Abstract
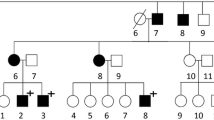
Familial neurohypophyseal diabetes insipidus (FNDI) is mostly an autosomal dominant inherited disorder presenting with severe polydipsia and polyuria typically in early childhood. To date, 69 different variations in the AVP gene encoding the AVP prohormone have been identified in autosomal dominant FNDI (adFNDI). In this study we present a family of seven generations, in which a novel variation in the AVP gene seems to cause adFNDI. Clinical assessment by 24 h urine collection, water deprivation test, desmopressin (dDAVP) challenge, and magnetic resonance imaging (MRI) of the posterior pituitary are presented. The diagnosis of adFNDI was confirmed by direct DNA sequence analysis of the AVP gene. Inheritance pattern and clinical history clearly pointed towards adFNDI. Inability of concentrating urine upon dehydration was demonstrated by a water deprivation test, and neurohypophyseal diabetes insipidus was strongly suspected after dDAVP administration, during which renal concentration ability quadrupled. MRI revealed a very weak pituitary “bright spot” in each of six subjects and a further reduction in the size of the neurohypophysis in a 7-year follow-up MRI scan in one subject. DNA sequence analysis revealed heterozygousity for a novel g.1785T > C gene variation predicting a p.Leu63Pro substitution in four affected subjects. Genetic testing in the diagnostic evaluation of families in which diabetes insipidus segregates is highly recommended in that interpretation of clinical assessments can be difficult. Furthermore, presymptomatic diagnosis can ease the parental concern of the carrier status of their offspring, and also avoid unnecessary surveillance of those being unaffected.



Similar content being viewed by others
References
Christensen JH et al (2004) Differential cellular handling of defective arginine vasopressin (AVP) prohormones in cells expressing mutations of the AVP gene associated with autosomal dominant and recessive familial neurohypophyseal diabetes insipidus. J Clin Endocrinol Metab 89(9):4521–4531
Robertson GL, Shelton RL, Athar S (1976) The osmoregulation of vasopressin. Kidney Int 10(1):25–37
Christensen JH, Rittig S (2006) Familial neurohypophyseal diabetes insipidus—an update. Semin Nephrol 26(3):209–223
Christensen JH et al (2004) Impaired trafficking of mutated AVP prohormone in cells expressing rare disease genes causing autosomal dominant familial neurohypophyseal diabetes insipidus. Clin Endocrinol (Oxf) 60(1):125–136
Siggaard C et al (2005) Expression of three different mutations in the arginine vasopressin gene suggests genotype-phenotype correlation in familial neurohypophyseal diabetes insipidus kindreds. Clin Endocrinol (Oxf) 63(2):207–216
Olias G, Richter D, Schmale H (1996) Heterologous expression of human vasopressin-neurophysin precursors in a pituitary cell line: defective transport of a mutant protein from patients with familial diabetes insipidus. DNA Cell Biol 15(11):929–935
Nijenhuis M, Zalm R, Burbach JP (1999) Mutations in the vasopressin prohormone involved in diabetes insipidus impair endoplasmic reticulum export but not sorting. J Biol Chem 274(30):21200–21208
Birk J et al (2009) Dominant pro-vasopressin mutants that cause diabetes insipidus form disulfide-linked fibrillar aggregates in the endoplasmic reticulum. J Cell Sci 122(Pt 21):3994–4002
Kubota T et al (2001) Hyperintensity of posterior pituitary on MR T1WI in a boy with central diabetes insipidus caused by missense mutation of neurophysin II gene. Endocr J 48(4):459–463
Miyamoto S, Sasaki N, Tanabe Y (1991) Magnetic resonance imaging in familial central diabetes insipidus. Neuroradiology 33(3):272–273
Mahoney CP et al (2002) Effects of aging on vasopressin production in a kindred with autosomal dominant neurohypophyseal diabetes insipidus due to the DeltaE47 neurophysin mutation. J Clin Endocrinol Metab 87(2):870–876
Braverman LE, Mancini JP, McGoldrick DM (1965) Hereditary idiopathic diabetes insipidus. A case report with autopsy findings. Ann Int Med 63:503–508
Bergeron C et al (1991) Hereditary diabetes insipidus: an immunohistochemical study of the hypothalamus and pituitary gland. Acta Neuropathol 81(3):345–348
Hayashi M et al (2009) Progressive polyuria without vasopressin neuron loss in a mouse model for familial neurohypophysial diabetes insipidus. Am J Physiol Regul Integr Comp Physiol 296(5):R1641–R1649
Si-Hoe SL et al (2000) Endoplasmic reticulum derangement in hypothalamic neurons of rats expressing a familial neurohypophyseal diabetes insipidus mutant vasopressin transgene. FASEB J 14(12):1680–1684
Morishita Y et al (2011) Poly(A) tail length of neurohypophysial hormones is shortened under endoplasmic reticulum stress. Endocrinology 152(12):4846–4855
Rittig S et al (1996) Identification of 13 new mutations in the vasopressin-neurophysin II gene in 17 kindreds with familial autosomal dominant neurohypophyseal diabetes insipidus. Am J Hum Genet 58(1):107–117
Baylis PH, Cheetham T (1998) Diabetes insipidus. Arch Dis Child 79(1):84–89
Robertson GL (1995) Diabetes insipidus. Endocrinol Metab Clin North Am 24(3):549–572
Zerbe RL, Robertson GL (1981) A comparison of plasma vasopressin measurements with a standard indirect test in the differential diagnosis of polyuria. N Engl J Med 305(26):1539–1546
Ito M et al (1991) A single base substitution in the coding region for neurophysin II associated with familial central diabetes insipidus. J Clin Invest 87(2):725–728
Bullmann C et al (2002) Identification of a novel mutation in the arginine vasopressin-neurophysin II gene in familial central diabetes insipidus. Exp Clin Endocrinol Diabetes 110(3):134–137
Rittig S et al (2002) Autosomal dominant neurohypophyseal diabetes insipidus due to substitution of histidine for tyrosine(2) in the vasopressin moiety of the hormone precursor. J Clin Endocrinol Metab 87(7):3351–3355
Santiprabhob J, Browning J, Repaske D (2002) A missense mutation encoding Cys73Phe in neurophysin II is associated with autosomal dominant neurohypophyseal diabetes insipidus. Mol Genet Metab 77(1–2):112–118
van den Akker EL et al (2000) Identification of a new mutation (CysII6Gly) in a family with neurogenic diabetes insipidus. Ned Tijdschr Geneeskd 144(20):941–945
Kanemitsu N et al (2002) Familial central diabetes insipidus detected by nocturnal enuresis. Pediatr Nephrol 17(12):1063–1065
Rutishauser J et al (2002) Clinical and molecular analysis of three families with autosomal dominant neurohypophyseal diabetes insipidus associated with a novel and recurrent mutations in the vasopressin-neurophysin II gene. Eur J Endocrinol 146(5):649–656
Boson WL et al (2003) A signal peptide mutation of the arginine vasopressin gene in monozygotic twins. Clin Endocrinol (Oxf) 58(1):108–110
Chen LQ et al (1991) Crystal structure of a bovine neurophysin II dipeptide complex at 2.8 A determined from the single-wavelength anomalous scattering signal of an incorporated iodine atom. Proc Natl Acad Sci USA 88(10):4240–4244
Rose JP et al (1996) Crystal structure of the neurophysin-oxytocin complex. Nat Struct Biol 3(2):163–169
Sitia R, Braakman I (2003) Quality control in the endoplasmic reticulum protein factory. Nature 426(6968):891–894
Lee MC et al (2004) Bi-directional protein transport between the ER and Golgi. Annu Rev Cell Dev Biol 20:87–123
Acknowledgments
We are indebted to the members of the family for their participation. We thank Jane H. Knudsen and Margrethe Kjeldsen for their skilled laboratory assistance. This work was supported by the Novo Nordisk Foundation and the Health Research Fund of Central Denmark Region.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Birkegaard, C., Christensen, J.H., Falorni, A. et al. A novel variation in the AVP gene resulting in familial neurohypophyseal diabetes insipidus in a large Italian kindred. Pituitary 16, 152–157 (2013). https://doi.org/10.1007/s11102-012-0392-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11102-012-0392-x