Abstract
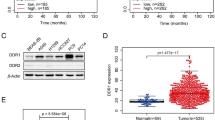
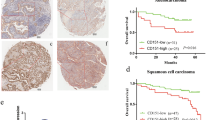
Discoidin domain receptors (DDRs) are a novel class of receptor tyrosine kinases that respond to several collagens and facilitate cell adhesion. DDR1 is highly expressed in a variety of human cancers, and it is clear that DDR1 is primarily expressed in epithelial cells including lung, colon and brain. Moreover, DDR1 expression can be stimulated by collagen types I, II, III, IV, V, VIII and XI, and aberrant signaling induced by DDR1 dysregulated expression is involved in various steps of tumorigenesis. However, the molecular mechanism underlying the role of DDR1 in cancer development is not well documented. In this study, we found that the expression of DDR1 is upregulated in non-small cell lung cancer (NSCLC) tissues and cells when compared with counterpart normal tissues and cells. Furthermore, collagen I could induce DDR1 expression, and activated DDR1 promoted NSCLC cell migration and invasion, while knockdown of DDR1 by transfection with siRNA resulted in a significant decrease in cell migrativeness and invasiveness. Enhanced DDR1 expression mediated by collagen I could activate MMP-2, N-cadherin and vimentin expression, but reduce E-cadherin expression; however, inhibition of DDR1 expression could suppress MMP-2, N-cadherin and vimentin expression and induce E-cadherin activation. In conclusion, our findings indicated that upregulation of DDR1 induced by collagen I may contribute to the development and progression of NSCLC and this effect may be associated with increased invasiveness, at least in part, via promoting epithelial-to-mesenchymal transition.




Similar content being viewed by others
References
Jemal A, Siegel R, Xu J, Ward E. Cancer statistics. CA Cancer J Clin. 2010;60(5):277–300. doi:10.3322/caac.20073.
Carney DN. Lung cancer–time to move on from chemotherapy. N Engl J Med. 2002;346(2):126–8. doi:10.1056/NEJM200201103460211.
Alves F, Vogel W, Mossie K, Millauer B, Hofler H, Ullrich A. Distinct structural characteristics of discoidin I subfamily receptor tyrosine kinases and complementary expression in human cancer. Oncogene. 1995;10(3):609–18.
Medici D, Nawshad A. Type I collagen promotes epithelial-mesenchymal transition through ILK-dependent activation of NF-kappaB and LEF-1. Matrix Biol. 2010;29(3):161–5. doi:10.1016/j.matbio.2009.12.003.
Ongusaha PP, Kim JI, Fang L, Wong TW, Yancopoulos GD, Aaronson SA, et al. p53 induction and activation of DDR1 kinase counteract p53-mediated apoptosis and influence p53 regulation through a positive feedback loop. EMBO J. 2003;22(6):1289–301. doi:10.1093/emboj/cdg129.
Vogel W, Gish GD, Alves F, Pawson T. The discoidin domain receptor tyrosine kinases are activated by collagen. Mol Cell. 1997;1(1):13–23.
Ram R, Lorente G, Nikolich K, Urfer R, Foehr E, Nagavarapu U. Discoidin domain receptor-1a (DDR1a) promotes glioma cell invasion and adhesion in association with matrix metalloproteinase-2. J Neurooncol. 2006;76(3):239–48. doi:10.1007/s11060-005-6874-1.
Hou G, Vogel W, Bendeck MP. The discoidin domain receptor tyrosine kinase DDR1 in arterial wound repair. J Clin Invest. 2001;107(6):727–35. doi:10.1172/JCI10720.
Vogel WF, Aszodi A, Alves F, Pawson T. Discoidin domain receptor 1 tyrosine kinase has an essential role in mammary gland development. Mol Cell Biol. 2001;21(8):2906–17. doi:10.1128/MCB.21.8.2906-2917.2001.
Johnson JD, Edman JC, Rutter WJ. A receptor tyrosine kinase found in breast carcinoma cells has an extracellular discoidin I-like domain. Proc Natl Acad Sci USA. 1993;90(22):10891.
Heinzelmann-Schwarz VA, Gardiner-Garden M, Henshall SM, Scurry J, Scolyer RA, Davies MJ, et al. Overexpression of the cell adhesion molecules DDR1, Claudin 3, and Ep-CAM in metaplastic ovarian epithelium and ovarian cancer. Clin Cancer Res. 2004;10(13):4427–36. doi:10.1158/1078-0432.CCR-04-0073.
Yang SH, Baek HA, Lee HJ, Park HS, Jang KY, Kang MJ, et al. Discoidin domain receptor 1 is associated with poor prognosis of non-small cell lung carcinomas. Oncol Rep. 2010;24(2):311–9.
Shimada K, Nakamura M, Ishida E, Higuchi T, Yamamoto H, Tsujikawa K, et al. Prostate cancer antigen-1 contributes to cell survival and invasion though discoidin receptor 1 in human prostate cancer. Cancer Sci. 2008;99(1):39–45. doi:10.1111/j.1349-7006.2007.00655.x.
Ford CE, Lau SK, Zhu CQ, Andersson T, Tsao MS, Vogel WF. Expression and mutation analysis of the discoidin domain receptors 1 and 2 in non-small cell lung carcinoma. Br J Cancer. 2007;96(5):808–14. doi:10.1038/sj.bjc.6603614.
Kamohara H, Yamashiro S, Galligan C, Yoshimura T. Discoidin domain receptor 1 isoform-a (DDR1alpha) promotes migration of leukocytes in three-dimensional collagen lattices. FASEB J. 2001;15(14):2724–6. doi:10.1096/fj.01-0359fje.
Zerlin M, Julius MA, Goldfarb M. NEP: a novel receptor-like tyrosine kinase expressed in proliferating neuroepithelia. Oncogene. 1993;8(10):2731–9.
L’Hote CG, Thomas PH, Ganesan TS. Functional analysis of discoidin domain receptor 1: effect of adhesion on DDR1 phosphorylation. FASEB J. 2002;16(2):234–6. doi:10.1096/fj.01-0414fje.
Park HS, Kim KR, Lee HJ, Choi HN, Kim DK, Kim BT, et al. Overexpression of discoidin domain receptor 1 increases the migration and invasion of hepatocellular carcinoma cells in association with matrix metalloproteinase. Oncol Rep. 2007;18(6):1435–41.
Ruiz PA, Jarai G. Collagen I induces discoidin domain receptor (DDR) 1 expression through DDR2 and a JAK2-ERK1/2-mediated mechanism in primary human lung fibroblasts. J Biol Chem. 2011;286(15):12912–23. doi:10.1074/jbc.M110.143693.
Koenig A, Mueller C, Hasel C, Adler G, Menke A. Collagen type I induces disruption of E-cadherin-mediated cell–cell contacts and promotes proliferation of pancreatic carcinoma cells. Cancer Res. 2006;66(9):4662–71. doi:10.1158/0008-5472.CAN-05-2804.
Shintani Y, Hollingsworth MA, Wheelock MJ, Johnson KR. Collagen I promotes metastasis in pancreatic cancer by activating c-Jun NH(2)-terminal kinase 1 and up-regulating N-cadherin expression. Cancer Res. 2006;66(24):11745–53. doi:10.1158/0008-5472.CAN-06-2322.
Maeda M, Johnson KR, Wheelock MJ. Cadherin switching: essential for behavioral but not morphological changes during an epithelium-to-mesenchyme transition. J Cell Sci. 2005;118(Pt 5):873–87. doi:10.1242/jcs.01634.
Kang Y, Massague J. Epithelial-mesenchymal transitions: twist in development and metastasis. Cell. 2004;118(3):277–9. doi:10.1016/j.cell.2004.07.011.
Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139(5):871–90. doi:10.1016/j.cell.2009.11.007.
Acloque H, Thiery JP, Nieto MA. The physiology and pathology of the EMT. Meeting on the epithelial-mesenchymal transition. EMBO Rep. 2008;9(4):322–6. doi:10.1038/embor.2008.30.
Lee JM, Dedhar S, Kalluri R, Thompson EW. The epithelial-mesenchymal transition: new insights in signaling, development, and disease. J Cell Biol. 2006;172(7):973–81. doi:10.1083/jcb.200601018.
Zavadil J, Bottinger EP. TGF-beta and epithelial-to-mesenchymal transitions. Oncogene. 2005;24(37):5764–74. doi:10.1038/sj.onc.1208927.
Bremnes RM, Veve R, Gabrielson E, Hirsch FR, Baron A, Bemis L, et al. High-throughput tissue microarray analysis used to evaluate biology and prognostic significance of the E-cadherin pathway in non-small-cell lung cancer. J Clin Oncol. 2002;20(10):2417–28.
Liu D, Huang C, Kameyama K, Hayashi E, Yamauchi A, Kobayashi S et al. E-cadherin expression associated with differentiation and prognosis in patients with non-small cell lung cancer. Ann Thorac Surg. 2001;71(3):949–54; discussion 54–5.
Acknowledgments
This research was supported by the Natural Science Fund of Jiangsu Province (BK2011658) to Song Yong.
Conflict of interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Miao, L., Zhu, S., Wang, Y. et al. Discoidin domain receptor 1 is associated with poor prognosis of non-small cell lung cancer and promotes cell invasion via epithelial-to-mesenchymal transition. Med Oncol 30, 626 (2013). https://doi.org/10.1007/s12032-013-0626-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-013-0626-4