Abstract
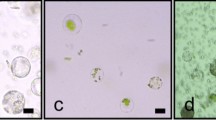
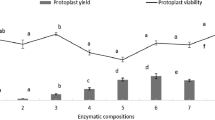
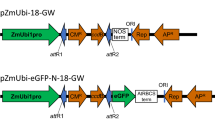
With the release of the Phalaenopsis equestris (Schauer) Rchb.f. genome database, more in-depth studies of Phalaenopsis spp. will be carried out in the future. Transient gene expression in protoplasts is a useful system for gene function analysis, which is especially true for Phalaenopsis, whose stable genetic transformation is difficult and extremely time-consuming. In this study, juvenile leaves from aseptic Phalaenopsis seedlings were used as the starting material for protoplast isolation. After protocol refinement, the highest yield of viable protoplasts [5.94 × 106 protoplasts g−1 fresh weight (FW)] was achieved with 1.0% (w/v) Cellulase Onozuka R-10, 0.7% (w/v) Macerozyme R-10, and 0.4 M D-mannitol, with an enzymolysis duration of 6 h. As indicated by transient expression of green fluorescent protein (GFP), a transformation efficiency of 41.7% was achieved with 20% (w/v) polyethylene glycol (PEG-4000), 20 μg plasmid DNA, 2 × 105 mL−1 protoplasts, and a transfection duration of 30 min. The protocol established here will be valuable for functional studies of Phalaenopsis genes.




Similar content being viewed by others
References
Cai J, Liu X, Vanneste K, Proost S, Tsai W-C, Liu K-W, Chen L-J, He Y, Xu Q, Bian C, Zheng Z, Sun F, Liu W, Hsiao Y-Y, Pan Z-J, Hsu C-C, Yang Y-P, Hsu Y-C, Chuang Y-C, Dievart A, Dufayard J-F, Xu X, Wang J-Y, Wang J, Xiao X-J, Zhao X-M, Du R, Zhang G-Q, Wang M, Y-Y S, Xie G-C, Liu G-H, Li L-Q, Huang L-Q, Luo Y-B, Chen H-H, Van de Peer Y, Liu Z-J (2015) The genome sequence of the orchid Phalaenopsis equestris. Nat Genet 47:65–72
Chen S, Tao L, Zeng L, Vega-Sanchez ME, Umemura K, Wang G-L (2006) A highly efficient transient protoplast system for analyzing defence gene expression and protein-protein interactions in rice. Mol Plant Pathol 7:417–427
Duquenne B, Eeckhaut T, Werbrouck S, Van Huylenbroeck J (2007) Effect of enzyme concentrations on protoplast isolation and protoplast culture of Spathiphyllum and Anthurium. Plant Cell Tissue Organ Cult 91:165–173
Hsieh M-H, Pan Z-J, Lai P-H, Lu H-C, Yeh H-H, Hsu C-C, Wu W-L, Chung M-C, Wang S-S, Chen W-H, Chen H-H (2013) Virus-induced gene silencing unravels multiple transcription factors involved in floral growth and development in Phalaenopsis orchids. J Exp Bot 64:3869–3884
Huang H, Wang Z, Cheng J, Zhao W, Li X, Wang H, Zhang Z, Sui X (2013) An efficient cucumber (Cucumis sativus L.) protoplast isolation and transient expression system. Sci Hortic 150:206–212
Julkifle AL, Rathinam X, Sinniah UR, Subramaniam S (2010) Optimisation of transient green fluorescent protein (GFP) gene expression in Phalaenopsis violacea orchid mediated by Agrobacterium tumefaciens-mediated transformation system. Aust J Basic Appl Sci 4:3424–3432
Kao KN, Michayluk MR (1974) A method for high-frequency intergeneric fusion of plant protoplasts. Planta 115:355–367
Khentry Y, Paradornuvat A, Tantiwiwat S, Phansiri S, Thaveechai N (2006) Protoplast isolation and culture of Dendrobium Sonia ‘Bom 17’. Kasetsart J –Nat Sci 40:361–369
Kobayashi T, Kameya T, Ichihashi S (1993) Plant regeneration from protoplasts derived from callus of Phalaenopsis. Plant Tissue Cult Lett 10:267–270
Locatelli F, Vannini C, Magnani E, Coraggio I, Bracale M (2003) Efficiency of transient transformation in tobacco protoplasts is independent of plasmid amount. Plant Cell Rep 21:865–871
Luehrsen KR, de Wet JR, Walbot V (1992) Transient expression analysis in plants using firefly luciferase reporter gene. Methods Enzymol 216:397–414
Maas C, Werr W (1989) Mechanism and optimized conditions for PEG mediated DNA transfection into plant protoplasts. Plant Cell Rep 8:148–151
Maddumage R, Fung RMW, Weir I, Ding H, Simons JL, Allan AC (2002) Efficient transient transformation of suspension culture-derived apple protoplasts. Plant Cell Tissue Organ Cult 70:77–82
Mishiba K-I, Chin DP, Mii M (2005) Agrobacterium-mediated transformation of Phalaenopsis by targeting protocorms at an early stage after germination. Plant Cell Rep 24:297–303
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497
Pindel A (2007) Optimization of isolation conditions of Cymbidium protoplasts. Folia Hort 19:79–88
Qiao Y-X, Zhang Y-P, Wang G-L, Chen C, Wang Y (2008) Optimization of the method for isolating protoplast of Phalaenopsis amabilisBl. Plant Physiol Comm 44:1177–1180 (in Chinese)
Ratanasanobon K, Seaton KA (2013) Protoplast isolation for species in the Chamelaucium group and the effect of antioxidant enzymes (superoxide dismutase and catalase) on protoplast viability. In Vitro Cell Dev Biol – Plant 49:593–598
Rezazadeh R, Niedz RP (2015) Protoplast isolation and plant regeneration of guava (Psidium guajava L.) using experiments in mixture-amount design. Plant Cell Tissue Organ Cult 122:585–604
Rose RJ (1980) Factors that influence the yield, stability in culture and cell wall regeneration of spinach mesophyll protoplasts. Aust J Plant Physiol 7:713–725
Shrestha BR, Tokuhara K, Mii M (2007) Plant regeneration from cell suspension-derived protoplasts of Phalaenopsis. Plant Cell Rep 26:719–725
Su V, Hsu BD (2003) Cloning and expression of a putative cytochrome P450 gene that influences the colour of Phalaenopsis flowers. Biotechnol Lett 25:1933–1939
Sun S, Furtula V, Nothnagel EA (1992) Mechanical release and lectin labeling of maize root protoplasts. Protoplasma 169:49–56
Xu J, Li J, Cui L, Zhang T, Wu Z, Zhu P-Y, Meng Y-J, Zhang K-J, Yu X-Q, Lou Q-F, Chen J-F (2017) New insights into the roles of cucumber TIR1 homologs and miR393 in regulating fruit/seed set development and leaf morphogenesis. BMC Plant Biol 17:130
Yao L, Liao X, Gan Z, Peng X, Wang P, Li S, Li T (2016) Protoplast isolation and development of a transient expression system for sweet cherry (Prunus avium L.) Sci Hortic 209:14–21
Yoo S-D, Cho Y-H, Sheen J (2007) Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nat Protoc 2:1565–1572
Zhang Y, Su J, Duan S, Ao Y, Dai J, Liu J, Wang P, Li Y, Liu B, Feng D, Wang J, Wang H (2011) A highly efficient rice green tissue protoplast system for transient gene expression and studying light/chloroplast-related processes. Plant Methods 7:30
Zhao W, Yang W, Wei C, Sun G (2011) A simple and efficient method for isolation of pineapple protoplasts. Biotechnol Biotechnol Equip 25:2464–2467
Zhou B, Nie Y-Z, Zhang X-L, Li Y-H (2008) Protoplast isolation of Brassica rapa ‘Tsuda’ turnip and transient expression of GFP. Lett Biotechnol 19:542–544 (in Chinese)
Acknowledgments
The authors would like to thank Drs. Ji Li and Jian Xu of Nanjing Agricultural University for providing vectors and help with transient expression experiments.
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 31372101).
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: Mark Jordan
Rights and permissions
About this article
Cite this article
Li, J., Liao, X., Zhou, S. et al. Efficient protoplast isolation and transient gene expression system for Phalaenopsis hybrid cultivar ‘Ruili Beauty’. In Vitro Cell.Dev.Biol.-Plant 54, 87–93 (2018). https://doi.org/10.1007/s11627-017-9872-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-017-9872-z