Abstract
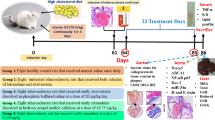
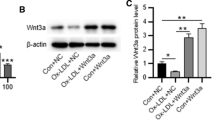
The purpose of this study was to investigate the antiatherosclerosis effects of ursolic acid (UA) in high-fat diet-fed quails (Coturnix coturnix) and potential mechanism. Quails were treated with high-fat diet (14 % pork oil, 1 % cholesterol w/w) with or without UA (50, 150, or 300 mg/kg/day) for 10 weeks. Serum lipid profile was assessed at 0, 4.5, and 10 weeks. After 10 weeks, serum antioxidant status and morphology of aorta were assessed. Additionally, human umbilical vein endothelial cells (HUVECs) were exposed to 100 μg/ml oxidized low-density lipoprotein (ox-LDL) for 24 h, with or without pretreatment with UA (5, 10 or 20 μM) for 16 h, autophagy inhibitor 3-MA 5 mM for 2 h, or SIRT1 inhibitor EX-527 10 μM for 2 h. Cell viability and oxidative stress status were assessed and autophagy status was determined. Acetylation of lysine residue on Atg5 was assessed with immunoprecipitation. In results, high-fat diet negatively affected serum lipid profile and antioxidant status in quails and induced significant histological changes. Cotreatment with UA remarkably alleviated such changes. In HUVECs, ox-LDL treatment induced significant cytotoxicity along with oxidative stress, while UA cotreatment alleviated such changes significantly. UA treatment induced autophagy, enhanced SIRT1 expression, and decreased acetylation of lysine residue on Atg5. Cotreatment with 3-MA or EX-527 effectively abolished UA’s protective effects. In summary, UA exerted antiatherosclerosis effects in quails and protected HUVECs from ox-LDL induced cytotoxicity, and the mechanism is associated with increased SIRT1 expression, decreased Atg5 acetylation on lysine residue, and increased autophagy.








Similar content being viewed by others
References
Sirimarco G, Amarenco P, Labreuche J, Touboul PJ, Alberts M, Goto S, Rother J, Mas JL, Bhatt DL, Steg PG, Investigators RR (2013) Carotid atherosclerosis and risk of subsequent coronary event in outpatients with atherothrombosis. Stroke 44:373–379. doi:10.1161/STROKEAHA.112.673129
Ding L, Biswas S, Morton RE, Smith JD, Hay N, Byzova TV, Febbraio M, Podrez EA (2012) Akt3 deficiency in macrophages promotes foam cell formation and atherosclerosis in mice. Cell Metab 15:861–872. doi:10.1016/j.cmet.2012.04.020
Goff DC Jr, Bertoni AG, Kramer H, Bonds D, Blumenthal RS, Tsai MY, Psaty BM (2006) Dyslipidemia prevalence, treatment, and control in the multi-ethnic study of atherosclerosis (MESA): gender, ethnicity, and coronary artery calcium. Circulation 113:647–656. doi:10.1161/CIRCULATIONAHA.105.552737
Lira FS, Rosa Neto JC, Antunes BM, Fernandes RA (2014) The relationship between inflammation, dyslipidemia and physical exercise: from the epidemiological to molecular approach. Curr Diabetes Rev 10:391–396
Kapur NK (2007) Rosuvastatin: a highly potent statin for the prevention and management of coronary artery disease. Expert Rev Cardiovasc Ther 5:161–175. doi:10.1586/14779072.5.2.161
Kones R, Rumana U (2015) Current treatment of dyslipidemia: a new paradigm for statin drug use and the need for additional therapies. Drugs 75:1187–1199. doi:10.1007/s40265-015-0428-4
Laufs U, Scharnagl H, Marz W (2015) Statin intolerance. Curr Opin Lipidol 26:492–501. doi:10.1097/MOL.0000000000000236
Xia EQ, Wang BW, Xu XR, Zhu L, Song Y, Li HB (2011) Microwave-assisted extraction of oleanolic acid and ursolic acid from Ligustrum lucidum Ait. Int J Mol Sci 12:5319–5329. doi:10.3390/ijms12085319
Lee JY, Moon H, Kim CJ (2010) Effects of hydroxy pentacyclic triterpene acids from Forsythia viridissima on asthmatic responses to ovalbumin challenge in conscious guinea pigs. Biol Pharm Bull 33:230–237
Zhang T, Su J, Wang K, Zhu T, Li X (2014) Ursolic acid reduces oxidative stress to alleviate early brain injury following experimental subarachnoid hemorrhage. Neurosci Lett 579:12–17. doi:10.1016/j.neulet.2014.07.005
Saraswati S, Agrawal SS, Alhaider AA (2013) Ursolic acid inhibits tumor angiogenesis and induces apoptosis through mitochondrial-dependent pathway in Ehrlich ascites carcinoma tumor. Chem Biol Interact 206:153–165. doi:10.1016/j.cbi.2013.09.004
Ma JQ, Ding J, Xiao ZH, Liu CM (2014) Ursolic acid ameliorates carbon tetrachloride-induced oxidative DNA damage and inflammation in mouse kidney by inhibiting the STAT3 and NF-kappaB activities. Int Immunopharmacol 21:389–395. doi:10.1016/j.intimp.2014.05.022
Jayaprakasam B, Olson LK, Schutzki RE, Tai MH, Nair MG (2006) Amelioration of obesity and glucose intolerance in high-fat-fed C57BL/6 mice by anthocyanins and ursolic acid in Cornelian cherry (Cornus mas). J Agric Food Chem 54:243–248. doi:10.1021/jf0520342
Ullevig SL, Zhao Q, Zamora D, Asmis R (2011) Ursolic acid protects diabetic mice against monocyte dysfunction and accelerated atherosclerosis. Atherosclerosis 219:409–416. doi:10.1016/j.atherosclerosis.2011.06.013
Messner B, Zeller I, Ploner C, Frotschnig S, Ringer T, Steinacher-Nigisch A, Ritsch A, Laufer G, Huck C, Bernhard D (2011) Ursolic acid causes DNA-damage, p53-mediated, mitochondria- and caspase-dependent human endothelial cell apoptosis, and accelerates atherosclerotic plaque formation in vivo. Atherosclerosis 219:402–408. doi:10.1016/j.atherosclerosis.2011.05.025
Wang GF, Shi CG, Sun MZ, Wang L, Wu SX, Wang HF, Xu ZQ, Chen DM (2013) Tetramethylpyrazine attenuates atherosclerosis development and protects endothelial cells from ox-LDL. Cardiovasc Drugs Ther 27:199–210. doi:10.1007/s10557-013-6440-6
Kao ES, Tseng TH, Lee HJ, Chan KC, Wang CJ (2009) Anthocyanin extracted from Hibiscus attenuate oxidized LDL-mediated foam cell formation involving regulation of CD36 gene. Chem Biol Interact 179:212–218
Zhang Y, Xie Y, You S, Han Q, Cao Y, Zhang X, Xiao G, Chen R, Liu C (2015) Autophagy and apoptosis in the response of human vascular endothelial cells to oxidized low-density lipoprotein. Cardiology 132:27–33. doi:10.1159/000381332
Razani B, Feng C, Coleman T, Emanuel R, Wen H, Hwang S, Ting JP, Virgin HW, Kastan MB, Semenkovich CF (2012) Autophagy links inflammasomes to atherosclerotic progression. Cell Metab 15:534–544. doi:10.1016/j.cmet.2012.02.011
Schrijvers DM, De Meyer GR, Martinet W (2011) Autophagy in atherosclerosis: a potential drug target for plaque stabilization. Arterioscler Thromb Vasc Biol 31:2787–2791. doi:10.1161/ATVBAHA.111.224899
Chen ML, Yi L, Jin X, Liang XY, Zhou Y, Zhang T, Xie Q, Zhou X, Chang H, Fu YJ, Zhu JD, Zhang QY, Mi MT (2013) Resveratrol attenuates vascular endothelial inflammation by inducing autophagy through the cAMP signaling pathway. Autophagy 9:2033–2045. doi:10.4161/auto.26336
Lee IH, Cao L, Mostoslavsky R, Lombard DB, Liu J, Bruns NE, Tsokos M, Alt FW, Finkel T (2008) A role for the NAD-dependent deacetylase Sirt1 in the regulation of autophagy. Proc Natl Acad Sci USA 105:3374–3379. doi:10.1073/pnas.0712145105
Wang X, Jiang Q, Wang W, Su L, Han Y, Wang C (2014) Molecular mechanism of polypeptides from Chlamys farreri (PCF)’s anti-apoptotic effect in UVA-exposed HaCaT cells involves HSF1/HSP70, JNK, XO, iNOS and NO/ROS. J Photochem Photobiol B 130:47–56. doi:10.1016/j.jphotobiol.2013.11.005
He SY, Qian ZY, Tang FT, Wen N, Xu GL, Sheng L (2005) Effect of crocin on experimental atherosclerosis in quails and its mechanisms. Life Sci 77:907–921. doi:10.1016/j.lfs.2005.02.006
Jia Y, Bhuiyan MJ, Jun HJ, Lee JH, Hoang MH, Lee HJ, Kim N, Lee D, Hwang KY, Hwang BY, Choi DW, Lee SJ (2011) Ursolic acid is a PPAR-alpha agonist that regulates hepatic lipid metabolism. Bioorg Med Chem Lett 21:5876–5880. doi:10.1016/j.bmcl.2011.07.095
Kim J, Jang DS, Kim H, Kim JS (2009) Anti-lipase and lipolytic activities of ursolic acid isolated from the roots of Actinidia arguta. Arch Pharm Res 32:983–987. doi:10.1007/s12272-009-1702-3
Shanmugasundaram R, Selvaraj RK (2011) Dietary lutein and fish oil interact to alter atherosclerotic lesions in a Japanese quail model of atherosclerosis. J Anim Physiol Anim Nutr (Berl) 95:762–770. doi:10.1111/j.1439-0396.2010.01106.x
Li X, Schulte P, Godin DV, Cheng KM (2012) Differential mRNA expression of seven genes involved in cholesterol metabolism and transport in the liver of atherosclerosis-susceptible and -resistant Japanese quail strains. Genet Sel Evol 44:20. doi:10.1186/1297-9686-44-20
Lee BS, Choi JY, Kim JY, Han SH, Park JE (2012) Simvastatin and losartan differentially and synergistically inhibit atherosclerosis in apolipoprotein e(−/−) mice. Korean Circ J 42:543–550. doi:10.4070/kcj.2012.42.8.543
Somova LO, Nadar A, Rammanan P, Shode FO (2003) Cardiovascular, antihyperlipidemic and antioxidant effects of oleanolic and ursolic acids in experimental hypertension. Phytomedicine 10:115–121. doi:10.1078/094471103321659807
Ma JQ, Ding J, Zhang L, Liu CM (2015) Protective effects of ursolic acid in an experimental model of liver fibrosis through Nrf2/ARE pathway. Clin Res Hepatol Gastroenterol 39:188–197. doi:10.1016/j.clinre.2014.09.007
Senaphan K, Kukongviriyapan U, Sangartit W, Pakdeechote P, Pannangpetch P, Prachaney P, Greenwald SE, Kukongviriyapan V (2015) Ferulic acid alleviates changes in a rat model of metabolic syndrome induced by high-carbohydrate, high-fat diet. Nutrients 7:6446–6464. doi:10.3390/nu7085283
Harris LK, Mann GE, Ruiz E, Mushtaq S, Leake DS (2006) Ascorbate does not protect macrophages against apoptosis induced by oxidised low density lipoprotein. Arch Biochem Biophys 455:68–76. doi:10.1016/j.abb.2006.07.019
Fraley AE, Tsimikas S (2006) Clinical applications of circulating oxidized low-density lipoprotein biomarkers in cardiovascular disease. Curr Opin Lipidol 17:502–509. doi:10.1097/01.mol.0000245255.40634.b5
Liu YX, Han GZ, Wu T, Liu P, Zhou Q, Liu KX, Sun HJ (2011) Protective effect of alpha-lipoic acid on oxidized low density lipoprotein-induced human umbilical vein endothelial cell injury. Pharmacol Rep 63:1180–1188
Jiang YR, Miao Y, Yang L, Xue M, Guo CY, Ma XJ, Yin HJ, Shi DZ, Chen KJ (2012) Effect of chinese herbal drug-containing serum for activating-blood and dispelling-toxin on ox-LDL-induced inflammatory factors’ expression in endothelial cells. Chin J Integr Med 18:30–33. doi:10.1007/s11655-011-0849-1
Yu W, Ying H, Tong F, Zhang C, Quan Y, Zhang Y (2013) Protective effect of the silkworm protein 30Kc6 on human vascular endothelial cells damaged by oxidized low density lipoprotein (Ox-LDL). PLoS ONE 8:e68746. doi:10.1371/journal.pone.0068746
Holvoet P, Kritchevsky SB, Tracy RP, Mertens A, Rubin SM, Butler J, Goodpaster B, Harris TB (2004) The metabolic syndrome, circulating oxidized LDL, and risk of myocardial infarction in well-functioning elderly people in the health, aging, and body composition cohort. Diabetes 53:1068–1073
Toyama T, Wada-Takahashi S, Takamichi M, Watanabe K, Yoshida A, Yoshino F, Miyamoto C, Maehata Y, Sugiyama S, Takahashi SS, Todoki K, Lee MC, Hamada N (2014) Reactive oxygen species scavenging activity of Jixueteng evaluated by electron spin resonance (ESR) and photon emission. Nat Prod Commun 9:1755–1759
Wen T, He W, Chong Y, Liu Y, Yin JJ, Wu X (2015) Exploring environment-dependent effects of Pd nanostructures on reactive oxygen species (ROS) using electron spin resonance (ESR) technique: implications for biomedical applications. Phys Chem Chem Phys 17:24937–24943. doi:10.1039/c5cp04046a
Leng S, Hao Y, Du D, Xie S, Hong L, Gu H, Zhu X, Zhang J, Fan D, Kung HF (2013) Ursolic acid promotes cancer cell death by inducing Atg5-dependent autophagy. Int J Cancer 133:2781–2790. doi:10.1002/ijc.28301
Shen S, Zhang Y, Zhang R, Tu X, Gong X (2014) Ursolic acid induces autophagy in U87MG cells via ROS-dependent endoplasmic reticulum stress. Chem Biol Interact 218:28–41. doi:10.1016/j.cbi.2014.04.017
Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, Tran H, Ross SE, Mostoslavsky R, Cohen HY, Hu LS, Cheng HL, Jedrychowski MP, Gygi SP, Sinclair DA, Alt FW, Greenberg ME (2004) Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science 303:2011–2015. doi:10.1126/science.1094637
Houtkooper RH, Pirinen E, Auwerx J (2012) Sirtuins as regulators of metabolism and healthspan. Nat Rev Mol Cell Biol 13:225–238. doi:10.1038/nrm3293
Yang L, Zhang J, Yan C, Zhou J, Lin R, Lin Q, Wang W, Zhang K, Yang G, Bian X, Zeng A (2012) SIRT1 regulates CD40 expression induced by TNF-alpha via NF-kB pathway in endothelial cells. Cell Physiol Biochem 30:1287–1298. doi:10.1159/000343318
Ota H, Eto M, Ogawa S, Iijima K, Akishita M, Ouchi Y (2010) SIRT1/eNOS axis as a potential target against vascular senescence, dysfunction and atherosclerosis. J Atheroscler Thromb 17:431–435
Guo Y, Xu A, Wang Y (2015) SIRT1 in endothelial cells as a novel target for the prevention of early vascular ageing. J Cardiovasc Pharmacol. doi:10.1097/FJC.0000000000000344
Zhang QJ, Wang Z, Chen HZ, Zhou S, Zheng W, Liu G, Wei YS, Cai H, Liu DP, Liang CC (2008) Endothelium-specific overexpression of class III deacetylase SIRT1 decreases atherosclerosis in apolipoprotein E-deficient mice. Cardiovasc Res 80:191–199. doi:10.1093/cvr/cvn224
Acknowledgments
The authors thank the affiliated hospital of Qingdao University for providing necessary instruments. This study was funded by the National Natural Science Foundation of China (Grant No. 81173593), received by Chunbo Wang.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report no other conflict of interest.
Additional information
Qixiao Jiang and Ranran Hao contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
11010_2016_2787_MOESM1_ESM.tif
Quantification method for the aorta histology. The areas of endothelium and/or smooth muscle that are rough and/or bulge into the aorta lumen were marked with black lines and indicated with arrows. ImageJ was used to measure the marked area. Atherosclerotic changes were quantified by normalizing the area with the total aorta area. Supplementary material 1 (TIFF 3040 kb)
Rights and permissions
About this article
Cite this article
Jiang, Q., Hao, R., Wang, W. et al. SIRT1/Atg5/autophagy are involved in the antiatherosclerosis effects of ursolic acid. Mol Cell Biochem 420, 171–184 (2016). https://doi.org/10.1007/s11010-016-2787-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-016-2787-x