No Heading
Purpose.
To characterize the pharmacokinetic/pharmacodynamic (PK/PD) properties of a new polyethylene glycol (PEG) conjugate formulation of interferon (IFN)-β 1a following subcutaneous (SC) administration in monkeys.
Methods.
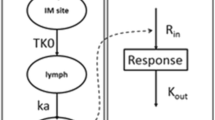
Single SC injections of 0.3, 1, and 3 million international units (MIU)/kg of PEG-IFN-β 1a were administered to 3 groups of cynomolgus monkeys (n = 4 each). Plasma concentrations of drug and neopterin, a classic biomarker for IFN-β PD, were measured at various time-points after dosing. PK/PD profiles were described by noncompartmental methods and pooled data by an integrated mathematical model, where fixed and delayed concentration-time profiles were used as driving functions in an indirect stimulatory response model.
Results.
PEG-IFN-β 1a was rapidly absorbed, with peak concentrations observed at about 4–5 h. Compared to previous identical SC doses of IFN-β 1a, administration of 1 and 3 MIU/kg of pegylated drug resulted in 27- and 16-fold increases in area under the concentration-time curves. Neopterin concentrations followed a typical dose-dependent biphasic pattern. Pooled PD profiles were well-described by the PK/PD model, and the neopterin elimination rate (0.0190 h−1) is consistent with previous estimates.
Conclusions.
The PEG-modification of IFN-β 1a provides enhanced drug exposure and similar pharmacodynamics of neopterin compared to the unmodified formulation.
Similar content being viewed by others
Abbreviations
- Ap:
-
amount of drug in central compartment
- Asc:
-
amount of drug in SC administration site
- At:
-
amount of drug in non-specific binding compartment
- k′:
-
first-order rate constant of drug absorption to and elimination from central compartment
- k12:
-
first-order rate constant of drug distribution from central to nonspecific binding compartment
- A21:
-
first-order rate constant of drug distribution from nonspecific binding to central compartment
- kin:
-
zero-order rate constant of neopterin production
- kout:
-
first-order rate constant of neopterin elimination
- N:
-
neopterin plasma concentration
- N0:
-
baseline or time-zero neopterin concentration
- SC50:
-
drug concentration producing 50% of Smax
- Smax:
-
capacity factor for drug stimulation of kin
- τ:
-
pharmacodynamic time-lag
- V/F:
-
volume of distribution of PEG-IFN-β 1a corrected for bioavailability
References
1. Y. Sugiyama and M. Hanano. Receptor-mediated transport of peptide hormones and its importance in the overall hormone disposition in the body. Pharm. Res. 6:192–202 (1989).
2. J. M. Harris and R. B. Chess. Effect of pegylation on pharmaceuticals. Nat. Rev. Drug Discov. 2:214–221 (2003).
3. R. Mehvar. Modulation of the pharmacokinetics and pharmacodynamics of proteins by polyethylene glycol conjugation. J. Pharm. Pharm. Sci. 3:125–136 (2000).
4. R. B. Pepinsky, D. J. LePage, A. Gill, A. Chakraborty, S. Vaidyanathan, M. Green, D. P. Baker, E. Whalley, P. S. Hochman, and P. Martin. Improved pharmacokinetic properties of a polyethylene glycol-modified form of interferon-beta-1a with preserved in vitro bioactivity. J. Pharmacol. Exp. Ther. 297:1059–1066 (2001).
5. N. L. Dayneka, V. Garg, and W. J. Jusko. Comparison of four basic models of indirect pharmacodynamic responses. J. Pharmacokinet. Biopharm. 21:457–478 (1993).
6. D. Z. D’Argenio and A. Schumitzky. ADAPT II User’s Guide, Biomedical Simulations Resource, Los Angeles, 1997.
7. J. Gabrielsson and D. Weiner. Pharmacokinetic and Pharmacodynamic Data Analysis: Concepts and Applications, Swedish Pharmaceutical Press, Stockholm, 1997.
8. D. E. Mager, B. Neuteboom, C. Efthymiopoulos, A. Munafo, and W. J. Jusko. Receptor-mediated pharmacokinetics and pharmacodynamics of interferon-β 1a in monkeys. J. Pharmacol. Exp. Ther. 306:262–270 (2003).
9. G. Levy. Pharmacologic target-mediated drug disposition. Clin. Pharmacol. Ther. 56:248–252 (1994).
10. K. Nieforth, R. Nadeau, I. Patel, and D. Mould. Use of an indirect pharmacodynamic stimulation model of MX protein induction to compare in vivo activity of interferon alfa-2a and a polyethylene glycol-modified derivative in healthy subjects. Clin. Pharmacol. Ther. 59:636–646 (1996).
11. K. A. Powers, N. M. Dixit, R. M. Ribeiro, P. Golia, A. H. Talal, and A. S. Perelson. Modeling viral and drug kinetics: hepatitis C virus treatment with pegylated interferon alfa-2b. Semin. Liver Dis. 23:13–18 (2003).
12. D. E. Mager and W. J. Jusko. Pharmacodynamic modeling of time-dependent transduction systems. Clin. Pharmacol. Ther. 70:210–216 (2001).
13. P. Glue, J. W. Fang, R. Rouzier-Panis, C. Raffanel, R. Sabo, S. K. Gupta, M. Salfi, and S. Jacobs. Pegylated interferon-alpha2b: pharmacokinetics, pharmacodynamics, safety, and preliminary efficacy data. Hepatitis C Intervention Therapy Group. Clin. Pharmacol. Ther. 68:556–567 (2000).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mager, D., Neuteboom, B. & Jusko, W. Pharmacokinetics and Pharmacodynamics of PEGylated IFN-β 1a Following Subcutaneous Administration in Monkeys. Pharm Res 22, 58–61 (2005). https://doi.org/10.1007/s11095-004-9009-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-004-9009-z