Abstract
Ablynx, a Sanofi Company, has developed the anti-von Willebrand factor Nanobody® caplacizumab (Cablivi™) for the treatment of acquired thrombotic thrombocytopenic purpura (aTTP). Based on positive results in phase II and III trials in patients with aTTP, caplacizumab was recently approved in the EU for the treatment of adults experiencing an episode of aTTP, in conjunction with plasma exchange and immunosuppression. This article summarizes the milestones in the development of caplacizumab leading to this first approval.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Caplacizumab (Cablivi™), a humanised single-variable-domain immunoglobulin (Nanobody®) targeting von Willebrand factor, has been developed by Ablynx, a Sanofi Company, for the treatment of acquired thrombotic thrombocytopenic purpura (aTTP). Caplacizumab received its first approval in the EU for the treatment of adults experiencing an episode of aTTP, in conjunction with plasma exchange and immunosuppression and is undergoing priority review in the USA for the treatment of patients aged ≥ 18 years experiencing an episode of aTTP [1, 2]. Caplacizumab was under development for the prevention of thrombosis in high risk patients with acute coronary syndrome (ACS) undergoing percutaneous coronary intervention (PCI); however, development for this indication has been discontinued.
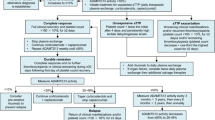
aTTP is a rare and potentially life-threatening autoimmune blood clotting disorder that is characterised by microvascular occlusions, and consequently thrombocytopenia, haemolytic anaemia, tissue ischemia and organ dysfunction [3]. It is caused by a severe deficiency of ADAMTS13 (a von Willebrand factor-cleaving metalloproteinase) due to the presence of anti-ADAMTS13 autoantibodies. Deficiency in ADAMTS13 activity leads to an accumulation of ultra-large von Willebrand factor multimers in the blood, which bind to platelets and thereby lead to the formation of platelet-rich microthrombi in the microvasculature. Standard therapy for aTTP includes plasma exchange until platelet counts normalise in addition to concurrent immunosuppression (e.g., corticosteroids and/or rituximab) [4].
The recommended caplacizumab dosage regimen in patients with aTTP is an initial intravenous (IV) injection of caplacizumab 10 mg prior to plasma exchange, and then daily subcutaneous (SC) caplacizumab 10 mg after completion of each plasma exchange for the duration of daily plasma exchange treatment, followed by daily SC injection of 10 mg of caplacizumab for 30 days after stopping daily plasma exchange treatment [1]. If at the end of this period there is evidence of unresolved immunological disease, it is recommended to optimise the immunosuppression regimen and continue daily SC administration of caplacizumab 10 mg until the signs of underlying immunological disease are resolved (e.g. sustained normalisation of ADAMTS13 activity level) [1]. This article summarizes the milestones in the development of caplacizumab leading to this first approval for the treatment of patients with aTTP.

Key milestones in the development of caplacizumab, leading to its first global approval in acquired thrombotic thrombocytopenic purpura
2 Scientific Summary
2.1 Pharmacodynamics
Caplacizumab has been developed as part of Ablynx’s Nanobody® programme; Nanobodies® are therapeutic proteins based on single-domain antibody fragments that contain the unique structural and functional properties of the naturally occurring heavy chain antibody [5]. Caplacizumab consists of two identical humanised building blocks that are genetically linked by a three-alanine linker [1]. The drug acts by targeting the A1-domain of the ultra-large von Willebrand factor, inhibiting the interaction between von Willebrand factor and platelets and thereby preventing the platelet adhesion [1]. In preclinical models of aTTP, caplacizumab was shown to target the A1-domain of von Willebrand factor, preventing its binding to the platelet glycoprotein Ib-IX-V receptor and inhibiting the interaction between von Willebrand factor and platelets [6, 7]. In in vitro collagen perfusion studies, caplacizumab completely inhibited platelet adhesion in blood obtained from patients undergoing PCI [7].
In a phase II trial in patients with aTTP (TITAN; NCT01151423), caplacizumab treatment resulted in a rapid suppression of von Willebrand factor–ristocetin cofactor (a biomarker for von Willebrand factor activity) activity to < 20% at day 1, with this effect sustained with once-daily caplacizumab throughout the 30 day treatment period [1, 8]. Levels of von Willebrand factor antigen and factor VIII were also transiently reduced with caplacizumab treatment compared with placebo, which is assumed to be linked with an increased clearance of the caplacizumab–von Willebrand factor complex versus unbound von Willebrand factor [8]. These parameters returned to baseline levels within 1 week of cessation of caplacizumab treatment [8].
2.2 Pharmacokinetics
Following IV administration in healthy volunteers, caplacizumab displayed non-linear pharmacokinetics and followed a two-compartment model, characteristic of target-mediated drug disposition (i.e. the pharmacokinetics of caplacizumab are dependent on the expression of the target von Willebrand factor) [1]. Following administration of SC caplacizumab 10 mg once-daily in healthy volunteers, Cmax was reached 6–7 h post-dose, with steady-state reached following the first administration.
Features and properties of caplacizumab
Alternative names | ALX-0081; ALX-0681; Anti-von Willebrand factor Nanobody® |
Class | Anticoagulants, antithrombotics, proteins, recombinant proteins, single-domain antibodies |
Mechanism of Action | Platelet aggregation inhibitors; Von Willebrand factor inhibitors |
Route of Administration | Intravenous, subcutaneous |
Pharmacodynamics | Targets the A1-domain of the von Willebrand factor and inhibits the interaction between von Willebrand factor and platelets |
Pharmacokinetics | Displays non-linear pharmacokinetics following a two-compartment model. Maximum plasma concentrations reached 6–7 h post-dose, with steady-state reached following the first administration. Rapidly absorbed, with a central volume of distribution of 6.33 L. Elimination half-life is concentration- and target-level dependent |
Adverse events | |
Most frequent | Gingival bleeding, epistaxis, headache |
Occasional | Fatigue, urticaria, injection site reaction |
ATC codes | |
WHO ATC code | B01A-X07 (Caplacizumab) |
EphMRA ATC code | B1C (Platelet Aggregation Inhibitors) |
Chemical name | Immunoglobulin, anti-(human von Willebrand’s blood-coagulation factor VIII domain A1) (human-Lama glama dimeric heavy chain fragment PMP12A2h1) |
Caplacizumab is rapidly and almost completely absorbed in the systemic circulation following SC administration, with a central volume of distribution of 6.33 L in patients with aTTP [1]. The elimination half-life of caplacizumab is concentration- and target-level dependent; the level of von Willebrand factor antigen present determines the level of caplacizumab-target complex retained in the circulation. Target-bound caplacizumab is thought to be catabolised hepatically, while unbound caplacizumab is thought to undergo renal clearance.
2.3 Therapeutic Trials
2.3.1 Phase III Trial
In the phase III, double-blind, placebo-controlled HERCULES (NCT02553317) trial, a significant reduction in time to platelet count response was seen in patients treated with daily caplacizumab 10 mg compared with placebo, with caplacizumab 10 mg recipients > 50% more likely to achieve a platelet count response (platelet count normalisation rate ratio 1.55; 95% CI 1.10–2.20 vs. placebo; p = 0.0099) [3, 9]. Caplacizumab 10 mg treatment also resulted in a significant reduction in the incidence of the composite endpoint of aTTP-related death, recurrence of aTTP (including exacerbation and relapse), or ≥ 1 major thromboembolic event during the study treatment period compared with placebo (incidence rate 12.7 vs. 49.3%, corresponding to a 74% reduction; p < 0.0001). A significant reduction in the incidence of aTTP recurrence was seen with caplacizumab 10 mg during the overall study period (12.7 vs. 38.4%, corresponding to a 67% reduction; p < 0.001) [9].
Caplacizumab recipients also experienced clinically meaningful reductions in plasma exchange use (mean 5.8 vs. 9.4 days for placebo), and length of stay in the intensive care unit (3.4 vs. 9.7 days) and the hospital (9.9 vs. 14.4 days) compared with placebo [1, 9].
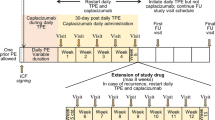
In HERCULES, patients with an acute episode of aTTP who had received one plasma exchange treatment were eligible for the trial [1, 9]. Patients were randomised 1:1 to treatment with daily caplacizumab 10 mg (n = 72) or placebo (n = 73) in addition to daily plasma exchange and immunosuppression treatment with corticosteroids [9]. Patients received the initial dose of IV caplacizumab 10 mg or placebo prior to the first plasma exchange and then received once-daily SC caplacizumab 10 mg or placebo following completion of each daily plasma exchange for the duration of the plasma exchange period, and for 30 days thereafter [1, 9]. If after 30 days there was evidence of ongoing disease (suppressed ADAMTS13 activity), extension of treatment for up to 4 weeks was encouraged. The primary endpoint was the time to platelet count response, which was defined as an initial platelet count of ≥ 150 × 109/L with subsequent stop of daily plasma exchange within 5 days.
2.3.2 Phase II Trial
In the phase II TITAN trial (NCT01151423), treatment with caplacizumab 10 mg significantly reduced the time to confirmed normalization of the platelet count (time to response) by 39% compared with placebo (median 2.97 vs. 4.79 days; event rate ratio 2.2 [95% CI 1.28–3.78], p = 0.005) in the overall intent-to-treat population (n = 75) [3, 8].
In terms of secondary endpoints, caplacizumab was associated with a higher frequency of complete remission after the initial course of daily plasma exchange compared with placebo (81 vs. 46%), according to data from a 1-month follow-up visit (n = 63). Three caplacizumab recipients (8.3%) experienced exacerbations compared with 11 placebo recipients (28.2%). During the 1-month follow-up period, eight patients in the caplacizumab treatment group had a relapse after cessation of treatment compared with no patients in the placebo treatment group (22.2 vs. 0%) [8]. In 7 of the 8 caplacizumab recipients with a relapse during this period, the recurrence occurred within 4–10 days of treatment cessation. ADAMTS13 activity levels were persistently < 10% in these patients, indicating that resolution of the underlying autoimmune disorder was incomplete [8].
In TITAN, patients with an acute episode of aTTP were randomised to an IV loading dose of caplacizumab 10 mg (n = 36) or placebo (n = 39) prior to the first plasma exchange following study enrolment, in addition to standard-of-care treatment [8]. Patients received once-daily SC caplacizumab 10 mg or placebo throughout the plasma exchange period, with treatment continuing for 30 days thereafter (maximum duration of treatment of 90 days). The primary endpoint was the time to a response, defined as an initial platelet count of ≥ 150 × 109/L and a lactate dehydrogenase level of 2 times the upper limit of normal.
2.4 Adverse Events
During the overall study period in the phase III HERCULES trial, the most frequently reported treatment-emergent adverse events (TEAEs) with caplacizumab that occurred at a greater frequency than with placebo were gingival bleeding, epistaxis and headache [3]. Other adverse reactions observed with caplacizumab treatment were pyrexia, fatigue, urticaria (all frequency ≥ 1/10), injection site reaction, myalgia, injection site pruritus, injection site erythema, cerebral infarction and dyspnoea (all frequency ≥ 1/100 to < 1/10) [1].
In HERCULES, the frequency of bleeding-related TEAEs (as assessed by Standardised MedDRA Queries) was 45.6% (n = 33) in the caplacizumab treatment group compared with 23.3% (n = 17) with placebo, with the most commonly reported (frequency > 5%) being epistaxis (23.9 vs. 1.4%), gingival bleeding (11.3 vs. 0%), bruising (7.0 vs. 4.1%) and haematuria (5.6 vs. 1.4%) [9]. Treatment-related serious bleeding events were reported in eight caplacizumab recipients (11%) and three placebo recipients (4%) [3]. Results from clinical trials have shown that bleeding events with caplacizumab occurred in different body systems and this was independent of duration of treatment; although some of these bleeding events were serious and required medical attention, most were self-limited or resolved [1].
In HERCULES, the frequency of patients experiencing at least one study drug-related TEAE was 57.7% for caplacizumab and 43.8% with placebo, while the corresponding frequency of at least one TEAE leading to study drug discontinuation was 7.0% and 12.3%, respectively [9]. At least one serious adverse event was reported in 39.4% of caplacizumab recipients and 53.4% of placebo recipients, with 14% and 6% considered at least possibly related to treatment [3, 9].
2.5 Ongoing Clinical Trials
There is currently one ongoing clinical trial for caplacizumab; the open label, phase IIIb, prospective follow-up Post-HERCULES (NCT02878603, EudraCT2016-001503-23) study to evaluate the long-term safety and efficacy of caplacizumab in patients with aTTP who have completed the HERCULES study.
3 Current Status
Caplacizumab received its first global approval on 3 September 2018 in the EU for the treatment of adults experiencing an episode of aTTP, in conjunction with plasma exchange and immunosuppression.
Change history
03 December 2018
The article Caplacizumab: First Global Approval, written by Sean Duggan, was originally published Online First without open access.
References
European Medicines Agency. Cablivi (caplacizumab): summary of product characteristics. 2018. http://www.ema.europa.eu. Accessed 7 Sept 2018.
Sanofi. Cablivi™ (caplacizumab) approved in Europe for adults with acquired thrombotic thrombocytopenic purpura (aTTP) [media release]. http://www.news.sanofi.us/. Accessed 3 Sept 2018.
European Medicines Agency. Cablivi (caplacizumab): EU assessment report. 2018. http://www.ema.europa.eu/ema/. Accessed 13 Jun 2018.
Joly BS, Coppo P, Veyradier A. Thrombotic thrombocytopenic purpura. Blood. 2017;129(21):2836–46.
Ablynx. Understanding nanobodies. 2018. http://www.ablynx.com/technology-innovation/understanding-nanobodies/. Accessed 14 Sept 2018.
Callewaert F, Roodt J, Ulrichts H, et al. Evaluation of efficacy and safety of the anti-VWF nanobody ALX-0681 in a preclinical baboon model of acquired thrombotic thrombocytopenic purpura. Blood. 2012;120(17):3603–10.
Ulrichts H, Silence K, Schoolmeester A, et al. Antithrombotic drug candidate ALX-0081 shows superior preclinical efficacy and safety compared with currently marketed antiplatelet drugs. Blood. 2011;118(3):757–65.
Peyvandi F, Scully M, Kremer Hovinga JA, et al. Caplacizumab for acquired thrombotic thrombocytopenic purpura. N Engl J Med. 2016;374(6):511–22.
Scully M, Cataland SR, Peyvandi F, et al. Results of the randomized, double-blind, placebo-controlled, phase 3 HERCULES study of caplacizumab in patients with acquired thrombotic thrombocytopenic purpura [abstract no. LBA-1 plus presentation]. Blood. 2017;130:1.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Conflict of interest
During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the author on the basis of scientific completeness and accuracy. Sean Duggan is a salaried employee of Adis/Springer, is responsible for the article content and declares no relevant conflicts of interest.
Additional information
This profile has been extracted and modified from the AdisInsight database. AdisInsight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch and beyond.
The original version of this article was revised due to a retrospective Open Access request.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, duplication, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
About this article
Cite this article
Duggan, S. Caplacizumab: First Global Approval. Drugs 78, 1639–1642 (2018). https://doi.org/10.1007/s40265-018-0989-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-018-0989-0