.
2.3 –. Immunoprecipitation
Dyn abeads protein G (Thermo Fisher Scientific) were incubated with an anti-HSP70 antibody (Proteintech 10995–1AP) for 30 minutes and then crosslinked with 20 mM dimethyl pimelimidate dihydrochloride for 30 minutes. The crosslinking reaction was quenched by incubation with 200 mM ethanolamine for 2 hours. Cell lysates were collected in Igepal lysis buffer (150 mM NaCl, 50 mM Tris-HCl pH 8.0, 1% Igepal) and precleared by incubation with Dynabeads for 20 minutes. The antibody-beads complex was then incubated with 300 μg protein for 1 hour at 4 °C. The complex was washed four times with lysis buffer for 5 minutes each at 4 °C. Immunoprecipitated protein was eluted with 0.15% trifluoroacetic acid.
2.4 –. Western blotting
Cell lysates were collected in 8M urea/0.2% SDS. A BCA assay (Pierce) was used to determine protein concentration. 5 μg of protein was separated on 4–12% bis-tris gradient gels (Invitrogen) and transferred onto nitrocellulose membrane. Revert Total Protein Stain (LI-COR Biosciences) was used to assess protein loading. The following primary antibodies/dilutions were used: BAG3 (Proteintech 10599–1AP, 1:4000), HSP70 (Proteintech 10995–1AP, 1:4000), HSPB8 (Proteintech 15287–1AP, 1:2500), HSPB5 (15808–1AP, 1:2500), SYNPO2 (Proteintech 25453–1AP, 1:2500), LC3 (Cell Signaling Technology 2775s, 1:1000). Secondary antibodies: LICOR IRDye 680RD and 800CW, 1:8000. Membranes were imaged on an Azure c600. Protein densitometry was quantified using ImageStudio (LI-COR Biosciences).
2.5 –. Quantitative RT-PCR
Cells were lysed in Trizol and the RNA was extracted using the RNeasy RNA extraction kit (Qiagen) with on-column DNase I treatment. cDNA was synthesized using Superscript IV Reverse Transcriptase (Thermo Fisher Scientific). Quantitative RT-PCR was performed with Quant Studio 3 using SYBR green mastermix and the primers listed in Table 1. Candidate mRNA expression was normalized to β-actin using the ΔΔCT method.
Table 1.
Primer sequences for target genes
| Cell Type | Gene | Primer Nucleotide Sequences | NCBI RefSeq |
|---|---|---|---|
| NRVM | bag3 | F: CTTGTTTGGGAGCCCTTTCT R: TCCTGTAACTCTCTGCCTATCC |
NM_001011936.1 |
| NRVM | hspa1a | F: CCATCGAGGAGGTGGATTAGA R: GAACTCCGGAGAGAAGGAGTAA |
NM_031971.2 |
| NRVM | hspb8 | F: GTCAGCCTTGGTCCTTCTTT R: AGGGATGGTCATTTGGTTCTC |
NM_053612.2 |
| NRVM | hspb5 | F: GTGGACAGAGAGCTAGTGAAAC R: GGGACTTGTGATCAGGGATTT |
NM_012935.4 |
| NRVM | synpo2 | F: CCTCTCAGCCCAACTTCTTT R: GGATGTGGAGGTCGTTTCTT |
NM_001191963.1 |
| NRVM | β-actin | F: CTTCCTTCCTGGGTATGGAATC R: CTGTGTTGGCATAGAGGTCTT |
NM_031144.3 |
| C2C12 | bag3 | F: TCTGGATGGGACCAGAAGT R: GGGTGGAAGTGTCTGGAAATAG |
NM_013863.5 |
| C2C12 | hspa1a | F: TGGTGCTGACGAAGATGAAG R: CGCTGAGAGTCGTTGAAGTAG |
NM_010479.2 |
| C2C12 | hspb8 | F: CCGGAAGAGCTGATGGTAAAG R: CTTCTGCAGGGAGCTGTATTT |
NM_030704.3 |
| C2C12 | hspb5 | F: TCTTCTCAACAGCCACTTCC R: TCCTTCTCCAAACGCATCTC |
NM_001289782.1 |
| C2C12 | synpo2 | F: CAGGTGACAAGGACGAGATAAG R: CCAGTCCAGAATCCCAATCAA |
NM_001388502.1 |
| C2C12 | β-actin | F: GAGGTATCCTGACCCTGAAGTA R: CACACGCAGCTCATTGTAGA |
NM_007393.5 |
2.6 –. Measurement of autophagy flux
The culture medium was replaced with fresh medium containing 1 μM JG-98 (MedChem Express) or DMSO, with or without 10 mM NH4Cl, and incubated for 18 hours. Following treatment, the cells were lysed in 8M urea/0.2% SDS. Western blotting for LC3-II was performed to assess autophagic flux.
2.7 –. Measurement of apoptosis
The Apoptosis/Necrosis Detection Kit (Abcam, ab176749) was used according to the manufacturer protocol. Briefly, myocytes were incubated in assay buffer containing Apopxin Green indicator for apoptosis and CytoCalcein indicator for healthy cells and incubated for one hour at room temperature. The cells were washed with assay buffer and imaged using a Zeiss LSM 880 at 10X magnification. Excitation/emission parameters: Apopxin Green – 488/525nm, CytoCalcein – 405/450nm. Image scoring was performed by an experimenter blinded to the treatment groups.
2.8 –. Measurement of protein half-life
Cells were treated with either DMSO or 1 μM JG-98 in the presence of 50 μM cycloheximide to block protein translation. Cell lysates were collected in 8M urea/0.2% SDS at the start of treatment (t = 0) and at 2-, 4-, 8-, 12-, and 24-hours post-treatment. Protein expression across the time points was assessed by western blot.
2.9 –. Assessment of sarcomere structural disarray
Myocytes were incubated with DMSO or 1 μM JG-98 for 18 hours or for 1 hour followed by 2-hour heat shock at 42 °C. Cells were then washed with PBS and fixed in ice-cold methanol for 1 minute, followed by 4% paraformaldehyde for 3 minutes. Permeabilization was performed with 0.5% triton for 20 minutes, followed by 0.1% triton for 30 minutes. 100 mM glycine (pH 7.4) for 30 minutes was used for antigen retrieval. The slides were washed with PBS and blocked with 1:1 PBS to BSA blocking solution (1% BSA, 1% gelatin, 1% tween-20, 0.001% NaN3) for one hour. Primary antibodies were added in blocking buffer and incubated overnight at 4 °C. Antibodies/dilutions: BAG3 (Proteintech 10599–1AP, 1:250), α-actinin (Millipore Sigma A7811, 1:150). Following overnight incubation, the slides were washed with PBS, and alexa-fluor secondary antibodies (Abcam) were added in blocking buffer at 1:1000 dilution for 1 hour at room temperature. Slides were washed with PBS, mounted with Vectashield containing DAPI (Vector Laboratories), and imaged at 63X magnification on a Zeiss LSM 880 microscope with Airyscan. Sarcomere scoring was performed as previously described (Judge et al., 2017), blinded to the treatment groups.
2.10 –. Statistical analysis
All experiments were replicated with myocytes from 3 or more separate isolations. The data are presented as the mean ± SEM and were analyzed using GraphPad Prism 9. For analyses of three or more groups, a one or two-way ANOVA was performed as annotated in the figure legends. If a significant interaction was identified, Tukey’s post-hoc test was used. For all comparisons of two groups, the data were analyzed using Student’s two-tailed t test. A p value of < 0.05 was considered statistically significant.
3. RESULTS
3.1. Disrupting the BAG3-HSP70 complex with JG-98 causes cardiomyocyte apoptosis
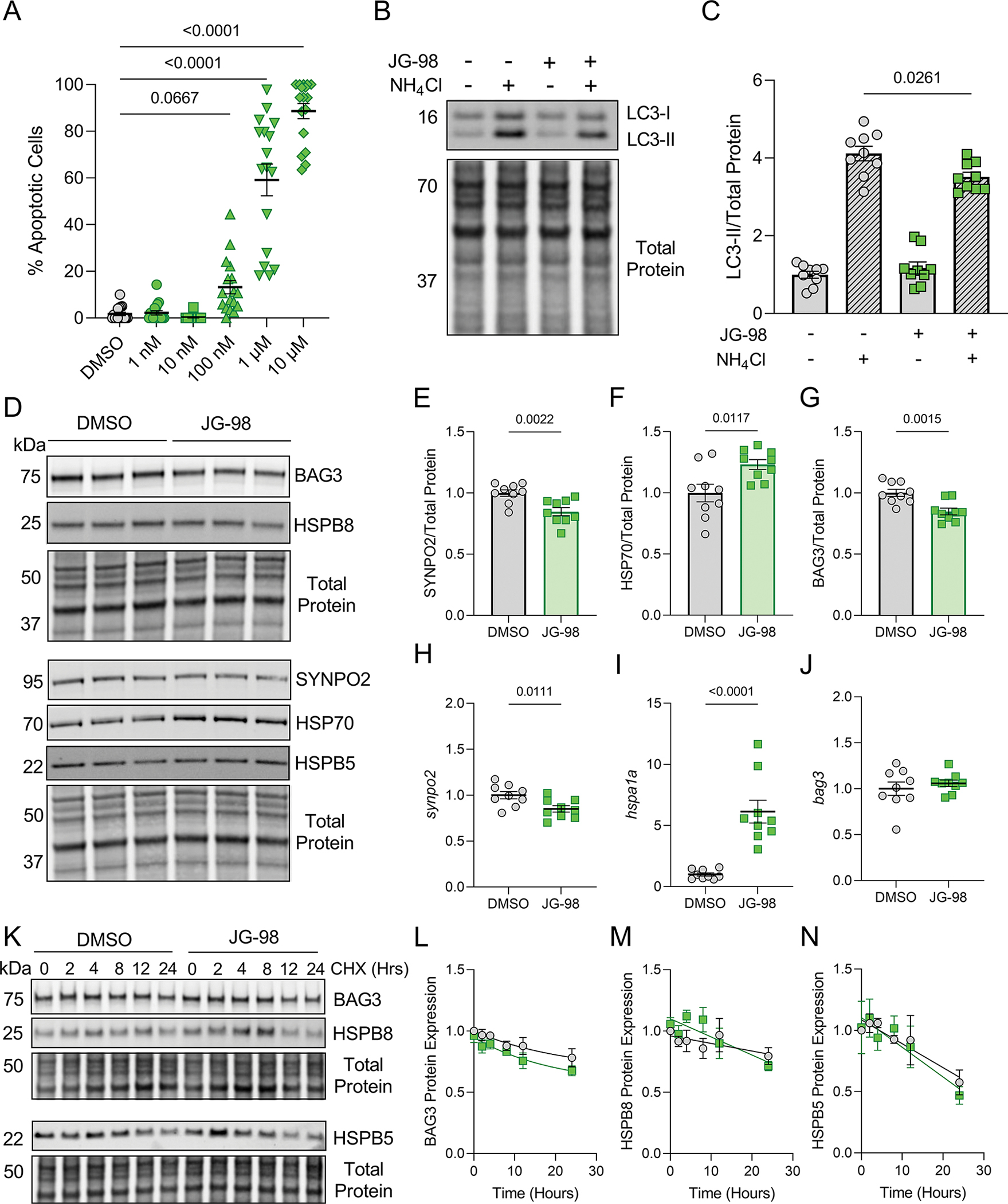
To determine the impact of JG-98 on cardiomyocyte viability, we treated NRVMs with doses of JG-98 ranging from 1 nM to 10 μM for 18 hours and assessed apoptosis using immunofluorescence microscopy for extracellular membrane-expressed phosphatidylserine. JG-98 treatment significantly increased cardiomyocyte apoptosis at doses from as low as 10 nM (15.95 ± 2.75%) up to 10 μM (92.97 ± 2.09%) compared to DMSO control (1.34 ± 0.32%) (Figure 1A–B). Based on the results of this assay, we chose to use 18 hours of 1 μM JG-98 for the remainder of the study as it caused moderate apoptosis (63.5 ± 2.7%) and is comparable to dose/duration used in cancer cell studies (Li et al., 2015; Yaglom et al., 2018). To confirm that 1 μM JG-98 reduced the association of BAG3-HSP70, we used immunoprecipitation (Figure 1C) and found that JG-98 significantly reduced their association as quantified by BAG3 signal normalized to HSP70 (1.00 ± 0.07 DMSO vs. 0.54 ± 0.05 JG-98, Figure 1D).
Figure 1. Disrupting the BAG3-HSP70 complex with JG-98 causes apoptosis in cardiomyocytes.
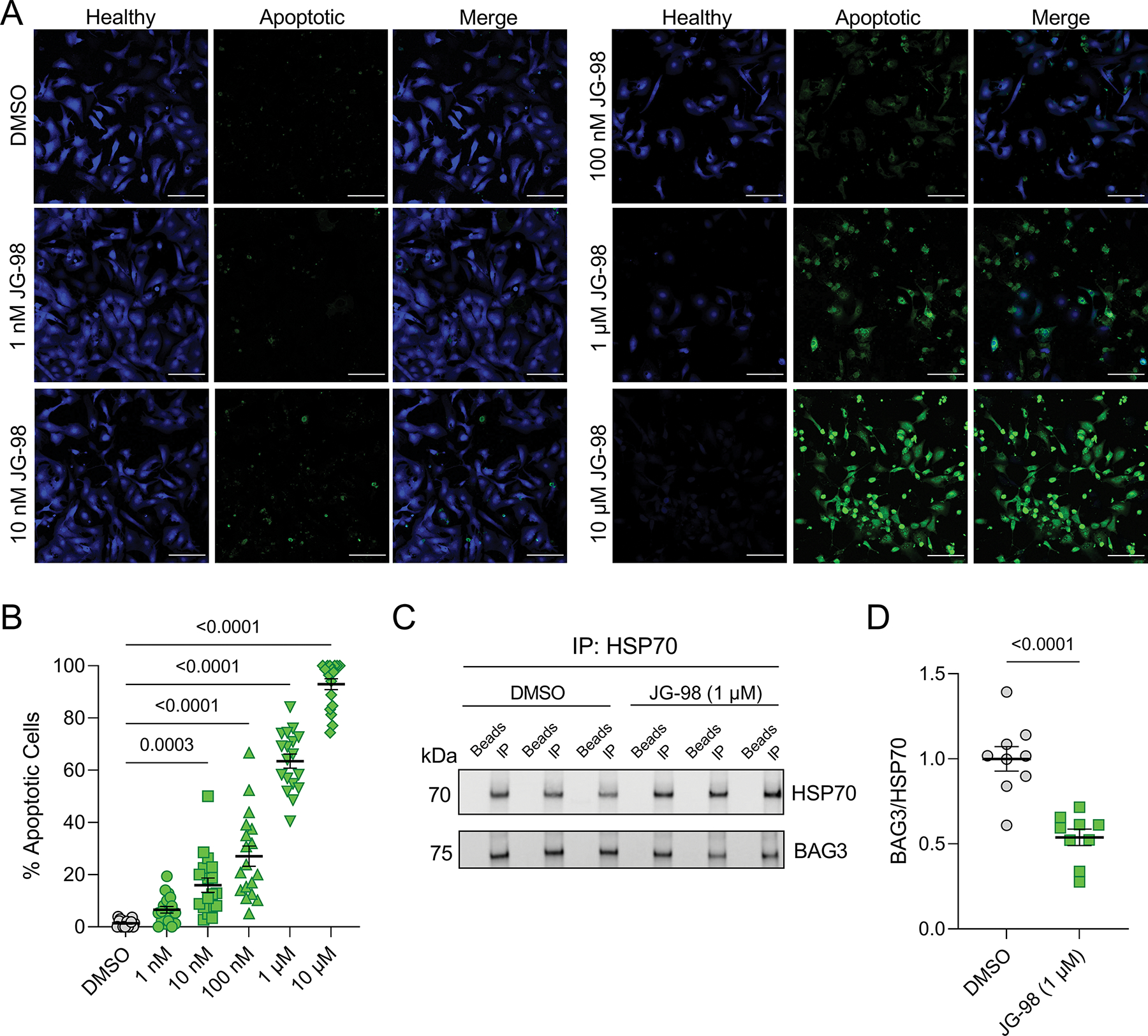
A, Representative immunofluorescence images for NRVMs treated with DMSO and 1 nM to 10 μM JG-98; green – extracellular membrane phosphatidylserine (apoptotic), blue – healthy; scale bars = 100 μm. B, Quantification of apoptosis in the immunofluorescence images; n = 18 images per treatment from 3 separate experiments; data were analyzed by one-way ANOVA with Tukey’s post-hoc test. C, Representative western blot for HSP70 and BAG3 in HSP70 immunoprecipitation experiments from cells treated with DMSO or 1 μM JG-98. D, Quantitative densitometry analysis of the BAG3 signal intensity normalized to HSP70; n = 9 per group from 3 separate experiments; data were analyzed by two-tailed t-test. All data are presented as the mean ± SEM.
3.2. Autophagy flux is impaired with JG-98
Since the BAG3-HSP70 complex had previously been identified to mediate autophagy in myocytes (Klimek et al., 2017), we next investigated whether inhibition of the BAG3-HSP70 complex with JG-98 affects autophagy flux. To measure flux, we treated NRVMs with DMSO or JG-98 in the presence or absence of 10 mM ammonium chloride (NH4Cl), which prohibits lysosome acidification and thus blocks autophagy. After 18 hours, cell lysates were collected and autophagy flux was assessed by western blotting for LC3-II (Figure 2A). LC3-II protein expression significantly increased in both the DMSO and JG-98 groups treated with NH4Cl, however, the extent of increase was reduced with JG-98 (2.09 ± 0.22 vs. 3.26 ± 0.29 DMSO) indicating impaired autophagy flux (Figure 2B).
Figure 2. JG-98 treatment reduces autophagy flux.
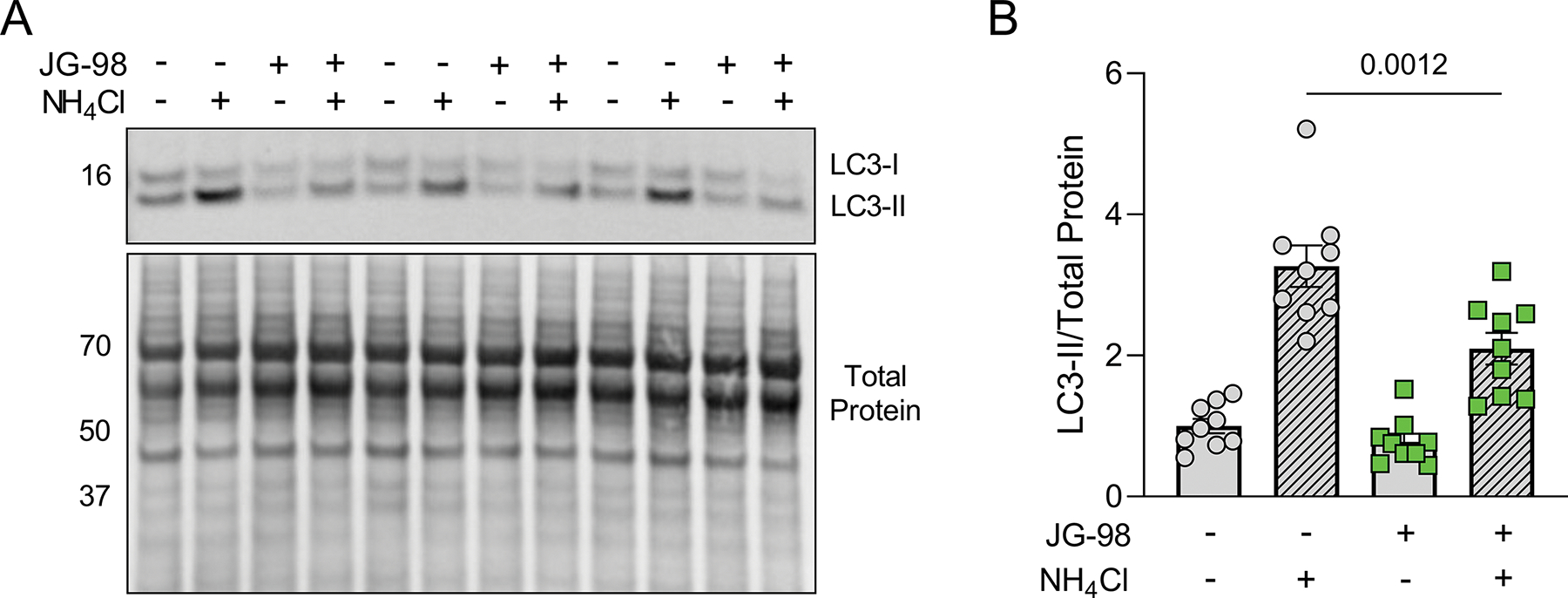
A, Representative western blot for LC3 in NRVMs treated with DMSO or JG-98 in the presence or absence of the autophagy inhibitor NH4Cl. B, Quantification of LC3-II expression normalized to total protein; n = 9 per group from 3 separate experiments; data were analyzed with two-way ANOVA (interaction, p = 0.023) with Tukey’s post-hoc test. Data are presented as the mean ± SEM.
3.3. JG-98 alters expression of BAG3 binding partners
To gain insight into the toxic effects of JG-98, we next assessed its impact on protein and gene expression of BAG3, HSP70, and several other BAG3-binding proteins that assemble with this complex to mediate autophagy: the small heat shock proteins HSPB8 and HSPB5, which mediate client recognition and connection with HSP70, and synaptopodin-2 (SYNPO2), which assists in autophagosome formation (Martin & Kirk, 2020). Using western blot and qPCR, we found no changes in BAG3 and HSP70 at the protein level with JG-98 (Figure 3A–D), while protein levels of HSPB8, HSPB5, and SYNPO2 decreased with JG-98 treatment (Figure 3E–G). At the transcript level we found a significant decrease in bag3 expression (Figure 3H), no change in hspa1a and hspb8 (Figure 3I–J), and decreases in hspb5 and synpo2 expression (Figure 3K–L). These findings suggest that the interaction of BAG3 with HSP70 regulates expression of BAG3 and its binding partners.
Figure 3. Inhibition of BAG3-HSP70 alters expression of proteins involved in BAG3-dependent autophagy pathway.
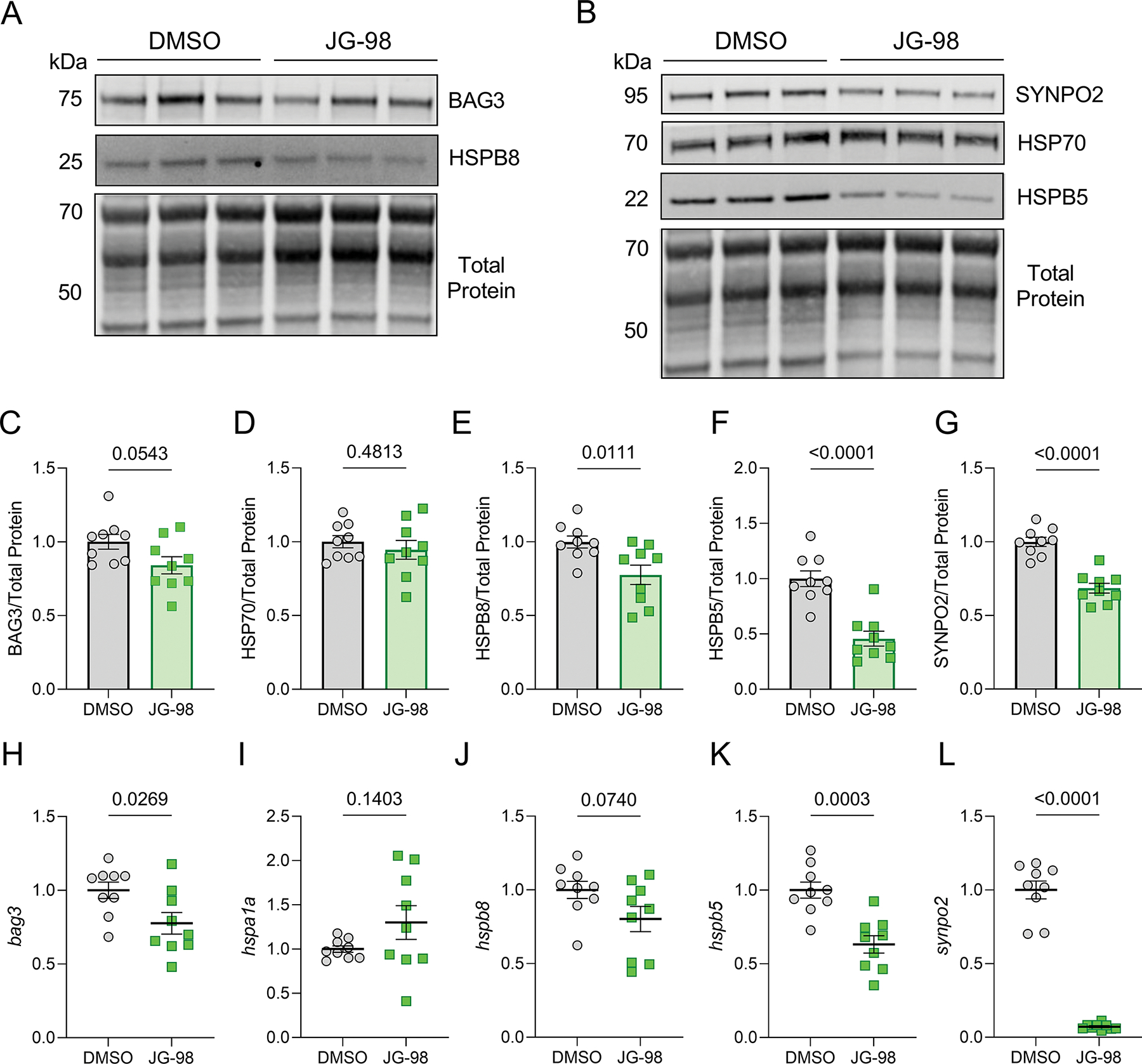
A-B, Representative western blots for BAG3, HSPB8, SYNPO2, HSP70, HSPB5 in NRVMs treated with DMSO or JG-98. C-G, Quantification of BAG3 (C), HSP70 (D), HSPB8 (E), HSPB5 (F), and SYNPO2 (G) protein expression normalized to total protein loading control. H-L, qPCR analysis of bag3 (H), hspa1a (I), hspb8 (J), hspb5 (K), and synpo2 (L) mRNA expression normalized to β-actin. All treatments were with 1 μM JG-98 for 18 hours. For all, n = 9 per group from 3 separate experiments; data were analyzed by two-tailed t-test and are presented as the mean ± SEM.
3.4. Maintaining protein stability of BAG3, HSPB8, and HSPB5 requires the BAG3-HSP70 complex
Previous work on a pathogenic BAG3 mutation that disrupts binding to HSP70 found that the BAG3-HSP70 interaction is required for stabilizing the HSPBs (Fang et al., 2017). We therefore next investigated whether JG-98 alters protein stability of HSPB5 and HSPB8, along with SYNPO2, HSP70, and BAG3. NRVMs were treated with DMSO or JG-98 in the presence of 50 μM cycloheximide (CHX) to block protein translation, cell lysates were collected at 0, 2, 4, 8, 12, and 24 hours post-CHX treatment, and protein expression was assessed by western blot (Figure 4A–B). JG-98 reduced the stability of BAG3, HSPB8, and HSPB5 (Figure 4C–E), while HSP70 and SYNPO2 stability were unaffected. Protein half-life decreased significantly for BAG3 (28.0 ± 0.3 to 3.04 ± 0.5 hours), HSPB8 (25.7 ± 4.3 to 4.0 ± 0.9 hours), and HSPB5 (14.0 ± 3.2 to 2.1 ± 0.6 hours) (Figure 4F), supporting that the BAG3-HSP70 interaction is fundamental for maintaining stability of these proteins.
Figure 4. The BAG3-HSP70 interaction is required for maintaining BAG3, HSPB8, and HSPB5 protein stability.
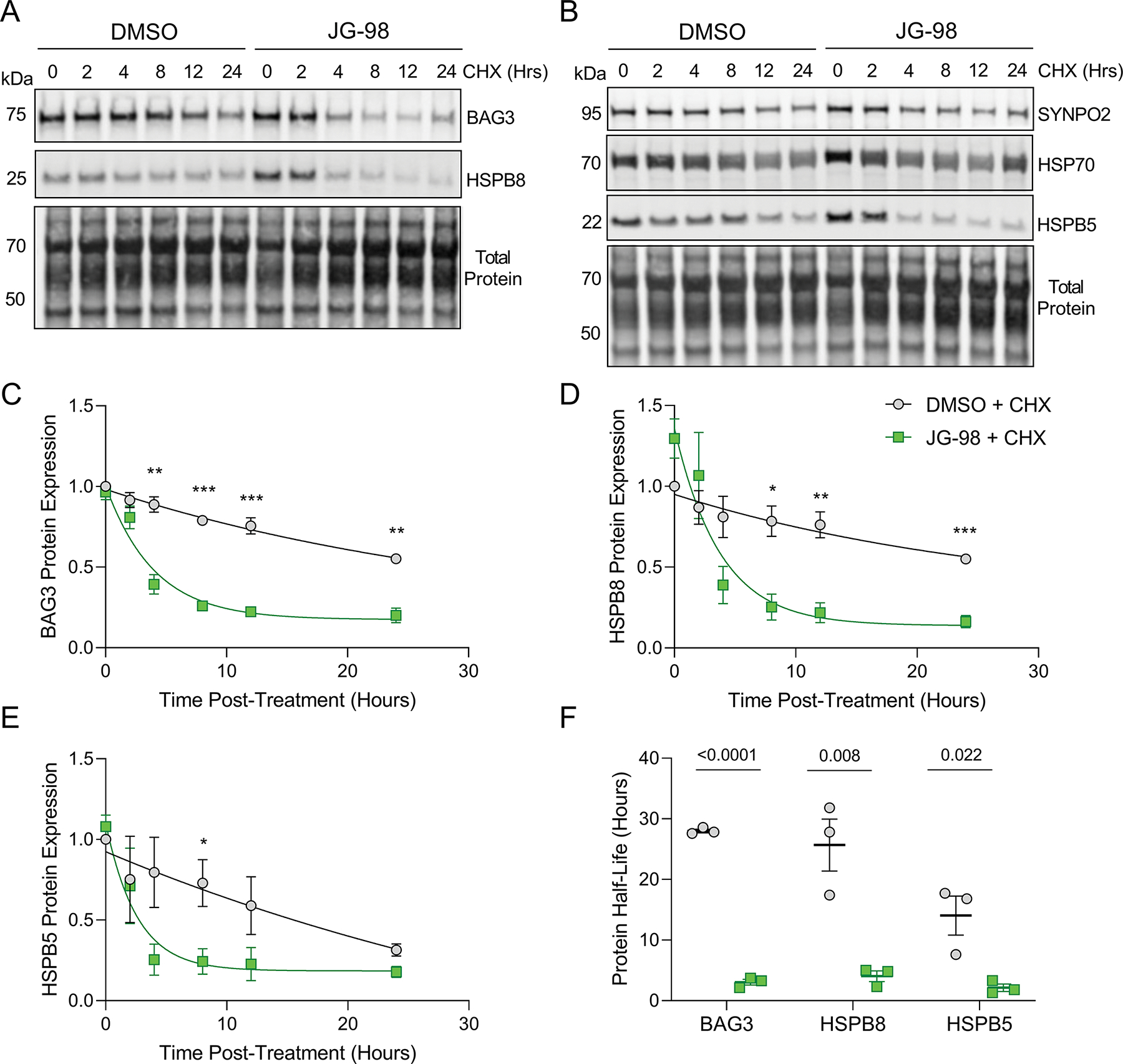
A-B, Representative western blots for BAG3, HSPB8, SYNPO2, HSP70, HSPB5 in NRVMs treated with DMSO or 1 μM JG-98 in the presence of cycloheximide (CHX) for 0–24 hours. C-E, Quantification of BAG3 (C), HSPB8 (D), and HSPB5 (E) protein expression normalized to total protein over the time-course of CHX treatment with DMSO or JG-98; n = 3 for each time point from 3 separate experiments; data were plotted using one-phase decay equation; data were analyzed by two-tailed t-test; *p < 0.05, **p < 0.01, ***p < 0.001. F, Presentation of protein half-life for BAG3, HSPB8, and HSPB5 obtained from the preceding graphs; each point for a given protein represents a separate experiment; data were analyzed by two-tailed t-test. All data are presented as the mean ± SEM.
3.5. JG-98 treatment causes sarcomere structural disintegration
We recently identified BAG3-dependent PQC at the cardiac sarcomere is required for maintaining cardiomyocyte contractile capacity (Martin et al., 2021). Since the interaction with HSP70 is integral to this process, we hypothesized that JG-98 treatment in NRVMs would be detrimental for sarcomere structure. Using immunofluorescence microscopy for BAG3 and the Z-disc protein α-actinin, we found a significant increase in sarcomere disarray with JG-98 (72.5 ± 5.6% vs. 25.0 ± 2.6% DMSO) (Figure 5A–B). To ensure this effect was directly due to impaired stress-responsive sarcomere maintenance and not a byproduct of apoptosis at the late time-point, we next treated NRVMs with JG-98 for just 1 hour followed by a 2-hour heat shock at 42 °C to induce stress. We again found significantly more sarcomere disarray in the JG-98 group (82.3 ± 4.6%) compared to DMSO (37.5 ± 4.0%) (Figure 5C–D), supporting that the BAG3-HSP70 complex is required for stress-responsive maintenance of the sarcomere.
Figure 5. BAG3-HSP70 is required for maintaining sarcomere structure.
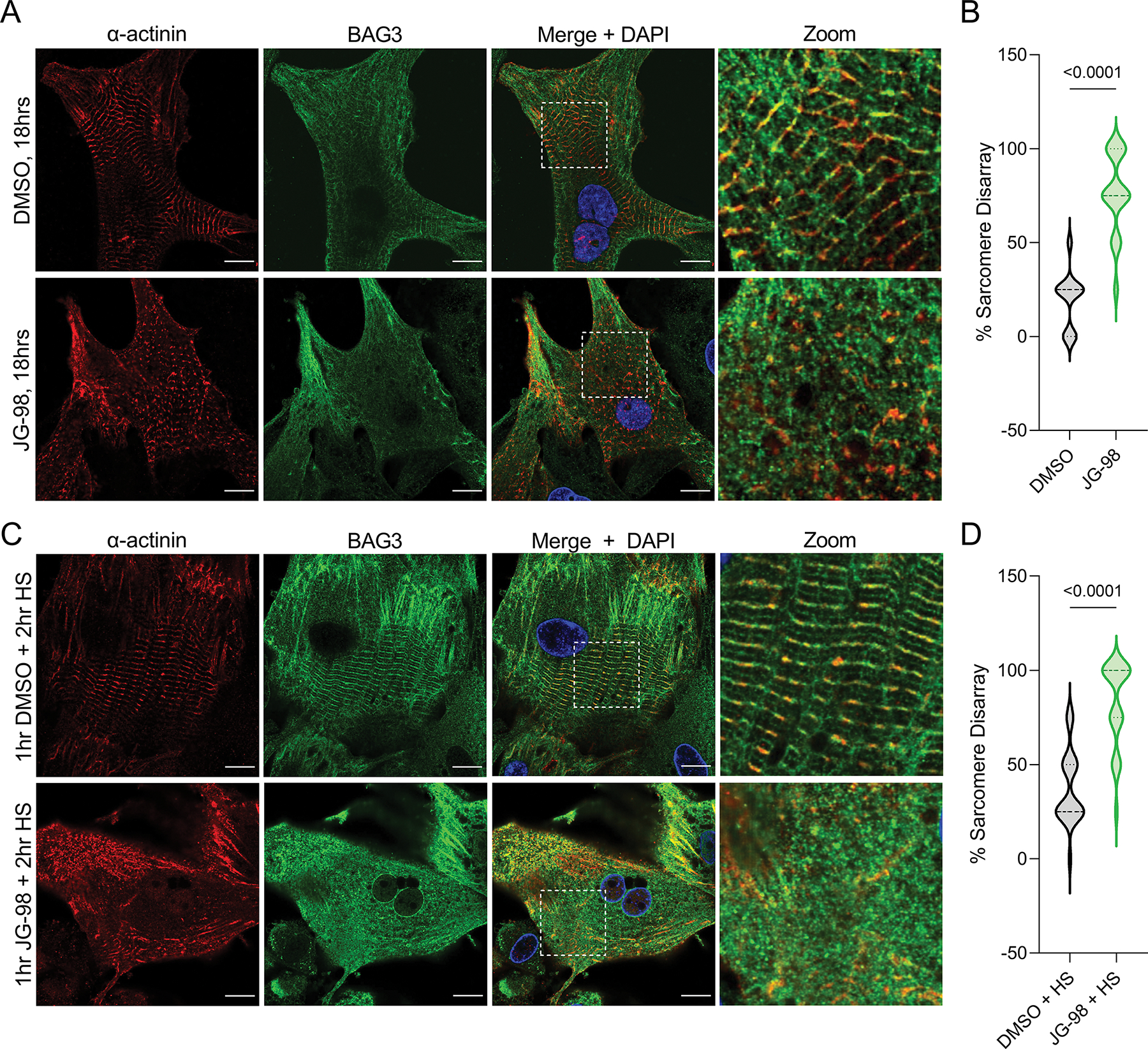
A, Representative immunofluorescence images of NRVMs treated with DMSO or 1 μM JG-98 for 18 hours and immunostained for BAG3 and α-actinin. B, Quantification of sarcomere disarray in the DMSO and JG-98 groups; n = 36 cells per group from 3 separate experiments. C, Representative immunofluorescence images of NRVMs treated with DMSO or 1 μM JG-98 for 1 hour, followed by heat shock (HS) at 42 °C for two hours, then fixed and immunostained for BAG3 and α-actinin. D, Quantification of sarcomere disarray in the DMSO/HS and JG-98/HS groups; n = 24 cells per group from 3 separate experiments. Images were acquired at 63X magnification; scale bars = 10 μm. Data were analyzed by two-tailed t-test.
3.6. Autophagy induction with rapamycin partially rescues toxic effects of JG-98
Earlier work in zebrafish with BAG3 loss-of-function mutations found protein aggregation was reduced with autophagy induction by rapamycin and with genetic haplo-insufficiency of mTOR (Ding et al., 2019; Ruparelia et al., 2014, 2020). We therefore investigated whether co-treatment with rapamycin could mitigate the cardiotoxicity of JG-98. We first assessed apoptosis by immunofluorescence with DMSO and 1 μM JG-98 with and without 30 nM rapamycin for 18 hours (Figure 6A). The extent of cell death caused by JG-98 (57.8 ± 4.1%) was partially rescued with rapamycin (42.8 ± 2.6%) (Figure 6B). We next assessed the effect of rapamycin on sarcomere structure and found it significantly reduced the negative impact of JG-98 (85.2 ± 3.3% disarray JG-98 vs. 67.2 ± 4.8% JG-98/rapamycin) (Figure 6C–D). These data support the earlier findings that rapamycin may be a potential therapeutic option when BAG3 function is compromised.
Figure 6. The negative effects of JG-98 on apoptosis and sarcomere structure are partially ameliorated with rapamycin co-treatment.
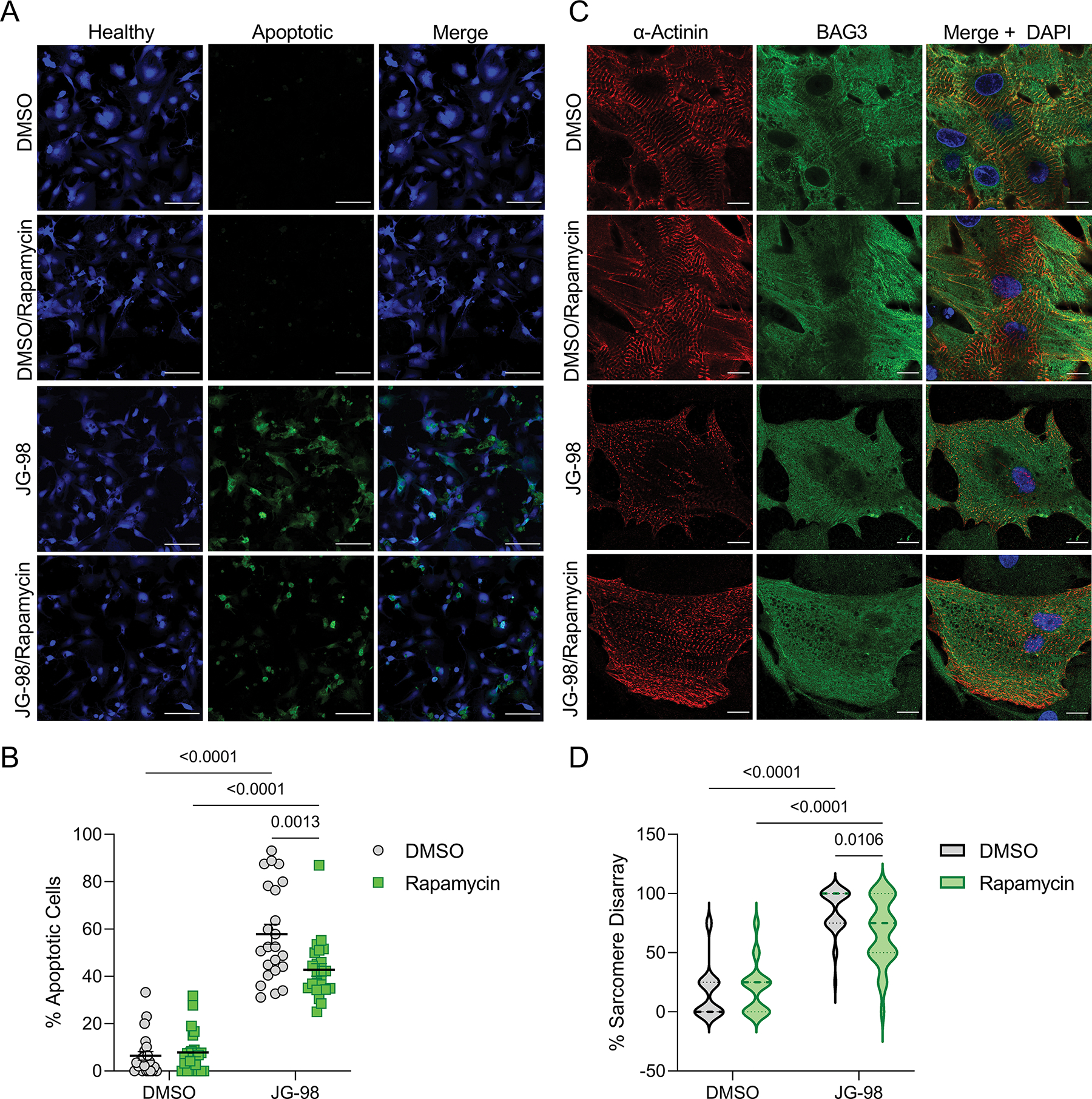
A, Representative immunofluorescence images of NRVMs treated with DMSO or 1 μM JG-98 with or without 30 nM rapamycin; green – extracellular membrane phosphatidylserine (apoptotic), blue – healthy; scale bars = 100 μm. B, Quantification of apoptosis in the four groups; n = 23 images per group from 3 separate experiments; data are presented as the mean ± SEM and were analyzed by two-way ANOVA (interaction = 0.0039) with Tukey’s post-hoc test. C, Representative immunofluorescence images of NRVMs treated with DMSO or 1 μM JG-98 with or without 30 nM rapamycin for 18 hours and immunostained for BAG3 and α-actinin; images were acquired at 63X magnification; scale bars = 10 μm. D, Quantification of sarcomere structural disarray in the four groups; n = 32 cells per group from 3 separate experiments; data were analyzed by two-way ANOVA (interaction = 0.0075) with Tukey’s post-hoc test.
3.7. JG-98 toxicity extends to skeletal myotubes
The importance of BAG3-dependent autophagy in myocytes has been well described in skeletal muscle (Arndt et al., 2010; Kathage et al., 2017; Ulbricht et al., 2015). Therefore, we next investigated whether the toxic effects of JG-98 extend to skeletal myocytes. Using C2C12 skeletal myotubes, we assessed apoptosis with JG-98 doses from 1 nM to 10 μM (Supplemental Figure 1). As in the cardiomyocytes, JG-98 caused significant apoptosis at 1 μM (59.2 ± 6.8%) and 10 μM (88.6 ± 3.3%), however, myotubes were viable at doses of 100 nM and lower (Figure 7A). We next measured autophagy flux with NH4Cl and found JG-98 reduced autophagy flux as indicated by decreased LC3-II expression (3.51 ± 0.12) compared to DMSO (4.12 ± 0.19) (Figure 7B–C). Using western blot and qPCR to assess expression of BAG3 and its binding partners, we found BAG3 and SYNPO2 decreased at the protein level, while HSP70 expression increased, changes that were borne out at the transcript level for synpo2 and hspa1a (Figure 7D–J). Unlike in cardiomyocytes, JG-98 had no impact on HSPB protein expression (Supplemental Figure 2), nor did it affect stability of BAG3, HSPB8, or HSPB5 (Figure 7K–N).
Figure 7. The effects of JG-98 on C2C12 skeletal myotubes.
A, Quantification of apoptosis identified by immunofluorescence imaging in C2C12 myotubes treated with DMSO or JG-98 for 18 hours; one-way ANOVA, Tukey post-hoc. B, Representative western blot for LC3 in C2C12s treated with DMSO or JG-98 in the presence or absence of NH4Cl. C, Quantification of LC3-II expression normalized to total protein; n = 9 per group from 3 separate experiments; data were analyzed by two-way ANOVA (interaction = 0.0104), Tukey post-hoc. D, Representative western blots for BAG3, HSPB8, SYNPO2, HSP70, and HSPB5 in C2C12s treated with DMSO or JG-98 for 18 hours. E-G, Quantification of SYNPO2 (E), HSP70 (F), and BAG3 (G) protein expression normalized to total protein; n = 9 per group from 3 separate experiments; two-tailed t-test. H-J, qPCR analysis of synpo2 (H), hspa1a (I), and bag3 (J) expression normalized to β-actin; n = 9 per group from 3 separate experiments; two-tailed t-test. K, Representative western blots for BAG3, HSPB8, SYNPO2, HSP70, and HSPB5 in C2C12s treated with DMSO or 1 μM JG-98 in the presence of cycloheximide (CHX). L-N, Quantification of BAG3 (L), HSPB8 (M), and HSPB5 (N) expression normalized to total protein over the time-course of CHX treatment; n = 3 for each time point from 3 separate experiments; data were plotted using one-phase decay equation. Data are presented as the mean ± SEM.
4. DISCUSSION
The multifunctional co-chaperone BAG3 maintains PQC through regulation of autophagy (Stürner & Behl, 2017). The role of BAG3 in PQC stems from its interaction with HSP70, where BAG3 binds in the nucleotide binding domain and connects HSP70 with misfolded protein aggregates and the macroautophagy pathway (Chakraborty et al., 2019; Gamerdinger et al., 2011). Unfortunately, the BAG3-HSP70 complex is co-opted in various cancers to promote cell survival and tumor proliferation (Colvin et al., 2014; Shields et al., 2018). This has led to the development of promising small molecule inhibitors of BAG3-HSP70, which display anti-proliferative effects in cancer models both in vitro and in vivo (Colvin et al., 2014; Das et al., 2018; Li et al., 2015; Yaglom et al., 2018). However, it is not clear if these proposed therapeutics have detrimental effects on other cell types, such as cardiomyocytes, which rely on BAG3-mediated macroautophagy for function (Mizushima & Sadoshima, 2017). Here, we studied the effects of the BAG3-HSP70 inhibitor JG-98 on cardiomyocytes in vitro and found that disrupting the complex caused apoptosis, reduced autophagic flux, and ablated sarcomere structure.
As terminally differentiated cells that must survive for decades while experiencing constant stress from the strain of contraction, cardiomyocytes critically rely on PQC systems (Henning & Brundel, 2017). When these systems are compromised, as often occurs with heart disease, cardiomyocytes exhibit increased apoptosis and contractile dysfunction (Maejima, 2020; Ranek et al., 2018). BAG3 has emerged in recent years as a key player in cardiac PQC and both mutations to BAG3 (Domínguez et al., 2018; Franaszczyk et al., 2014; Myers et al., 2018; Selcen et al., 2009) and its decreased expression (Feldman et al., 2014; Martin et al., 2021) are associated with heart failure. Interestingly, pathogenic BAG3 mutations are commonly found in the C-terminal BAG domain, which mediates binding to HSP70 (Sondermann et al., 2001), and thus compromise BAG3-dependent protein turnover (Fang et al., 2017). In this study, we show that the effects of JG-98 in cardiomyocytes are similar to BAG domain mutants, causing apoptosis, reducing autophagy, and destabilizing the small heat shock proteins. Additionally, we show that the interaction with HSP70 is critical for BAG3’s protein stability. The BAG3-HSP70 association may also be important for regulating gene expression of bag3, synpo2, and the hspbs, which decreased at the transcript level with JG-98. The mechanism of this decrease it not clear, but previous work has shown that both BAG3 and HSP70 modulate the stress response through regulation of heat shock factor-1 (Jin et al., 2015; Masser et al., 2019). However, whether the BAG3-HSP70 complex is involved in gene expression regulation remains to be elucidated.
BAG3 localization in cardiomyocytes is predominantly to the sarcomere Z-disc. Our earlier work, informed by studies in skeletal myocytes (Arndt et al., 2010; Ulbricht et al., 2015), found that sarcomere-localized BAG3 orchestrates chaperone-assisted selective autophagy (CASA) of sarcomeric proteins to maintain contractile function in the mouse heart (Martin et al., 2021). Others have shown that BAG3 maintains myocyte contractile force in zebrafish (Ding et al., 2019) and human iPSC-cardiomyocytes (Judge et al., 2017), clearly delineating the importance of BAG3-mediated PQC for contractile function. Our findings with JG-98 exposure in NRVMs show that disrupting the BAG3-HSP70 complex causes sarcomere structural disintegration and suggest that JG-98 impairs stress responsive sarcomere turnover. These findings complement earlier work using NRVMs, which showed that siRNA knockdown of BAG3 caused sarcomere structural disarray in the context of mechanical strain (Hishiya et al., 2010). While functional readouts are difficult to obtain from immature cardiomyocyte models like NRVMs, our data on the sarcomeric structural effects of JG-98 strongly suggest this compound will impair cardiomyocyte contractile function.
Due to the importance of PQC for their survival, cardiomyocytes engage numerous semi-overlapping processes to maintain proteostasis. Thus, if one fails, its effects may be ameliorated by upregulating a different arm. Indeed, studies in zebrafish showed administration of autophagy inducing compounds such as metformin and rapamycin restore clearance of protein aggregates when BAG3-mediated autophagy is compromised by pathogenic mutations or haplo-insufficiency (Ruparelia et al., 2014, 2020). In this study, we investigated if co-treatment of NRVMs with JG-98 and rapamycin could reduce the toxic effects of the compound and found that a 30 nM dose of rapamycin, chosen based on previous work in NRVMs (Marin et al., 2008, 2011), reduced apoptosis and sarcomere disarray by 15 and 20%, respectively. While these effects were modest, future studies should explore the optimal doses of JG-98 and rapamycin co-treatment that effectively reduce tumor proliferation while minimizing effects on cardiomyocytes.
Another tissue where BAG3-HSP70 mediates protein turnover is skeletal muscle, where CASA mediates the degradation of denatured filamin C (Arndt et al., 2010) and BAG3 knockout causes sarcomere structural disintegration (Homma et al., 2006). We used C2C12 myotubes to assess whether the detrimental effects of JG-98 extend to skeletal myocytes and found that, while less affected at low doses, 1 μM JG-98 caused significant apoptosis and reduced autophagic flux in these cells. Future studies on the effects of JG-98 and similar compounds are warranted in skeletal muscle and in nervous tissue where BAG3-HSP70 has also been shown to regulate macroautophagy (Ji et al., 2019; Lei et al., 2015; Zhou et al., 2020).
In summary, we show that the proposed cancer therapeutic JG-98 is harmful for cardiomyocytes and support careful consideration of cardiotoxicity before advancing such compounds to the clinic. Our data also suggest that the detrimental impact of JG-98 may extend to skeletal muscle. However, it is important to note several limitations with this study. First, adult cardiomyocytes are not equipped for long-term culture, which leads to immature cardiomyocyte models such as NRVMs and iPSC-CMs being the only methods to do so. While NRVMs share many aspects of mature cardiomyocytes, it is possible that immature myocytes are more sensitive to the effects of disrupting BAG3-HSP70. Second, C2C12 skeletal myotubes are a stable cell line, which may not fully represent the physiology of skeletal muscle. Additionally, while JG-98 has been shown to reliably prevent the BAG3-HSP70 interaction, the specificity of JG-98 for this complex is not clear and earlier work suggests it may elicit effects on other processes (Yaglom et al., 2018). Future studies should investigate the effects of JG-98 in vivo on adult cardiomyocytes and skeletal muscle and characterize its effects on BAG3-dependent and independent processes.
Supplementary Material
ACKNOWLEDGEMENTS
This study was supported by the National Institute of Health (R01HL136737 to J.A.K.) and the American Heart Association (Predoctoral Fellowships 35170045 to T.G.M. and 831515 to M.J.S.). We thank Dr. Ivana Kuo from Loyola University Chicago for providing the C2C12 cell line for this study and Dr. Jordan Beach from Loyola University Chicago for allowing access to the Zeiss LSM 880 in his laboratory for our imaging needs.
Footnotes
CONFLICTS OF INTEREST
The authors declare that there are no conflicts of interest.
DATA AVAILABILITY STATEMENT
The data in support of this study are available in the manuscript and associated data files. Any data not readily available or reagents used for this study will be provided from the corresponding author upon reasonable request.
REFERENCES
- Arndt V, Dick N, Tawo R, Dreiseidler M, Wenzel D, Hesse M, Fürst DO, Saftig P, Saint R, Fleischmann BK, Hoch M, & Höhfeld J (2010). Chaperone-Assisted Selective Autophagy Is Essential for Muscle Maintenance. Current Biology, 20(2), 143–148. [DOI] [PubMed] [Google Scholar]
- Chakraborty D, Felzen V, Hiebel C, Stürner E, Perumal N, Manicam C, Sehn E, Grus F, Wolfrum U, & Behl C (2019). Enhanced autophagic-lysosomal activity and increased BAG3-mediated selective macroautophagy as adaptive response of neuronal cells to chronic oxidative stress. Redox Biology, 24. 10.1016/j.redox.2019.101181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colvin TA, Gabai VL, Gong J, Calderwood SK, Li H, Gummuluru S, Matchuk ON, Smirnova SG, Orlova NV, Zamulaeva IA, Garcia-Marcos M, Li X, Young ZT, Rauch JN, Gestwicki JE, Takayama S, & Sherman MY (2014). Hsp70-Bag3 interactions regulate cancer-related signaling networks. Cancer Research, 74(17). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das CK, Linder B, Bonn F, Rothweiler F, Dikic I, Michaelis M, Cinatl J, Mandal M, & Kögel D (2018). BAG3 Overexpression and Cytoprotective Autophagy Mediate Apoptosis Resistance in Chemoresistant Breast Cancer Cells. Neoplasia (United States), 20(3). 10.1016/j.neo.2018.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Marco M, Basile A, Iorio V, Festa M, Falco A, Ranieri B, Pascale M, Sala G, Remondelli P, Capunzo M, Firpo MA, Pezzilli R, Marzullo L, Cavallo P, De Laurenzi V, Turco MC, & Rosati A (2018). Role of BAG3 in cancer progression: A therapeutic opportunity. In Seminars in Cell and Developmental Biology (Vol. 78). [DOI] [PubMed] [Google Scholar]
- De Marco M, Turco MC, & Marzullo L (2020). BAG3 in Tumor Resistance to Therapy. In Trends in Cancer (Vol. 6, Issue 12). [DOI] [PubMed] [Google Scholar]
- Ding Y, Dvornikov AV, Ma X, Zhang H, Wang Y, Lowerison M, Packard RR, Wang L, Chen J, Zhang Y, Hsiai T, Lin X, & Xu X (2019). Haploinsufficiency of mechanistic target of rapamycin ameliorates bag3 cardiomyopathy in adult zebrafish. DMM Disease Models and Mechanisms. 10.1242/dmm.040154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domínguez F, Cuenca S, Bilińska Z, Toro R, Villard E, Barriales-Villa R, Ochoa JP, Asselbergs F, Sammani A, Franaszczyk M, Akhtar M, Coronado-Albi MJ, Rangel-Sousa D, Rodriguez-Palomares JF, Jiménez-Jáimez J, Garcia-Pinilla JM, Ripoll-Vera T, Mogollón-Jiménez MV, Fontalba-Romero A, … Sammani A (2018). Dilated Cardiomyopathy Due to BLC2-Associated Athanogene 3 (BAG3) Mutations. Journal of the American College of Cardiology, 72(20), 2471–2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang X, Bogomolovas J, Wu T, Zhang W, Liu C, Veevers J, Stroud MJ, Zhang Z, Ma X, Mu Y, Lao DH, Dalton ND, Gu Y, Wang C, Wang M, Liang Y, Lange S, Ouyang K, Peterson KL, … Chen J (2017). Loss-of-function mutations in co-chaperone BAG3 destabilize small HSPs and cause cardiomyopathy. Journal of Clinical Investigation, 127(8), 3189–3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman AM, Begay RL, Knezevic T, Myers VD, Slavov DB, Zhu W, Gowan K, Graw SL, Jones KL, Tilley DG, Coleman RC, Walinsky P, Cheung JY, Mestroni L, Khalili K, & Taylor MRG (2014). Decreased Levels of BAG3 in a Family With a Rare Variant and in Idiopathic Dilated Cardiomyopathy. Journal of Cellular Physiology, 229(11), 1697–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franaszczyk M, Bilinska ZT, Sobieszczańska-Małek M, Michalak E, Sleszycka J, Sioma A, Małek ŁA, Kaczmarska D, Walczak E, Włodarski P, Hutnik Ł, Milanowska B, Dzielinska Z, Religa G, Grzybowski J, Zieliński T, & Ploski R (2014). The BAG3 gene variants in Polish patients with dilated cardiomyopathy: Four novel mutations and a genotype-phenotype correlation. Journal of Translational Medicine, 12(1). 10.1186/1479-5876-12-192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamerdinger M, Kaya AM, Wolfrum U, Clement AM, & Behl C (2011). BAG3 mediates chaperone-based aggresome-targeting and selective autophagy of misfolded proteins. EMBO Reports, 12(2), 149–156. 10.1038/embor.2010.203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henning RH, & Brundel BJJM (2017). Proteostasis in cardiac health and disease. In Nature Reviews Cardiology (Vol. 14, Issue 11, pp. 637–653). 10.1038/nrcardio.2017.89 [DOI] [PubMed] [Google Scholar]
- Hishiya A, Kitazawa T, & Takayama S (2010). BAG3 and Hsc70 interact with actin capping protein CapZ to maintain myofibrillar integrity under mechanical stress. Circulation Research. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homma S, Iwasaki M, Shelton GD, Engvall E, Reed JC, & Takayama S (2006). BAG3 deficiency results in fulminant myopathy and early lethality. American Journal of Pathology, 169(3), 761–773. 10.2353/ajpath.2006.060250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji C, Tang M, Zeidler C, Höhfeld J, & Johnson GVW (2019). BAG3 and SYNPO (synaptopodin) facilitate phospho-MAPT/Tau degradation via autophagy in neuronal processes. Autophagy, 15(7). 10.1080/15548627.2019.1580096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin YH, Ahn SG, & Kim SA (2015). BAG3 affects the nucleocytoplasmic shuttling of HSF1 upon heat stress. Biochemical and Biophysical Research Communications, 464(2). 10.1016/j.bbrc.2015.07.006 [DOI] [PubMed] [Google Scholar]
- Judge LM, Perez-Bermejo JA, Truong A, Ribeiro AJS, Yoo JC, Jensen CL, Mandegar MA, Huebsch N, Kaake RM, So P-L, Srivastava D, Pruitt BL, Krogan NJ, & Conklin BR (2017). A BAG3 chaperone complex maintains cardiomyocyte function during proteotoxic stress. JCI Insight, 2(14), e94623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kathage B, Gehlert S, Ulbricht A, Lüdecke L, Tapia VE, Orfanos Z, Wenzel D, Bloch W, Volkmer R, Fleischmann BK, Fürst DO, & Höhfeld J (2017). The cochaperone BAG3 coordinates protein synthesis and autophagy under mechanical strain through spatial regulation of mTORC1. Biochimica et Biophysica Acta - Molecular Cell Research, 1864(1), 62–75. 10.1016/j.bbamcr.2016.10.007 [DOI] [PubMed] [Google Scholar]
- Klimek C, Kathage B, Wördehoff J, & Höhfeld J (2017). BAG3-mediated proteostasis at a glance. Journal of Cell Science, 130(17). 10.1242/jcs.203679 [DOI] [PubMed] [Google Scholar]
- Lei Z, Brizzee C, & Johnson GVW (2015). BAG3 facilitates the clearance of endogenous tau in primary neurons. Neurobiology of Aging, 36(1), 241–248. 10.1016/j.neurobiolaging.2014.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Colvin T, Rauch JN, Acosta-Alvear D, Kampmann M, Dunyak B, Hann B, Aftab BT, Murnane M, Cho M, Walter P, Weissman JS, Sherman MY, & Gestwicki JE (2015). Validation of the Hsp70-Bag3 protein-protein interaction as a potential therapeutic target in cancer. Molecular Cancer Therapeutics, 14(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maejima Y (2020). The critical roles of protein quality control systems in the pathogenesis of heart failure. In Journal of Cardiology (Vol. 75, Issue 3). 10.1016/j.jjcc.2019.09.019 [DOI] [PubMed] [Google Scholar]
- Marin TM, Clemente CFMZ, Santos AM, Picardi PK, Pascoal VDB, Lopes-Cendes I, Saad MJA, & Franchini KG (2008). Shp2 negatively regulates growth in cardiomyocytes by controlling focal adhesion kinase/src and mTOR pathways. Circulation Research, 103(8). 10.1161/CIRCRESAHA.108.179754 [DOI] [PubMed] [Google Scholar]
- Marin TM, Keith K, Davies B, Conner DA, Guha P, Kalaitzidis D, Wu X, Lauriol J, Wang B, Bauer M, Bronson R, Franchini KG, Neel BG, & Kontaridis MI (2011). Rapamycin reverses hypertrophic cardiomyopathy in a mouse model of LEOPARD syndrome-associated PTPN11 mutation. Journal of Clinical Investigation, 121(3). 10.1172/JCI44972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin TG, & Kirk JA (2020). Under construction: The dynamic assembly, maintenance, and degradation of the cardiac sarcomere. Journal of Molecular and Cellular Cardiology, 148, 89–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin TG, Myers VD, Dubey P, Dubey S, Perez E, Moravec CS, Willis MS, Feldman AM, & Kirk JA (2021). Cardiomyocyte Contractile Impairment in Heart Failure Results from Reduced BAG3-mediated Sarcomeric Protein Turnover. Nature Communications. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin TG, Tawfik S, Moravec CS, Pak TR, & Kirk JA (2021). BAG3 expression and sarcomere localization in the human heart are linked to HSF-1 and are differentially affected by sex and disease. American Journal of Physiology - Heart and Circulatory Physiology, 320(6). 10.1152/AJPHEART.00419.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masser AE, Kang W, Roy J, Kaimal JM, Quintana-Cordero J, Friedländer MR, & Andréasson C (2019). Cytoplasmic protein misfolding titrates Hsp70 to activate nuclear Hsf1. ELife, 8. 10.7554/eLife.47791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima W, & Sadoshima J (2017). BAG3 plays a central role in proteostasis in the heart. In Journal of Clinical Investigation (Vol. 127, Issue 8). 10.1172/JCI95839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers VD, Gerhard GS, McNamara DM, Tomar D, Madesh M, Kaniper S, Ramsey FV, Fisher SG, Ingersoll RG, Kasch-Semenza L, Wang J, Hanley-Yanez K, Lemster B, Schwisow JA, Ambardekar AV, Degann SH, Bristow MR, Sheppard R, Alexis JD, … Feldman AM (2018). Association of Variants in BAG3 with Cardiomyopathy Outcomes in African American Individuals. JAMA Cardiology, 3(10), 929–938. 10.1001/jamacardio.2018.2541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranek MJ, Stachowski MJ, Kirk JA, & Willis MS (2018). The role of heat shock proteins and co-chaperones in heart failure. In Philosophical Transactions of the Royal Society B: Biological Sciences (Vol. 373, Issue 1738). 10.1098/rstb.2016.0530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruparelia AA, McKaige EA, Williams C, Schulze KE, Fuchs M, Oorschot V, Lacene E, Meregalli M, Lee C, Serrano RJ, Baxter EC, Monro K, Torrente Y, Ramm G, Stojkovic T, Lavoie JN, & Bryson-Richardson RJ (2020). Metformin rescues muscle function in BAG3 myofibrillar myopathy models. Autophagy. 10.1080/15548627.2020.1833500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruparelia AA, Oorschot V, Vaz R, Ramm G, & Bryson-Richardson RJ (2014). Zebrafish models of BAG3 myofibrillar myopathy suggest a toxic gain of function leading to BAG3 insufficiency. Acta Neuropathologica, 128(6). 10.1007/s00401-014-1344-5 [DOI] [PubMed] [Google Scholar]
- Selcen D, Muntoni F, Burton BK, Pegoraro E, Sewry C, Bite AV, & Engel AG (2009). Mutation in BAG3 causes severe dominant childhood muscular dystrophy. Annals of Neurology, 65(1), 83–89. 10.1002/ana.21553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields S, Conroy E, O’Grady T, McGoldrick A, Connor K, Ward MP, Useckaite Z, Dempsey E, Reilly R, Fan Y, Chubb A, Matallanas DG, Kay EW, O’Connor D, McCann A, Gallagher WM, & Coppinger JA (2018). BAG3 promotes tumour cell proliferation by regulating EGFR signal transduction pathways in triple negative breast cancer. Oncotarget, 9(21). 10.18632/oncotarget.24590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sondermann H, Scheufler C, Schneider C, Höhfeld J, Hartl FU, & Moarefi I (2001). Structure of a Bag/Hsc70 complex: Convergent functional evolution of Hsp70 nucleotide exchange factors. Science. 10.1126/science.1057268 [DOI] [PubMed] [Google Scholar]
- Stürner E, & Behl C (2017). The role of the multifunctional bag3 protein in cellular protein quality control and in disease. In Frontiers in Molecular Neuroscience (Vol. 10). 10.3389/fnmol.2017.00177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulbricht A, Gehlert S, Leciejewski B, Schiffer T, Bloch W, & Höhfeld J (2015). Induction and adaptation of chaperone-assisted selective autophagy CASA in response to resistance exercise in human skeletal muscle. Autophagy, 11(3), 538–546. 10.1080/15548627.2015.1017186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulbricht A, & Höhfeld J (2013). Tension-induced autophagy: May the chaperone be with you. Autophagy, 9(6), 920–922. 10.4161/auto.24213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaglom JA, Wang Y, Li A, Li Z, Monti S, Alexandrov I, Lu X, & Sherman MY (2018). Cancer cell responses to Hsp70 inhibitor JG-98: Comparison with Hsp90 inhibitors and finding synergistic drug combinations. Scientific Reports, 8(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Chow HM, Liu Y, Wu D, Shi M, Li J, Wen L, Gao Y, Chen G, Zhuang K, Lin H, Zhang G, Xie W, Li H, Leng L, Wang M, Zheng N, Sun H, Zhao Y, … Zhang J (2020). Cyclin-Dependent Kinase 5–Dependent BAG3 Degradation Modulates Synaptic Protein Turnover. Biological Psychiatry, 87(8). 10.1016/j.biopsych.2019.11.013 [DOI] [PubMed] [Google Scholar]
- Zhu H, Liu P, & Li J (2012). BAG3: A new therapeutic target of human cancers? In Histology and Histopathology (Vol. 27, Issue 3). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data in support of this study are available in the manuscript and associated data files. Any data not readily available or reagents used for this study will be provided from the corresponding author upon reasonable request.


