Abstract
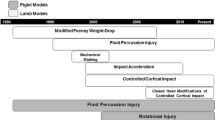
Traumatic brain injury (TBI) contributes a major cause of death, disability, and mental health disorders. Most TBI patients suffer long-term post-traumatic stress disorder, cognitive dysfunction, and disability. The underlying molecular and cellular mechanisms of such neuropathology progression in TBI remain elusive. In part, it is due to non-standardized classification of mild, moderate, and severe injury in various animal models of TBI. Thus, a better diagnosis and treatment requires a better understanding of the injury mechanisms in a well-defined severity of mild, moderate, and severe injury in different models that may potentially reflect the various types of human brain injuries. The purpose of this review article is to highlight the classification of mild, moderate, and severe injury in various animal models of TBI with special focus on mixed injury that represents a translational concussive head injury. We will classify animal models of TBI broadly into focal injury, diffuse injury, and mixed injury. Focal injury, a localized injury, is represented by animal models of controlled cortical impact, penetrating ballistic-like brain injury, and Feeney or Shohami weight drop injury. A global diffuse injury is best represented by shock tube model of primary blast injury, and Marmarou or Maryland weight drop model. A mixed injury consists of focal and diffuse injury which reproduces the concussive clinical syndrome, and it is best studied in animal model of lateral fluid percussion injury.





Similar content being viewed by others
References
Thurman DJ, Alverson C, Dunn KA, Guerrero J, Sniezek JE (1999) Traumatic brain injury in the United States: a public health perspective. J Head Trauma Rehabil 14(6):602–615
Faul M, Coronado V (2015) Epidemiology of traumatic brain injury. Handb Clin Neurol 127:3–13. https://doi.org/10.1016/b978-0-444-52892-6.00001-5
Taylor CA, Bell JM, Breiding MJ, Xu L (2017) Traumatic brain injury-related emergency department visits, hospitalizations, and deaths - United States, 2007 and 2013. Morb Mortal Wkly Rep Surveill Summ 66(9):1–16. https://doi.org/10.15585/mmwr.ss6609a1
Dewan MC, Rattani A, Gupta S, Baticulon RE, Hung YC, Punchak M, Agrawal A, Adeleye AO, Shrime MG, Rubiano AM, Rosenfeld JV, Park KB (2018) Estimating the global incidence of traumatic brain injury. J Neurosurg 1–18. https://doi.org/10.3171/2017.10.Jns17352
Arciniegas DB, Held K, Wagner P (2002) Cognitive impairment following traumatic brain injury. Curr Treat Options Neurol 4(1):43–57
Barman A, Chatterjee A, Bhide R (2016) Cognitive impairment and rehabilitation strategies after traumatic brain injury. Indian J Psychol Med 38(3):172–181. https://doi.org/10.4103/0253-7176.183086
Chen Y, Huang W (2011) Non-impact, blast-induced mild TBI and PTSD: concepts and caveats. Brain Inj 25(7–8):641–650. https://doi.org/10.3109/02699052.2011.580313
Bryant R (2011) Post-traumatic stress disorder vs traumatic brain injury. Dialogues Clin Neurosci 13(3):251–262
Prasad KN, Bondy SC (2015) Common biochemical defects linkage between post-traumatic stress disorders, mild traumatic brain injury (TBI) and penetrating TBI. Brain Res 1599:103–114. https://doi.org/10.1016/j.brainres.2014.12.038
Johnson VE, Stewart W, Smith DH (2012) Widespread tau and amyloid-beta pathology many years after a single traumatic brain injury in humans. Brain Pathol (Zurich, Switzerland) 22(2):142–149. https://doi.org/10.1111/j.1750-3639.2011.00513.x
Hay J, Johnson VE, Smith DH, Stewart W (2016) Chronic traumatic encephalopathy: the neuropathological legacy of traumatic brain injury. Annu Rev Pathol 11:21–45. https://doi.org/10.1146/annurev-pathol-012615-044116
Stern RA, Riley DO, Daneshvar DH, Nowinski CJ, Cantu RC, McKee AC (2011) Long-term consequences of repetitive brain trauma: chronic traumatic encephalopathy. PM & R 3(10 Suppl 2):S460–S467. https://doi.org/10.1016/j.pmrj.2011.08.008
DeGrauw X, Thurman D, Xu L, Kancherla V, DeGrauw T (2018) Epidemiology of traumatic brain injury-associated epilepsy and early use of anti-epilepsy drugs: an analysis of insurance claims data, 2004-2014. Epilepsy Res 146:41–49. https://doi.org/10.1016/j.eplepsyres.2018.07.012
Semple BD, Zamani A, Rayner G, Shultz SR, Jones NC (2018) Affective, neurocognitive and psychosocial disorders associated with traumatic brain injury and post-traumatic epilepsy. Neurobiol Dis. https://doi.org/10.1016/j.nbd.2018.07.018
Fleminger S, Oliver DL, Lovestone S, Rabe-Hesketh S, Giora A (2003) Head injury as a risk factor for Alzheimer’s disease: the evidence 10 years on; a partial replication. J Neurol Neurosurg Psychiatry 74(7):857–862
Salib E, Hillier V (1997) Head injury and the risk of Alzheimer’s disease: a case control study. Int J Geriatr Psychiatry 12(3):363–368
Guo Z, Cupples LA, Kurz A, Auerbach SH, Volicer L, Chui H, Green RC, Sadovnick AD et al (2000) Head injury and the risk of AD in the MIRAGE study. Neurology 54(6):1316–1323
Narayan RK, Michel ME, Ansell B, Baethmann A, Biegon A, Bracken MB, Bullock MR, Choi SC et al (2002) Clinical trials in head injury. J Neurotrauma 19(5):503–557. https://doi.org/10.1089/089771502753754037
Menon DK (2009) Unique challenges in clinical trials in traumatic brain injury. Crit Care Med 37(1 Suppl):S129–S135. https://doi.org/10.1097/CCM.0b013e3181921225
Marklund N, Hillered L (2011) Animal modelling of traumatic brain injury in preclinical drug development: where do we go from here? Br J Pharmacol 164(4):1207–1229. https://doi.org/10.1111/j.1476-5381.2010.01163.x
Briones TL (2015) Chapter 3 animal models of traumatic brain injury: is there an optimal model that parallels human brain injury? Annu Rev Nurs Res 33:31–73. https://doi.org/10.1891/0739-6686.33.31
Maas AI, Lingsma HF (2008) New approaches to increase statistical power in TBI trials: insights from the IMPACT study. Acta Neurochir Suppl 101:119–124
Lu J, Marmarou A, Lapane K, Turf E, Wilson L (2010) A method for reducing misclassification in the extended Glasgow Outcome Score. J Neurotrauma 27(5):843–852. https://doi.org/10.1089/neu.2010.1293
Xiong Y, Mahmood A, Chopp M (2013) Animal models of traumatic brain injury. Nat Rev Neurosci 14(2):128–142. https://doi.org/10.1038/nrn3407
Eakin K, Rowe RK, Lifshitz J (2015) Modeling fluid percussion injury: relevance to human traumatic brain injury. In: Kobeissy FH (ed) Brain neurotrauma: molecular, neuropsychological, and rehabilitation aspects. Frontiers in Neuroengineering, Boca Raton (FL)
Cernak I (2005) Animal models of head trauma. NeuroRx 2(3):410–422. https://doi.org/10.1602/neurorx.2.3.410
Johnson VE, Meaney DF, Cullen DK, Smith DH (2015) Animal models of traumatic brain injury. Handb Clin Neurol 127:115–128. https://doi.org/10.1016/b978-0-444-52892-6.00008-8
Osier ND, Carlson SW, DeSana A, Dixon CE (2015) Chronic histopathological and behavioral outcomes of experimental traumatic brain injury in adult male animals. J Neurotrauma 32(23):1861–1882. https://doi.org/10.1089/neu.2014.3680
Gennarelli TA (1994) Animate models of human head injury. J Neurotrauma 11(4):357–368. https://doi.org/10.1089/neu.1994.11.357
Bhowmick S, D’Mello V, Abdul-Muneer PM (2018) Synergistic inhibition of ERK1/2 and JNK, not p38, phosphorylation ameliorates neuronal damages after traumatic brain injury. Mol Neurobiol. https://doi.org/10.1007/s12035-018-1132-7
Patel RK, Prasad N, Kuwar R, Haldar D, Abdul-Muneer PM (2017) Transforming growth factor-beta 1 signaling regulates neuroinflammation and apoptosis in mild traumatic brain injury. Brain Behav Immun 64:244–258. https://doi.org/10.1016/j.bbi.2017.04.012
Gaetz M (2004) The neurophysiology of brain injury. Clin Neurophysiol 115(1):4–18
Wang HC, Duan ZX, Wu FF, Xie L, Zhang H, Ma YB (2010) A new rat model for diffuse axonal injury using a combination of linear acceleration and angular acceleration. J Neurotrauma 27(4):707–719. https://doi.org/10.1089/neu.2009.1071
Bramlett HM, Dietrich WD (2007) Progressive damage after brain and spinal cord injury: pathomechanisms and treatment strategies. Prog Brain Res 161:125–141. https://doi.org/10.1016/s0079-6123(06)61009-1
DeWitt DS, Prough DS (2009) Blast-induced brain injury and posttraumatic hypotension and hypoxemia. J Neurotrauma 26(6):877–887. https://doi.org/10.1089/neu.2007.0439
Kaur C, Singh J, Lim MK, Ng BL, Yap EP, Ling EA (1995) The response of neurons and microglia to blast injury in the rat brain. Neuropathol Appl Neurobiol 21(5):369–377
Bauman RA, Ling G, Tong L, Januszkiewicz A, Agoston D, Delanerolle N, Kim Y, Ritzel D et al (2009) An introductory characterization of a combat-casualty-care relevant swine model of closed head injury resulting from exposure to explosive blast. J Neurotrauma 26(6):841–860. https://doi.org/10.1089/neu.2009-089810.1089/neu.2008.0898
de Lanerolle NC, Bandak F, Kang D, Li AY, Du F, Swauger P, Parks S, Ling G et al (2011) Characteristics of an explosive blast-induced brain injury in an experimental model. J Neuropathol Exp Neurol 70(11):1046–1057. https://doi.org/10.1097/NEN.0b013e318235bef2
Zhu F, Skelton P, Chou CC, Mao H, Yang KH, King AI (2013) Biomechanical responses of a pig head under blast loading: a computational simulation. Int J Numer Methods Biomed Eng 29(3):392–407. https://doi.org/10.1002/cnm.2518
Lu J, Ng KC, Ling G, Wu J, Poon DJ, Kan EM, Tan MH, Wu YJ et al (2012) Effect of blast exposure on the brain structure and cognition in Macaca fascicularis. J Neurotrauma 29(7):1434–1454. https://doi.org/10.1089/neu.2010.1591
Reneer DV, Hisel RD, Hoffman JM, Kryscio RJ, Lusk BT, Geddes JW (2011) A multi-mode shock tube for investigation of blast-induced traumatic brain injury. J Neurotrauma 28(1):95–104. https://doi.org/10.1089/neu.2010.1513
Axelsson H, Hjelmqvist H, Medin A, Persson JK, Suneson A (2000) Physiological changes in pigs exposed to a blast wave from a detonating high-explosive charge. Mil Med 165(2):119–126
Savic J, Tatic V, Ignjatovic D, Mrda V, Erdeljan D, Cernak I, Vujnov S, Simovic M et al (1991) Pathophysiologic reactions in sheep to blast waves from detonation of aerosol explosives. Vojnosanit Pregl 48(6):499–506
Elder GA, Cristian A (2009) Blast-related mild traumatic brain injury: mechanisms of injury and impact on clinical care. Mt. Sinai J Med 76(2):111–118. https://doi.org/10.1002/msj.20098
Garman RH, Jenkins LW, Switzer RC 3rd, Bauman RA, Tong LC, Swauger PV, Parks SA, Ritzel DV et al (2011) Blast exposure in rats with body shielding is characterized primarily by diffuse axonal injury. J Neurotrauma 28(6):947–959. https://doi.org/10.1089/neu.2010.1540
Ryu J, Horkayne-Szakaly I, Xu L, Pletnikova O, Leri F, Eberhart C, Troncoso JC, Koliatsos VE (2014) The problem of axonal injury in the brains of veterans with histories of blast exposure. Acta Neuropathol Commun 2:153. https://doi.org/10.1186/s40478-014-0153-3
Sundaramurthy A, Alai A, Ganpule S, Holmberg A, Plougonven E, Chandra N (2012) Blast-induced biomechanical loading of the rat: an experimental and anatomically accurate computational blast injury model. J Neurotrauma 29(13):2352–2364. https://doi.org/10.1089/neu.2012.2413
Svetlov SI, Prima V, Kirk DR, Gutierrez H, Curley KC, Hayes RL, Wang KK (2010) Morphologic and biochemical characterization of brain injury in a model of controlled blast overpressure exposure. J Trauma 69(4):795–804. https://doi.org/10.1097/TA.0b013e3181bbd885
Alay E, Skotak M, Misistia A, Chandra N (2018) Dynamic loads on human and animal surrogates at different test locations in compressed-gas-driven shock tubes. Shock Waves 28(1):51–62. https://doi.org/10.1007/s00193-017-0762-4
Dal Cengio Leonardi A, Keane NJ, Bir CA, Ryan AG, Xu L, Vandevord PJ (2012) Head orientation affects the intracranial pressure response resulting from shock wave loading in the rat. J Biomech 45(15):2595–2602. https://doi.org/10.1016/j.jbiomech.2012.08.024
Mishra V, Skotak M, Schuetz H, Heller A, Haorah J, Chandra N (2016) Primary blast causes mild, moderate, severe and lethal TBI with increasing blast overpressures: experimental rat injury model. Sci Rep 6:26992. https://doi.org/10.1038/srep26992
Flierl MA, Stahel PF, Beauchamp KM, Morgan SJ, Smith WR, Shohami E (2009) Mouse closed head injury model induced by a weight-drop device. Nat Protoc 4(9):1328–1337. https://doi.org/10.1038/nprot.2009.148
Beit-Yannai E, Zhang R, Trembovler V, Samuni A, Shohami E (1996) Cerebroprotective effect of stable nitroxide radicals in closed head injury in the rat. Brain Res 717(1–2):22–28
Cernak I, Merkle AC, Koliatsos VE, Bilik JM, Luong QT, Mahota TM, Xu L, Slack N et al (2011) The pathobiology of blast injuries and blast-induced neurotrauma as identified using a new experimental model of injury in mice. Neurobiol Dis 41(2):538–551. https://doi.org/10.1016/j.nbd.2010.10.025
Abdul-Muneer PM, Schuetz H, Wang F, Skotak M, Jones J, Gorantla S, Zimmerman MC, Chandra N et al (2013) Induction of oxidative and nitrosative damage leads to cerebrovascular inflammation in an animal model of mild traumatic brain injury induced by primary blast. Free Radic Biol Med 60:282–291. https://doi.org/10.1016/j.freeradbiomed.2013.02.029
Lighthall JW (1988) Controlled cortical impact: a new experimental brain injury model. J Neurotrauma 5(1):1–15. https://doi.org/10.1089/neu.1988.5.1
Dixon CE, Clifton GL, Lighthall JW, Yaghmai AA, Hayes RL (1991) A controlled cortical impact model of traumatic brain injury in the rat. J Neurosci Methods 39(3):253–262
Smith DH, Soares HD, Pierce JS, Perlman KG, Saatman KE, Meaney DF, Dixon CE, McIntosh TK (1995) A model of parasagittal controlled cortical impact in the mouse: cognitive and histopathologic effects. J Neurotrauma 12(2):169–178. https://doi.org/10.1089/neu.1995.12.169
Manley GT, Rosenthal G, Lam M, Morabito D, Yan D, Derugin N, Bollen A, Knudson MM et al (2006) Controlled cortical impact in swine: pathophysiology and biomechanics. J Neurotrauma 23(2):128–139. https://doi.org/10.1089/neu.2006.23.128
King C, Robinson T, Dixon CE, Rao GR, Larnard D, Nemoto CE (2010) Brain temperature profiles during epidural cooling with the ChillerPad in a monkey model of traumatic brain injury. J Neurotrauma 27(10):1895–1903. https://doi.org/10.1089/neu.2009.1178
Chen Y, Mao H, Yang KH, Abel T, Meaney DF (2014) A modified controlled cortical impact technique to model mild traumatic brain injury mechanics in mice. Front Neurol 5:100. https://doi.org/10.3389/fneur.2014.00100
Smith SL, Hall ED (1996) Mild pre- and posttraumatic hypothermia attenuates blood-brain barrier damage following controlled cortical impact injury in the rat. J Neurotrauma 13(1):1–9. https://doi.org/10.1089/neu.1996.13.1
Osier ND, Dixon CE (2016) The controlled cortical impact model: applications, considerations for researchers, and future directions. Front Neurol 7:134. https://doi.org/10.3389/fneur.2016.00134
Yu S, Kaneko Y, Bae E, Stahl CE, Wang Y, van Loveren H, Sanberg PR, Borlongan CV (2009) Severity of controlled cortical impact traumatic brain injury in rats and mice dictates degree of behavioral deficits. Brain Res 1287:157–163. https://doi.org/10.1016/j.brainres.2009.06.067
Washington PM, Forcelli PA, Wilkins T, Zapple DN, Parsadanian M, Burns MP (2012) The effect of injury severity on behavior: a phenotypic study of cognitive and emotional deficits after mild, moderate, and severe controlled cortical impact injury in mice. J Neurotrauma 29(13):2283–2296. https://doi.org/10.1089/neu.2012.2456
Wang X, Gao X, Michalski S, Zhao S, Chen J (2016) Traumatic brain injury severity affects neurogenesis in adult mouse hippocampus. J Neurotrauma 33(8):721–733. https://doi.org/10.1089/neu.2015.4097
Siebold L, Obenaus A, Goyal R (2018) Criteria to define mild, moderate, and severe traumatic brain injury in the mouse controlled cortical impact model. Exp Neurol 310:48–57. https://doi.org/10.1016/j.expneurol.2018.07.004
O’Connor WT, Smyth A, Gilchrist MD (2011) Animal models of traumatic brain injury: a critical evaluation. Pharmacol Ther 130(2):106–113. https://doi.org/10.1016/j.pharmthera.2011.01.001
Shapira Y, Shohami E, Sidi A, Soffer D, Freeman S, Cotev S (1988) Experimental closed head injury in rats: mechanical, pathophysiologic, and neurologic properties. Crit Care Med 16(3):258–265
Foda MA, Marmarou A (1994) A new model of diffuse brain injury in rats. Part II: morphological characterization. J Neurosurg 80(2):301–313. https://doi.org/10.3171/jns.1994.80.2.0301
Feeney DM, Boyeson MG, Linn RT, Murray HM, Dail WG (1981) Responses to cortical injury: I. Methodology and local effects of contusions in the rat. Brain Res 211(1):67–77
Dail WG, Feeney DM, Murray HM, Linn RT, Boyeson MG (1981) Responses to cortical injury: II. Widespread depression of the activity of an enzyme in cortex remote from a focal injury. Brain Res 211(1):79–89
Morales DM, Marklund N, Lebold D, Thompson HJ, Pitkanen A, Maxwell WL, Longhi L, Laurer H et al (2005) Experimental models of traumatic brain injury: do we really need to build a better mousetrap? Neuroscience 136(4):971–989. https://doi.org/10.1016/j.neuroscience.2005.08.030
Mikawa S, Kinouchi H, Kamii H, Gobbel GT, Chen SF, Carlson E, Epstein CJ, Chan PH (1996) Attenuation of acute and chronic damage following traumatic brain injury in copper, zinc-superoxide dismutase transgenic mice. J Neurosurg 85(5):885–891. https://doi.org/10.3171/jns.1996.85.5.0885
Bellander BM, von Holst H, Fredman P, Svensson M (1996) Activation of the complement cascade and increase of clusterin in the brain following a cortical contusion in the adult rat. J Neurosurg 85(3):468–475. https://doi.org/10.3171/jns.1996.85.3.0468
Uhl MW, Biagas KV, Grundl PD, Barmada MA, Schiding JK, Nemoto EM, Kochanek PM (1994) Effects of neutropenia on edema, histology, and cerebral blood flow after traumatic brain injury in rats. J Neurotrauma 11(3):303–315. https://doi.org/10.1089/neu.1994.11.303
Holmin S, Schalling M, Hojeberg B, Nordqvist AC, Skeftruna AK, Mathiesen T (1997) Delayed cytokine expression in rat brain following experimental contusion. J Neurosurg 86(3):493–504. https://doi.org/10.3171/jns.1997.86.3.0493
Chen Y, Constantini S, Trembovler V, Weinstock M, Shohami E (1996) An experimental model of closed head injury in mice: pathophysiology, histopathology, and cognitive deficits. J Neurotrauma 13(10):557–568. https://doi.org/10.1089/neu.1996.13.557
Kalish BT, Whalen MJ (2016) Weight drop models in traumatic brain injury. Methods Mol Biol (Clifton, NJ) 1462:193–209. https://doi.org/10.1007/978-1-4939-3816-2_12
Marmarou A, Foda MA, van den Brink W, Campbell J, Kita H, Demetriadou K (1994) A new model of diffuse brain injury in rats. Part I: pathophysiology and biomechanics. J Neurosurg 80(2):291–300. https://doi.org/10.3171/jns.1994.80.2.0291
Albert-Weissenberger C, Varrallyay C, Raslan F, Kleinschnitz C, Siren AL (2012) An experimental protocol for mimicking pathomechanisms of traumatic brain injury in mice. Exp Transl Stroke Med 4:1. https://doi.org/10.1186/2040-7378-4-1
Kilbourne M, Kuehn R, Tosun C, Caridi J, Keledjian K, Bochicchio G, Scalea T, Gerzanich V et al (2009) Novel model of frontal impact closed head injury in the rat. J Neurotrauma 26(12):2233–2243. https://doi.org/10.1089/neu.2009.0968
Viano DC, Hamberger A, Bolouri H, Saljo A (2009) Concussion in professional football: animal model of brain injury--part 15. Neurosurgery 64(6):1162–1173; discussion 1173. https://doi.org/10.1227/01.neu.0000345863.99099.c7
Khalin I, Jamari NL, Razak NB, Hasain ZB, Nor MA, Zainudin MH, Omar AB, Alyautdin R (2016) A mouse model of weight-drop closed head injury: emphasis on cognitive and neurological deficiency. Neural Regen Res 11(4):630–635. https://doi.org/10.4103/1673-5374.180749
Hsieh TH, Kang JW, Lai JH, Huang YZ, Rotenberg A, Chen KY, Wang JY, Chan SY et al (2017) Relationship of mechanical impact magnitude to neurologic dysfunction severity in a rat traumatic brain injury model. PLoS One 12(5):e0178186. https://doi.org/10.1371/journal.pone.0178186
Thompson HJ, Lifshitz J, Marklund N, Grady MS, Graham DI, Hovda DA, McIntosh TK (2005) Lateral fluid percussion brain injury: a 15-year review and evaluation. J Neurotrauma 22(1):42–75. https://doi.org/10.1089/neu.2005.22.42
Ziebell JM, Rowe RK, Harrison JL, Eakin KC, Colburn T, Willyerd FA, Lifshitz J (2016) Experimental diffuse brain injury results in regional alteration of gross vascular morphology independent of neuropathology. Brain Inj 30(2):217–224. https://doi.org/10.3109/02699052.2015.1090012
McIntosh TK, Vink R, Noble L, Yamakami I, Fernyak S, Soares H, Faden AL (1989) Traumatic brain injury in the rat: characterization of a lateral fluid-percussion model. Neuroscience 28(1):233–244
Floyd CL, Golden KM, Black RT, Hamm RJ, Lyeth BG (2002) Craniectomy position affects Morris water maze performance and hippocampal cell loss after parasagittal fluid percussion. J Neurotrauma 19(3):303–316. https://doi.org/10.1089/089771502753594873
Sanders MJ, Dietrich WD, Green EJ (1999) Cognitive function following traumatic brain injury: effects of injury severity and recovery period in a parasagittal fluid-percussive injury model. J Neurotrauma 16(10):915–925. https://doi.org/10.1089/neu.1999.16.915
Alder J, Fujioka W, Lifshitz J, Crockett DP, Thakker-Varia S (2011) Lateral fluid percussion: model of traumatic brain injury in mice. J Vis Exp 54. https://doi.org/10.3791/3063
Hicks R, Soares H, Smith D, McIntosh T (1996) Temporal and spatial characterization of neuronal injury following lateral fluid-percussion brain injury in the rat. Acta Neuropathol 91(3):236–246
Bramlett HM, Dietrich WD (2002) Quantitative structural changes in white and gray matter 1 year following traumatic brain injury in rats. Acta Neuropathol 103(6):607–614. https://doi.org/10.1007/s00401-001-0510-8
Liu YR, Cardamone L, Hogan RE, Gregoire MC, Williams JP, Hicks RJ, Binns D, Koe A et al (2010) Progressive metabolic and structural cerebral perturbations after traumatic brain injury: an in vivo imaging study in the rat. J Nucl Med 51(11):1788–1795. https://doi.org/10.2967/jnumed.110.078626
Hamm RJ (2001) Neurobehavioral assessment of outcome following traumatic brain injury in rats: an evaluation of selected measures. J Neurotrauma 18(11):1207–1216. https://doi.org/10.1089/089771501317095241
Pierce JE, Smith DH, Trojanowski JQ, McIntosh TK (1998) Enduring cognitive, neurobehavioral and histopathological changes persist for up to one year following severe experimental brain injury in rats. Neuroscience 87(2):359–369
Wahab RA, Neuberger EJ, Lyeth BG, Santhakumar V, Pfister BJ (2015) Fluid percussion injury device for the precise control of injury parameters. J Neurosci Methods 248:16–26. https://doi.org/10.1016/j.jneumeth.2015.03.010
Kabadi SV, Hilton GD, Stoica BA, Zapple DN, Faden AI (2010) Fluid-percussion-induced traumatic brain injury model in rats. Nat Protoc 5(9):1552–1563. https://doi.org/10.1038/nprot.2010.112
Prins ML, Lee SM, Cheng CL, Becker DP, Hovda DA (1996) Fluid percussion brain injury in the developing and adult rat: a comparative study of mortality, morphology, intracranial pressure and mean arterial blood pressure. Brain Res Dev Brain Res 95(2):272–282
Shultz SR, MacFabe DF, Foley KA, Taylor R, Cain DP (2011) A single mild fluid percussion injury induces short-term behavioral and neuropathological changes in the Long-Evans rat: support for an animal model of concussion. Behav Brain Res 224(2):326–335. https://doi.org/10.1016/j.bbr.2011.06.012
Griesbach GS, Hovda DA, Gomez-Pinilla F (2009) Exercise-induced improvement in cognitive performance after traumatic brain injury in rats is dependent on BDNF activation. Brain Res 1288:105–115. https://doi.org/10.1016/j.brainres.2009.06.045
Chitturi J, Li Y, Santhakumar V, Kannurpatti SS (2018) Early behavioral and metabolomic change after mild to moderate traumatic brain injury in the developing brain. Neurochem Int 120:75–86. https://doi.org/10.1016/j.neuint.2018.08.003
Li Y, Korgaonkar AA, Swietek B, Wang J, Elgammal FS, Elkabes S, Santhakumar V (2015) Toll-like receptor 4 enhancement of non-NMDA synaptic currents increases dentate excitability after brain injury. Neurobiol Dis 74:240–253. https://doi.org/10.1016/j.nbd.2014.11.021
Kinoshita K, Chatzipanteli i K, Vitarbo E, Truettner JS, Alonso OF, Dietrich WD (2002) Interleukin-1beta messenger ribonucleic acid and protein levels after fluid-percussion brain injury in rats: importance of injury severity and brain temperature. Neurosurgery 51(1):195–203 discussion 203
Vitarbo EA, Chatzipanteli K, Kinoshita K, Truettner JS, Alonso OF, Dietrich WD (2004) Tumor necrosis factor alpha expression and protein levels after fluid percussion injury in rats: the effect of injury severity and brain temperature. Neurosurgery 55(2):416–424 discussion 424-415
D’Ambrosio R, Fairbanks JP, Fender JS, Born DE, Doyle DL, Miller JW (2004) Post-traumatic epilepsy following fluid percussion injury in the rat. Brain J Neurol 127(Pt 2):304–314. https://doi.org/10.1093/brain/awh038
Sanders MJ, Sick TJ, Perez-Pinzon MA, Dietrich WD, Green EJ (2000) Chronic failure in the maintenance of long-term potentiation following fluid percussion injury in the rat. Brain Res 861(1):69–76
Schmidt RH, Grady MS (1993) Regional patterns of blood-brain barrier breakdown following central and lateral fluid percussion injury in rodents. J Neurotrauma 10(4):415–430. https://doi.org/10.1089/neu.1993.10.415
Morehead M, Bartus RT, Dean RL, Miotke JA, Murphy S, Sall J, Goldman H (1994) Histopathologic consequences of moderate concussion in an animal model: correlations with duration of unconsciousness. J Neurotrauma 11(6):657–667. https://doi.org/10.1089/neu.1994.11.657
Schmidt RH, Scholten KJ, Maughan PH (1999) Time course for recovery of water maze performance and central cholinergic innervation after fluid percussion injury. J Neurotrauma 16(12):1139–1147. https://doi.org/10.1089/neu.1999.16.1139
Fujimoto ST, Longhi L, Saatman KE, Conte V, Stocchetti N, McIntosh TK (2004) Motor and cognitive function evaluation following experimental traumatic brain injury. Neurosci Biobehav Rev 28(4):365–378. https://doi.org/10.1016/j.neubiorev.2004.06.002
Bramlett HM, Green EJ, Dietrich WD (1997) Hippocampally dependent and independent chronic spatial navigational deficits following parasagittal fluid percussion brain injury in the rat. Brain Res 762(1–2):195–202
Rabinowitz AR, Levin HS (2014) Cognitive sequelae of traumatic brain injury. Psychiatr Clin North Am 37(1):1–11. https://doi.org/10.1016/j.psc.2013.11.004
Metz GA, Whishaw IQ (2009) The ladder rung walking task: a scoring system and its practical application. J Vis Exp (28): e1204. https://doi.org/10.3791/1204
Hicks RR, Boggs A, Leider D, Kraemer P, Brown R, Scheff SW, Seroogy KB (1998) Effects of exercise following lateral fluid percussion brain injury in rats. Restor Neurol Neurosci 12(1):41–47
Clough RW, Neese SL, Sherill LK, Tan AA, Duke A, Roosevelt RW, Browning RA, Smith DC (2007) Cortical edema in moderate fluid percussion brain injury is attenuated by vagus nerve stimulation. Neuroscience 147(2):286–293. https://doi.org/10.1016/j.neuroscience.2007.04.043
Alessandri B, Rice AC, Levasseur J, DeFord M, Hamm RJ, Bullock MR (2002) Cyclosporin A improves brain tissue oxygen consumption and learning/memory performance after lateral fluid percussion injury in rats. J Neurotrauma 19(7):829–841. https://doi.org/10.1089/08977150260190429
Gold EM, Su D, Lopez-Velazquez L, Haus DL, Perez H, Lacuesta GA, Anderson AJ, Cummings BJ (2013) Functional assessment of long-term deficits in rodent models of traumatic brain injury. Regen Med 8(4):483–516. https://doi.org/10.2217/rme.13.41
Hamm RJ, Pike BR, O’Dell DM, Lyeth BG, Jenkins LW (1994) The rotarod test: an evaluation of its effectiveness in assessing motor deficits following traumatic brain injury. J Neurotrauma 11(2):187–196. https://doi.org/10.1089/neu.1994.11.187
Smith DH, Okiyama K, Thomas MJ, Claussen B, McIntosh TK (1991) Evaluation of memory dysfunction following experimental brain injury using the Morris water maze. J Neurotrauma 8(4):259–269. https://doi.org/10.1089/neu.1991.8.259
Lyeth BG, Jenkins LW, Hamm RJ, Dixon CE, Phillips LL, Clifton GL, Young HF, Hayes RL (1990) Prolonged memory impairment in the absence of hippocampal cell death following traumatic brain injury in the rat. Brain Res 526(2):249–258
Harrison JL, Rowe RK, Ellis TW, Yee NS, O’Hara BF, Adelson PD, Lifshitz J (2015) Resolvins AT-D1 and E1 differentially impact functional outcome, post-traumatic sleep, and microglial activation following diffuse brain injury in the mouse. Brain Behav Immun 47:131–140. https://doi.org/10.1016/j.bbi.2015.01.001
Hallam TM, Floyd CL, Folkerts MM, Lee LL, Gong QZ, Lyeth BG, Muizelaar JP, Berman RF (2004) Comparison of behavioral deficits and acute neuronal degeneration in rat lateral fluid percussion and weight-drop brain injury models. J Neurotrauma 21(5):521–539. https://doi.org/10.1089/089771504774129865
Peterson TC, Maass WR, Anderson JR, Anderson GD, Hoane MR (2015) A behavioral and histological comparison of fluid percussion injury and controlled cortical impact injury to the rat sensorimotor cortex. Behav Brain Res 294:254–263. https://doi.org/10.1016/j.bbr.2015.08.007
Vink R, Mullins PG, Temple MD, Bao W, Faden AI (2001) Small shifts in craniotomy position in the lateral fluid percussion injury model are associated with differential lesion development. J Neurotrauma 18(8):839–847. https://doi.org/10.1089/089771501316919201
Kuo JR, Cheng YH, Chen YS, Chio CC, Gean PW (2013) Involvement of extracellular signal regulated kinases in traumatic brain injury-induced depression in rodents. J Neurotrauma 30(14):1223–1231. https://doi.org/10.1089/neu.2012.2689
Deng-Bryant Y, Leung LY, Caudle K, Tortella F, Shear D (2016) Cognitive evaluation using Morris water maze in neurotrauma. Methods Mol Biol(Clifton, NJ) 1462:539–551. https://doi.org/10.1007/978-1-4939-3816-2_29
Lyeth BG (2016) Historical review of the fluid-percussion TBI model. Front Neurol 7:217. https://doi.org/10.3389/fneur.2016.00217
Zeeshan M, Jehan F, O’Keeffe T, Khan M, Zakaria ER, Hamidi M, Gries L, Kulvatunyou N et al (2018) The novel oral anticoagulants (NOACs) have worse outcomes compared to warfarin in patients with intracranial hemorrhage after TBI. J Trauma Acute Care Surg 85:915–920. https://doi.org/10.1097/ta.0000000000001995
Funding
This work was supported by grant 1R21AA022734-01A1 (to JH).
Author information
Authors and Affiliations
Contributions
XM carried out the studies of literature research and performed the acquisition of data and writing in manuscript preparation. AA wrote the part of “behavioral assessment” and helped XM in manuscript preparation. BJF and NC proofread the manuscript and gave important comments from their area of expertise. JH supervised the development of work, gave critical revisions, and edited the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ma, X., Aravind, A., Pfister, B.J. et al. Animal Models of Traumatic Brain Injury and Assessment of Injury Severity. Mol Neurobiol 56, 5332–5345 (2019). https://doi.org/10.1007/s12035-018-1454-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-018-1454-5