Abstract
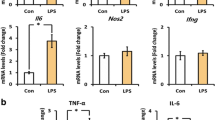
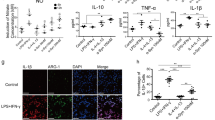
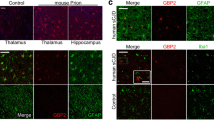
Prion diseases are fatal neurodegenerative diseases characterized by spongiform change, neuronal loss, and gliosis involving microglial activation in the central nervous system. Microglial activation is thought to play a key role in the pathogenesis of prion disease; however, the molecular mechanisms underlying prion-induced microglial activation are not well understood. The present study underlines the importance of toll-like receptor (TLR)-2 in mediating PrP106-126-induced microglial activation. We found that PrP106-126 induced expression of proinflammatory molecules and TLR2 in microglial cells; however, functional blocking antibodies against TLR2 suppressed PrP106-126-induced expression of proinflammatory molecules. PrP106-126-induced expression of proinflammatory molecules was also reduced in microglial cells isolated from TLR2−/− mice compared to those isolated from wild-type mice. Consistent with the importance of nuclear factor kappa B (NF-κB) mediating TLR functions, NF-κB inhibition also inhibited PrP106-126-induced expression of proinflammatory molecules. To better understand the effect of TLR2 deficiency on active microglial cells, we studied the expression of Arg1 and Mrc1 and anti-inflammatory cytokines, which indicated that TLR2 deficiency in microglial cells results in a shift from neurotoxic to neuroprotective phenotype. Taken together, our results indicate that the TLR2 signaling pathway mediates PrP106-126-induced microglial activation and potentially reveal new therapeutic strategies for prion diseases that modulate TLR2 signaling.





Similar content being viewed by others
Abbreviations
- GSS:
-
Gerstmann-Sträussler syndrome
- CJD:
-
Creutzfeldt-Jakob disease
- CNS:
-
Central nervous system
- PD:
-
Parkinson’s disease
- MS:
-
Multiple sclerosis
- AD:
-
Alzheimer’s disease
- TLR:
-
Toll-like receptor
- KO:
-
Knockout
- WT:
-
Wild type
- Aβ:
-
Amyloid β
- SCC:
-
Strongly connected component
- MAPK:
-
Mitogen-activated protein kinase
- ERK:
-
Extracellular signal-regulated kinase
- mFPR2:
-
Formyl peptide receptor 2
- miR:
-
mRNA
- RAGE:
-
Receptor for advanced glycation end products
- NF-κB:
-
Nuclear factor kappa B
- CCK-8:
-
Cell counting Kit-8
- DMEM:
-
Dulbecco’s modified Eagle’s medium
- cDNA:
-
Complementary DNA
- qPCR:
-
Quantitative polymerase chain reaction
- ROS:
-
Reactive oxygen species
- TNF:
-
Tumor necrosis factor
- IL:
-
Interleukin
- iNOS:
-
Inducible isoform of nitric oxide synthase
- SDS-PAGE:
-
Sodium dodecyl sulfate polyacrylamide gel electrophoresis
- TBS:
-
Tris-buffered saline
- ECF:
-
Chemifluorescence
- ThT:
-
Thioflavine-T
- PDTC:
-
Pyrrolidine dithiocarbamate
- Arg:
-
Arginase
- Mrc:
-
Macrophage mannose receptor
- MyD88:
-
Myeloid differentiation factor 88
- TGF:
-
Transforming growth factor
- PAMPs:
-
Pathogen-associated molecular pattern molecules
- DAMPs:
-
Damage-associated molecular pattern molecules
- NTF:
-
Neurotrophic factors
- TIRAP:
-
TIR domain-containing adaptor protein
References
Aguzzi A, Polymenidou M (2004) Mammalian prion biology: one century of evolving concepts. Cell 116:313–327
Brown DR, Schmidt B, Kretzschmar HA (1996) Role of microglia and host prion protein in neurotoxicity of a prion protein fragment. Nature 380:345–347
Chang J, Yang L, Kouadir M et al (2012) Antibody-mediated inhibition of integrin alpha5beta1 blocks neurotoxic prion peptide PrP106-126-induced activation of BV2 microglia. J Mol Neurosci 48:248–252
Chen K, Iribarren P, Hu J et al (2006) Activation of Toll-like receptor 2 on microglia promotes cell uptake of Alzheimer disease-associated amyloid beta peptide. J Biol Chem 281:3651–3659
Colton C, Wilcock DM (2010) Assessing activation states in microglia. CNS Neurol Disord Drug Targets 9:174–191
Crespo I, Roomp K, Jurkowski W et al (2012) Gene regulatory network analysis supports inflammation as a key neurodegeneration process in prion disease. BMC Syst Biol 6:132
Ettaiche M, Pichot R, Vincent JP et al (2000) In vivo cytotoxicity of the prion protein fragment 106–126. J Biol Chem 275:36487–36490
Forloni G, Angeretti N, Chiesa R et al (1993) Neurotoxicity of a prion protein fragment. Nature 362:543–546
Garcao P, Oliveira CR, Agostinho P (2006) Comparative study of microglia activation induced by amyloid-beta and prion peptides: role in neurodegeneration. J Neurosci Res 84:182–193
Gonzalez-Scarano F, Baltuch G (1999) Microglia as mediators of inflammatory and degenerative diseases. Annu Rev Neurosci 22:219–240
Gu Y, Fujioka H, Mishra RS et al (2002) Prion peptide 106–126 modulates the aggregation of cellular prion protein and induces the synthesis of potentially neurotoxic transmembrane PrP. J Biol Chem 277:2275–2286
Hanke ML, Kielian T (2011) Toll-like receptors in health and disease in the brain: mechanisms and therapeutic potential. Clin Sci (Lond) 121:367–387
Ibrahim ZA, Armour CL, Phipps S et al (2013) RAGE and TLRs: relatives, friends or neighbours? Mol Immunol 56:739–744
Julius C, Heikenwalder M, Schwarz P et al (2008) Prion propagation in mice lacking central nervous system NF-kappaB signalling. J Gen Virol 89:1545–1550
Kouadir M, Yang L, Tan R et al (2012) CD36 participates in PrP(106–126)-induced activation of microglia. PLoS One 7:e30756
Kuo HS, Tsai MJ, Huang MC et al (2011) Acid fibroblast growth factor and peripheral nerve grafts regulate Th2 cytokine expression, macrophage activation, polyamine synthesis, and neurotrophin expression in transected rat spinal cords. J Neurosci 31:4137–4147
Liu S, Liu Y, Hao W et al (2012) TLR2 is a primary receptor for Alzheimer’s amyloid beta peptide to trigger neuroinflammatory activation. J Immunol 188:1098–1107
Lu Y, Liu A, Zhou X et al (2012) Prion peptide PrP106-126 induces inducible nitric oxide synthase and proinflammatory cytokine gene expression through the activation of NF-kappaB in macrophage cells. DNA Cell Biol 31:833–838
Ma TC, Campana A, Lange PS et al (2010) A large-scale chemical screen for regulators of the arginase 1 promoter identifies the soy isoflavone daidzeinas a clinically approved small molecule that can promote neuronal protection or regeneration via a cAMP-independent pathway. J Neurosci 30:739–748
Martinez FO, Helming L, Gordon S (2009) Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol 27:451–483
Monden M, Koyama H, Otsuka Y et al (2013) Receptor for advanced glycation end products regulates adipocyte hypertrophy and insulin sensitivity in mice: involvement of toll-like receptor 2. Diabetes 62:478–489
Neniskyte U, Neher JJ, Brown GC (2011) Neuronal death induced by nanomolar amyloid beta is mediated by primary phagocytosis of neurons by microglia. J Biol Chem 286:39904–39913
Nimmerjahn A, Kirchhoff F, Helmchen F (2005) Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 308:1314–1318
Ofodile O (2011) Toll-like receptors (TLRs) and prion disease: relevance to pathology and novel therapy
Oliveira-Nascimento L, Massari P, Wetzler LM (2012) The role of TLR2 in infection and immunity. Front Immunol 3:79
Pan B, Yang L, Wang J et al (2014) c-Abl tyrosine kinase mediates neurotoxic prion peptide-induced neuronal apoptosis via regulating mitochondrial homeostasis. Mol Neurobiol 49:1102–1116
Perry VH, Nicoll JA, Holmes C (2010) Microglia in neurodegenerative disease. Nat Rev Neurol 6:193–201
Prinz M, Heikenwalder M, Schwarz P et al (2003) Prion pathogenesis in the absence of toll-like receptor signalling. EMBO Rep 4:195–199
Prusiner SB (1998) Prions. Proc Natl Acad Sci U S A 95:13363–13383
Ransohoff RM, Perry VH (2009) Microglial physiology: unique stimuli, specialized responses. Annu Rev Immunol 27:119–145
Richard KL, Filali M, Prefontaine P et al (2008) Toll-like receptor 2 acts as a natural innate immune receptor to clear amyloid beta 1–42 and delay the cognitive decline in a mouse model of Alzheimer’s disease. J Neurosci 28:5784–5793
Saba R, Gushue S, Huzarewich RL et al (2012) MicroRNA 146a (miR-146a) is over-expressed during prion disease and modulates the innate immune response and the microglial activation state. PLoS One 7:e30832
Sakai K, Hasebe R, Takahashi Y et al (2013) Absence of CD14 delays progression of prion diseases accompanied by increased microglial activation. J Virol 87:13433–13445
Scott JR (1993) Scrapie pathogenesis. Br Med Bull 49:778–791
Shi F, Yang L, Kouadir M et al (2012) The NALP3 inflammasome is involved in neurotoxic prion peptide-induced microglial activation. J Neuroinflammation 9:73
Shi F, Yang L, Wang J et al (2013) Inhibition of phagocytosis reduced the classical activation of BV2 microglia induced by amyloidogenic fragments of beta-amyloid and prion proteins. Acta Biochim Biophys Sin (Shanghai) 45:973–978
Singh N, Gu Y, Bose S et al (2002) Prion peptide 106–126 as a model for prion replication and neurotoxicity. Front Biosci 7:a60–a71
Soto C (2003) Unfolding the role of protein misfolding in neurodegenerative diseases. Nat Rev Neurosci 4:49–60
Trudler D, Farfara D, Frenkel D (2010) Toll-like receptors expression and signaling in glia cells in neuro-amyloidogenic diseases: towards future therapeutic application. Mediators Inflamm
Yang L, Zhou X, Yang J et al (2008) Aspirin inhibits cytotoxicity of prion peptide PrP106-126 to neuronal cells associated with microglia activation in vitro. J Neuroimmunol 199:10–17
Yang H, Hreggvidsdottir HS, Palmblad K et al (2010) A critical cysteine is required for HMGB1 binding to Toll-like receptor 4 and activation of macrophage cytokine release. Proc Natl Acad Sci U S A 107:11942–11947
Acknowledgments
This work was supported by the National “Twelfth Five-Year” Plan for Science & Technology Support (Project No. 2012AA101302), MoSTRCUK international cooperation project (Project No. 2013DFG32500), the National Natural Science Foundation (Project No. 31172293, No. 31272532), Chinese Universities Scientific Fund (Project No. 2013QT004), and CAU Foreign Experts Major Projects (Project No. 2012Z018)
Conflict of interest
The authors declare no financial or commercial conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Jihong Wang and Deming Zhao contributed equally to this work.
Rights and permissions
About this article
Cite this article
Wang, J., Zhao, D., Pan, B. et al. Toll-Like Receptor 2 Deficiency Shifts PrP106-126-Induced Microglial Activation from a Neurotoxic to a Neuroprotective Phenotype. J Mol Neurosci 55, 880–890 (2015). https://doi.org/10.1007/s12031-014-0442-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-014-0442-0