Abstract
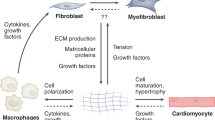
Cardiac fibroblasts are key cellular effectors of cardiac repair; their phenotype and function are modulated by interactions with extracellular matrix proteins. This review manuscript discusses the effects of the extracellular matrix on the inflammatory and reparative properties of fibroblasts in the infarcted myocardium. Early generation of matrix fragments in the infarct induces a pro-inflammatory and matrix-degrading fibroblast phenotype. Formation of a fibrin/fibronectin-rich provisional matrix serves as a conduit for migration of fibroblasts into the infarcted area. Induction of ED-A fibronectin and nonfibrillar collagens may contribute to myofibroblast transdifferentiation. Upregulation of matricellular proteins promotes transduction of growth factor and cytokine-mediated signals. As the scar matures, matrix cross-linking, clearance of matricellular proteins, and reduced growth factor signaling cause deactivation and apoptosis of reparative infarct fibroblasts. Understanding the effects of matrix components on infarct fibroblasts may guide the design of peptides that reproduce, or inhibit, specific matricellular functions, attenuating adverse remodeling.



Similar content being viewed by others
References
Nag, A. C. (1980). Study of non-muscle cells of the adult mammalian heart: a fine structural analysis and distribution. Cytobios, 28, 41–61.
Baudino, T. A., Carver, W., Giles, W., & Borg, T. K. (2006). Cardiac fibroblasts: friend or foe? Am J Physiol Heart Circ Physiol, 291, H1015–1026.
Porter, K. E., & Turner, N. A. (2009). Cardiac fibroblasts: at the heart of myocardial remodeling. Pharmacol Ther, 123, 255–278.
Snider, P., Standley, K. N., Wang, J., Azhar, M., Doetschman, T., & Conway, S. J. (2009). Origin of cardiac fibroblasts and the role of periostin. Circ Res, 105, 934–947.
Camelliti, P., Borg, T. K., & Kohl, P. (2005). Structural and functional characterisation of cardiac fibroblasts. Cardiovasc Res, 65, 40–51.
Kakkar, R., & Lee, R. T. (2010). Intramyocardial fibroblast myocyte communication. Circ Res, 106, 47–57.
Smith, R. S., Smith, T. J., Blieden, T. M., & Phipps, R. P. (1997). Fibroblasts as sentinel cells. Synthesis of chemokines and regulation of inflammation. Am J Pathol, 151, 317–322.
Leonard, B. L., Smaill, B. H., & Legrice, I. J. (2012). Structural remodeling and mechanical function in heart failure. Microsc Microanal, 18, 50–67.
Jugdutt, B. I. (2003). Ventricular remodeling after infarction and the extracellular collagen matrix: when is enough enough? Circulation, 108, 1395–1403.
Weber, K. T. (1989). Cardiac interstitium in health and disease: the fibrillar collagen network. J Am Coll Cardiol, 13, 1637–1652.
Ieda, M., Tsuchihashi, T., Ivey, K. N., Ross, R. S., Hong, T. T., Shaw, R. M., & Srivastava, D. (2009). Cardiac fibroblasts regulate myocardial proliferation through beta1 integrin signaling. Dev Cell, 16, 233–244.
Eghbali, M., Blumenfeld, O. O., Seifter, S., Buttrick, P. M., Leinwand, L. A., Robinson, T. F., Zern, M. A., & Giambrone, M. A. (1989). Localization of types I, III and IV collagen mRNAs in rat heart cells by in situ hybridization. J Mol Cell Cardiol, 21, 103–113.
Banerjee, I., Fuseler, J. W., Price, R. L., Borg, T. K., & Baudino, T. A. (2007). Determination of cell types and numbers during cardiac development in the neonatal and adult rat and mouse. Am J Physiol Heart Circ Physiol, 293, H1883–1891.
Norris, R. A., Borg, T. K., Butcher, J. T., Baudino, T. A., Banerjee, I., & Markwald, R. R. (2008). Neonatal and adult cardiovascular pathophysiological remodeling and repair: developmental role of periostin. Ann N Y Acad Sci, 1123, 30–40.
Oka, T., Xu, J., Kaiser, R. A., Melendez, J., Hambleton, M., Sargent, M. A., Lorts, A., Brunskill, E. W., Dorn, G. W., 2nd, Conway, S. J., Aronow, B. J., Robbins, J., & Molkentin, J. D. (2007). Genetic manipulation of periostin expression reveals a role in cardiac hypertrophy and ventricular remodeling. Circ Res, 101, 313–321.
Berk, B. C., Fujiwara, K., & Lehoux, S. (2007). ECM remodeling in hypertensive heart disease. J Clin Invest, 117, 568–575.
Tomasek, J. J., Gabbiani, G., Hinz, B., Chaponnier, C., & Brown, R. A. (2002). Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol, 3, 349–363.
Roberts, C. S., Maclean, D., Maroko, P., & Kloner, R. A. (1984). Early and late remodeling of the left ventricle after acute myocardial infarction. Am J Cardiol, 54, 407–410.
Pfeffer, M. A., & Braunwald, E. (1990). Ventricular remodeling after myocardial infarction. Experimental observations and clinical implications. Circulation, 81, 1161–1172.
St John Sutton, M., Lee, D., Rouleau, J. L., Goldman, S., Plappert, T., Braunwald, E., & Pfeffer, M. A. (2003). Left ventricular remodeling and ventricular arrhythmias after myocardial infarction. Circulation, 107, 2577–2582.
Swynghedauw, B. (1991). Remodeling of the heart in chronic pressure overload. Basic Res Cardiol, 86(Suppl 1), 99–105.
Frangogiannis, N. G. (2012). Regulation of the inflammatory response in cardiac repair. Circ Res, 110, 159–173.
Frangogiannis, N. G. (2007). Chemokines in ischemia and reperfusion. Thromb Haemost, 97, 738–747.
Frangogiannis, N. G., Mendoza, L. H., Ren, G., Akrivakis, S., Jackson, P. L., Michael, L. H., Smith, C. W., & Entman, M. L. (2003). MCSF expression is induced in healing myocardial infarcts and may regulate monocyte and endothelial cell phenotype. Am J Physiol Heart Circ Physiol, 285, H483–492.
Bujak, M., Dobaczewski, M., Gonzalez-Quesada, C., Xia, Y., Leucker, T., Zymek, P., Veeranna, V., Tager, A. M., Luster, A. D., & Frangogiannis, N. G. (2009). Induction of the CXC chemokine interferon-gamma-inducible protein 10 regulates the reparative response following myocardial infarction. Circ Res, 105, 973–983.
Dobaczewski, M., Xia, Y., Bujak, M., Gonzalez-Quesada, C., & Frangogiannis, N. G. (2010). CCR5 signaling suppresses inflammation and reduces adverse remodeling of the infarcted heart, mediating recruitment of regulatory T cells. Am J Pathol, 176, 2177–2187.
Ng, F. (2008). The immune system and cardiac repair. Pharmacol Res, 58, 88–111.
Bujak, M., Dobaczewski, M., Chatila, K., Mendoza, L. H., Li, N., Reddy, A., & Frangogiannis, N. G. (2008). Interleukin-1 receptor type i signaling critically regulates infarct healing and cardiac remodeling. Am J Pathol, 173, 57–67.
Frangogiannis, N. G., Michael, L. H., & Entman, M. L. (2000). Myofibroblasts in reperfused myocardial infarcts express the embryonic form of smooth muscle myosin heavy chain (SMemb). Cardiovasc Res, 48, 89–100.
Willems, I. E., Havenith, M. G., De Mey, J. G., & Daemen, M. J. (1994). The alpha-smooth muscle actin-positive cells in healing human myocardial scars. Am J Pathol, 145, 868–875.
Cleutjens, J. P., Verluyten, M. J., Smiths, J. F., & Daemen, M. J. (1995). Collagen remodeling after myocardial infarction in the rat heart. Am J Pathol, 147, 325–338.
Dobaczewski, M., Gonzalez-Quesada, C., & Frangogiannis, N. G. (2010). The extracellular matrix as a modulator of the inflammatory and reparative response following myocardial infarction. J Mol Cell Cardiol, 48, 504–511.
Mann, D. L. (2011). The emerging role of innate immunity in the heart and vascular system: for whom the cell tolls. Circ Res, 108, 1133–1145.
Kawaguchi, M., Takahashi, M., Hata, T., Kashima, Y., Usui, F., Morimoto, H., Izawa, A., Takahashi, Y., Masumoto, J., Koyama, J., Hongo, M., Noda, T., Nakayama, J., Sagara, J., Taniguchi, S., & Ikeda, U. (2011). Inflammasome activation of cardiac fibroblasts is essential for myocardial ischemia/reperfusion injury. Circulation, 123, 594–604.
Cannon, R. O., 3rd, Butany, J. W., Mcmanus, B. M., Speir, E., Kravitz, A. B., Bolli, R., & Ferrans, V. J. (1983). Early degradation of collagen after acute myocardial infarction in the rat. Am J Cardiol, 52, 390–395.
Whittaker, P., Boughner, D. R., & Kloner, R. A. (1991). Role of collagen in acute myocardial infarct expansion. Circulation, 84, 2123–2134.
Etoh, T., Joffs, C., Deschamps, A. M., Davis, J., Dowdy, K., Hendrick, J., Baicu, S., Mukherjee, R., Manhaini, M., & Spinale, F. G. (2001). Myocardial and interstitial matrix metalloproteinase activity after acute myocardial infarction in pigs. Am J Physiol Heart Circ Physiol, 281, H987–994.
Takahashi, S., Barry, A. C., & Factor, S. M. (1990). Collagen degradation in ischaemic rat hearts. Biochem J, 265, 233–241.
Villarreal, F., Omens, J., Dillmann, W., Risteli, J., Nguyen, J., & Covell, J. (2004). Early degradation and serum appearance of type I collagen fragments after myocardial infarction. J Mol Cell Cardiol, 36, 597–601.
Adair-Kirk, T. L., & Senior, R. M. (2008). Fragments of extracellular matrix as mediators of inflammation. Int J Biochem Cell Biol, 40, 1101–1110.
Gaggar, A., Jackson, P. L., Noerager, B. D., O'reilly, P. J., Mcquaid, D. B., Rowe, S. M., Clancy, J. P., & Blalock, J. E. (2008). A novel proteolytic cascade generates an extracellular matrix-derived chemoattractant in chronic neutrophilic inflammation. J Immunol, 180, 5662–5669.
Riley, D. J., Berg, R. A., Soltys, R. A., Kerr, J. S., Guss, H. N., Curran, S. F., & Laskin, D. L. (1988). Neutrophil response following intratracheal instillation of collagen peptides into rat lungs. Exp Lung Res, 14, 549–563.
Senior, R. M., Griffin, G. L., & Mecham, R. P. (1980). Chemotactic activity of elastin-derived peptides. J Clin Invest, 66, 859–862.
Trial, J., Baughn, R. E., Wygant, J. N., Mcintyre, B. W., Birdsall, H. H., Youker, K. A., Evans, A., Entman, M. L., & Rossen, R. D. (1999). Fibronectin fragments modulate monocyte VLA-5 expression and monocyte migration. J Clin Invest, 104, 419–430.
Clark, R. A., Wikner, N. E., Doherty, D. E., & Norris, D. A. (1988). Cryptic chemotactic activity of fibronectin for human monocytes resides in the 120-kDa fibroblastic cell-binding fragment. J Biol Chem, 263, 12115–12123.
Huttenlocher, A., Werb, Z., Tremble, P., Huhtala, P., Rosenberg, L., & Damsky, C. H. (1996). Decorin regulates collagenase gene expression in fibroblasts adhering to vitronectin. Matrix Biol, 15, 239–250.
Kapila, Y. L., Kapila, S., & Johnson, P. W. (1996). Fibronectin and fibronectin fragments modulate the expression of proteinases and proteinase inhibitors in human periodontal ligament cells. Matrix Biol, 15, 251–261.
Kong, W., Longaker, M. T., & Lorenz, H. P. (2004). Cyclophilin C-associated protein is a mediator for fibronectin fragment-induced matrix metalloproteinase-13 expression. J Biol Chem, 279, 55334–55340.
Dobaczewski, M., Bujak, M., Zymek, P., Ren, G., Entman, M. L., & Frangogiannis, N. G. (2006). Extracellular matrix remodeling in canine and mouse myocardial infarcts. Cell Tissue Res, 324, 475–488.
Taylor, K. R., Trowbridge, J. M., Rudisill, J. A., Termeer, C. C., Simon, J. C., & Gallo, R. L. (2004). Hyaluronan fragments stimulate endothelial recognition of injury through TLR4. J Biol Chem, 279, 17079–17084.
Campo, G. M., Avenoso, A., D'ascola, A., Scuruchi, M., Prestipino, V., Nastasi, G., Calatroni, A., & Campo, S. (2012). The inhibition of hyaluronan degradation reduced pro-inflammatory cytokines in mouse synovial fibroblasts subjected to collagen-induced arthritis. J Cell Biochem, 113, 1852–1867.
Teder, P., Vandivier, R. W., Jiang, D., Liang, J., Cohn, L., Pure, E., Henson, P. M., & Noble, P. W. (2002). Resolution of lung inflammation by CD44. Science, 296, 155–158.
Ponta, H., Sherman, L., & Pa, H. (2003). CD44: from adhesion molecules to signalling regulators. Nat Rev Mol Cell Biol, 4, 33–45.
Huebener, P., Abou-Khamis, T., Zymek, P., Bujak, M., Ying, X., Chatila, K., Haudek, S., Thakker, G., & Frangogiannis, N. G. (2008). CD44 is critically involved in infarct healing by regulating the inflammatory and fibrotic response. J Immunol, 180, 2625–2633.
Liu, X., & Piela-Smith, T. H. (2000). Fibrin(ogen)-induced expression of ICAM-1 and chemokines in human synovial fibroblasts. J Immunol, 165, 5255–5261.
Welch, M. P., Odland, G. F., & Clark, R. A. (1990). Temporal relationships of F-actin bundle formation, collagen and fibronectin matrix assembly, and fibronectin receptor expression to wound contraction. J Cell Biol, 110, 133–145.
Clark, R. A. (1995). Wound repair. Overview and general considerations. In C. Ra (Ed.), The molecular and cellular biology of wound repair (pp. 3–50). New York: Plenum.
Rybarczyk, B. J., Lawrence, S. O., & Simpson-Haidaris, P. J. (2003). Matrix–fibrinogen enhances wound closure by increasing both cell proliferation and migration. Blood, 102, 4035–4043.
Greiling, D., & Clark, R. A. (1997). Fibronectin provides a conduit for fibroblast transmigration from collagenous stroma into fibrin clot provisional matrix. J Cell Sci, 110(Pt 7), 861–870.
Lin, F., Ren, X. D., Doris, G., & Clark, R. A. (2005). Three-dimensional migration of human adult dermal fibroblasts from collagen lattices into fibrin/fibronectin gels requires syndecan-4 proteoglycan. J Invest Dermatol, 124, 906–913.
Corbett, S. A., & Schwarzbauer, J. E. (1998). Fibronectin–fibrin cross-linking: a regulator of cell behavior. Trends Cardiovasc Med, 8, 357–362.
Xu, J., & Clark, R. A. (1996). Extracellular matrix alters PDGF regulation of fibroblast integrins. J Cell Biol, 132, 239–249.
Hinz, B. (2007). Formation and function of the myofibroblast during tissue repair. J Invest Dermatol, 127, 526–537.
Hinz, B. (2010). The myofibroblast: paradigm for a mechanically active cell. J Biomech, 43, 146–155.
Yano, T., Miura, T., Ikeda, Y., Matsuda, E., Saito, K., Miki, T., Kobayashi, H., Nishino, Y., Ohtani, S., & Shimamoto, K. (2005). Intracardiac fibroblasts, but not bone marrow derived cells, are the origin of myofibroblasts in myocardial infarct repair. Cardiovasc Pathol, 14, 241–246.
Haudek, S. B., Xia, Y., Huebener, P., Lee, J. M., Carlson, S., Crawford, J. R., Pilling, D., Gomer, R. H., Trial, J., Frangogiannis, N. G., & Entman, M. L. (2006). Bone marrow-derived fibroblast precursors mediate ischemic cardiomyopathy in mice. Proc Natl Acad Sci U S A, 103, 18284–18289.
Zeisberg, E. M., Tarnavski, O., Zeisberg, M., Dorfman, A. L., Mcmullen, J. R., Gustafsson, E., Chandraker, A., Yuan, X., Pu, W. T., Roberts, A. B., Neilson, E. G., Sayegh, M. H., Izumo, S., & Kalluri, R. (2007). Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med, 13, 952–961.
Knowlton, A. A., Connelly, C. M., Romo, G. M., Mamuya, W., Apstein, C. S., & Brecher, P. (1992). Rapid expression of fibronectin in the rabbit heart after myocardial infarction with and without reperfusion. J Clin Invest, 89, 1060–1068.
Arslan, F., Smeets, M. B., Riem Vis, P. W., Karper, J. C., Quax, P. H., Bongartz, L. G., Peters, J. H., Hoefer, I. E., Doevendans, P. A., Pasterkamp, G., & De Kleijn, D. P. (2011). Lack of fibronectin-EDA promotes survival and prevents adverse remodeling and heart function deterioration after myocardial infarction. Circ Res, 108, 582–592.
Serini, G., Bochaton-Piallat, M. L., Ropraz, P., Geinoz, A., Borsi, L., Zardi, L., & Gabbiani, G. (1998). The fibronectin domain ED-A is crucial for myofibroblastic phenotype induction by transforming growth factor-beta1. J Cell Biol, 142, 873–881.
Hinz, B., Phan, S. H., Thannickal, V. J., Galli, A., Bochaton-Piallat, M. L., & Gabbiani, G. (2007). The myofibroblast: one function, multiple origins. Am J Pathol, 170, 1807–1816.
Naugle, J. E., Olson, E. R., Zhang, X., Mase, S. E., Pilati, C. F., Maron, M. B., Folkesson, H. G., Horne, W. I., Doane, K. J., & Meszaros, J. G. (2006). Type VI collagen induces cardiac myofibroblast differentiation: implications for postinfarction remodeling. Am J Physiol Heart Circ Physiol, 290, H323–330.
Luther, D. J., Thodeti, C. K., Shamhart, P. E., Adapala, R. K., Hodnichak, C., Weihrauch, D., Bonaldo, P., Chilian, W. M., & Meszaros, J. G. (2012). Absence of type VI collagen paradoxically improves cardiac function, structure, and remodeling after myocardial infarction. Circ Res, 110, 851–856.
Webber, J., Meran, S., Steadman, R., & Phillips, A. (2009). Hyaluronan orchestrates transforming growth factor-beta1-dependent maintenance of myofibroblast phenotype. J Biol Chem, 284, 9083–9092.
Meran, S., Thomas, D., Stephens, P., Martin, J., Bowen, T., Phillips, A., & Steadman, R. (2007). Involvement of hyaluronan in regulation of fibroblast phenotype. J Biol Chem, 282, 25687–25697.
Zhao, X. H., Laschinger, C., Arora, P., Szaszi, K., Kapus, A., & Mcculloch, C. A. (2007). Force activates smooth muscle alpha-actin promoter activity through the Rho signaling pathway. J Cell Sci, 120, 1801–1809.
Bornstein, P. (2009). Matricellular proteins: an overview. J Cell Commun Signal, 3, 163–165.
Frangogiannis, N. G. (2012). Matricellular proteins in cardiac adaptation and disease. Physiol Rev, 92, 635–688.
Adams, J. C., & Lawler, J. (2004). The thrombospondins. Int J Biochem Cell Biol, 36, 961–968.
Frangogiannis, N. G., Ren, G., Dewald, O., Zymek, P., Haudek, S., Koerting, A., Winkelmann, K., Michael, L. H., Lawler, J., & Entman, M. L. (2005). The critical role of endogenous thrombospondin (TSP)-1 in preventing expansion of healing myocardial infarcts. Circulation, 111, 2935–2942.
Rodriguez-Manzaneque, J. C., Lane, T. F., Ortega, M. A., Hynes, R. O., Lawler, J., & Iruela-Arispe, M. L. (2001). Thrombospondin-1 suppresses spontaneous tumor growth and inhibits activation of matrix metalloproteinase-9 and mobilization of vascular endothelial growth factor. Proc Natl Acad Sci U S A, 98, 12485–12490.
Hogg, P. J. (1994). Thrombospondin 1 as an enzyme inhibitor. Thromb Haemost, 72, 787–792.
Xia, Y., Dobaczewski, M., Gonzalez-Quesada, C., Chen, W., Biernacka, A., Li, N., Lee, D. W., & Frangogiannis, N. G. (2011). Endogenous thrombospondin 1 protects the pressure-overloaded myocardium by modulating fibroblast phenotype and matrix metabolism. Hypertension, 58, 902–911.
Willems, I. E., Arends, J. W., & Daemen, M. J. (1996). Tenascin and fibronectin expression in healing human myocardial scars. J Pathol, 179, 321–325.
Tamaoki, M., Imanaka-Yoshida, K., Yokoyama, K., Nishioka, T., Inada, H., Hiroe, M., Sakakura, T., & Yoshida, T. (2005). Tenascin-C regulates recruitment of myofibroblasts during tissue repair after myocardial injury. Am J Pathol, 167, 71–80.
Nishioka, T., Onishi, K., Shimojo, N., Nagano, Y., Matsusaka, H., Ikeuchi, M., Ide, T., Tsutsui, H., Hiroe, M., Yoshida, T., & Imanaka-Yoshida, K. (2010). Tenascin-C may aggravate left ventricular remodeling and function after myocardial infarction in mice. Am J Physiol Heart Circ Physiol, 298, H1072–1078.
Schellings, M. W., Vanhoutte, D., Swinnen, M., Cleutjens, J. P., Debets, J., Van Leeuwen, R. E., D'hooge, J., Van De Werf, F., Carmeliet, P., Pinto, Y. M., Sage, E. H., & Heymans, S. (2009). Absence of SPARC results in increased cardiac rupture and dysfunction after acute myocardial infarction. J Exp Med, 206, 113–123.
Murry, C. E., Giachelli, C. M., Schwartz, S. M., & Vracko, R. (1994). Macrophages express osteopontin during repair of myocardial necrosis. Am J Pathol, 145, 1450–1462.
Krishnamurthy, P., Peterson, J. T., Subramanian, V., Singh, M., & Singh, K. (2009). Inhibition of matrix metalloproteinases improves left ventricular function in mice lacking osteopontin after myocardial infarction. Mol Cell Biochem, 322, 53–62.
Trueblood, N. A., Xie, Z., Communal, C., Sam, F., Ngoy, S., Liaw, L., Jenkins, A. W., Wang, J., Sawyer, D. B., Bing, O. H., Apstein, C. S., Colucci, W. S., & Singh, K. (2001). Exaggerated left ventricular dilation and reduced collagen deposition after myocardial infarction in mice lacking osteopontin. Circ Res, 88, 1080–1087.
Ashizawa, N., Graf, K., Do, Y. S., Nunohiro, T., Giachelli, C. M., Meehan, W. P., Tuan, T. L., & Hsueh, W. A. (1996). Osteopontin is produced by rat cardiac fibroblasts and mediates A(II)-induced DNA synthesis and collagen gel contraction. J Clin Invest, 98, 2218–2227.
Zohar, R., Zhu, B., Liu, P., Sodek, J., & Mcculloch, C. A. (2004). Increased cell death in osteopontin-deficient cardiac fibroblasts occurs by a caspase-3-independent pathway. Am J Physiol Heart Circ Physiol, 287, H1730–1739.
Shimazaki, M., Nakamura, K., Kii, I., Kashima, T., Amizuka, N., Li, M., Saito, M., Fukuda, K., Nishiyama, T., Kitajima, S., Saga, Y., Fukayama, M., Sata, M., & Kudo, A. (2008). Periostin is essential for cardiac healing after acute myocardial infarction. J Exp Med, 205, 295–303.
Ren, G., Michael, L. H., Entman, M. L., & Frangogiannis, N. G. (2002). Morphological characteristics of the microvasculature in healing myocardial infarcts. J Histochem Cytochem, 50, 71–79.
Petzelbauer, P., Zacharowski, P. A., Miyazaki, Y., Friedl, P., Wickenhauser, G., Castellino, F. J., Groger, M., Wolff, K., & Zacharowski, K. (2005). The fibrin-derived peptide Bbeta15-42 protects the myocardium against ischemia–reperfusion injury. Nat Med, 11, 298–304.
Henkin, J., & Volpert, O. V. (2011). Therapies using anti-angiogenic peptide mimetics of thrombospondin-1. Expert Opin Ther Targets, 15, 1369–1386.
Belmadani S, Bernal J, Wei CC, Pallero MA, Dell'italia L, Murphy-Ullrich JE, Berecek KH (2007) A thrombospondin-1 antagonist of transforming growth factor-{beta} activation blocks cardiomyopathy in rats with diabetes and elevated angiotensin II. Am J Pathol.
Frangogiannis, N. G. (2012). Biomarkers: hopes and challenges in the path from discovery to clinical practice. Transl Res, 159, 197–204.
Acknowledgments
Dr. Frangogiannis’ laboratory is supported by NIH grants R01 HL-76246 and HL-85440, the Wilf Family Cardiovascular Research Institute, and the Edmond J Safra/Republic National Bank of New York Chair in Cardiovascular Medicine.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dobaczewski, M., de Haan, J.J. & Frangogiannis, N.G. The Extracellular Matrix Modulates Fibroblast Phenotype and Function in the Infarcted Myocardium. J. of Cardiovasc. Trans. Res. 5, 837–847 (2012). https://doi.org/10.1007/s12265-012-9406-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12265-012-9406-3