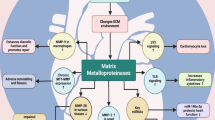
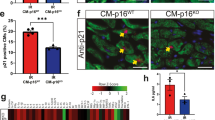
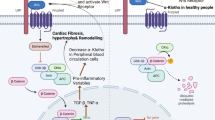
Abstract
Heart failure is strongly associated with aging. Elderly patients with heart failure often have preserved systolic function exhibiting left ventricular hypertrophy accompanied by a decline in diastolic function. Experimental studies have demonstrated that age-related cardiac fibrosis plays an important role in the pathogenesis of diastolic heart failure in senescent hearts. Reactive oxygen species and angiotensin II are critically involved in fibrotic remodeling of the aging ventricle; their fibrogenic actions may be mediated, at least in part, through transforming growth factor (TGF)-β. The increased prevalence of heart failure in the elderly is also due to impaired responses of the senescent heart to cardiac injury. Aging is associated with suppressed inflammation, delayed phagocytosis of dead cardiomyocytes, and markedly diminished collagen deposition following myocardial infarction, due to a blunted response of fibroblasts to fibrogenic growth factors. Thus, in addition to a baseline activation of fibrogenic pathways, senescent hearts exhibit an impaired reparative reserve due to decreased responses of mesenchymal cells to stimulatory signals. Impaired scar formation in senescent hearts is associated with accentuated dilative remodeling and worse systolic dysfunction. Understanding the pathogenesis of interstitial fibrosis in the aging heart and dissecting the mechanisms responsible for age-associated healing defects following cardiac injury are critical in order to design new strategies for prevention of adverse remodeling and heart failure in elderly patients.


Similar content being viewed by others
References
Chen MA (2009) Heart failure with preserved ejection fraction in older adults. Am J Med 122:713–723
Thomas S, Rich MW (2007) Epidemiology, pathophysiology, and prognosis of heart failure in the elderly. Clin Geriatr Med 23:1–10
DeFrances CJ, Cullen KA, Kozak LJ (2007) National hospital discharge survey: 2005 annual summary with detailed diagnosis and procedure data. Vital Health Stat 13:1–209
Lakatta EG, Levy D (2003) Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: part II: the aging heart in health: links to heart disease. Circulation 107:346–354
Kitzman DW, Gardin JM, Gottdiener JS, Arnold A, Boineau R, Aurigemma G, Marino EK, Lyles M, Cushman M, Enright PL (2001) Importance of heart failure with preserved systolic function in patients > or = 65 years of age. CHS research group. Cardiovascular health study. Am J Cardiol 87:413–419
Dannenberg AL, Levy D, Garrison RJ (1989) Impact of age on echocardiographic left ventricular mass in a healthy population (the Framingham Study). Am J Cardiol 64:1066–1068
Lakatta EG (2002) Age-associated cardiovascular changes in health: impact on cardiovascular disease in older persons. Heart Fail Rev 7:29–49
Lakatta EG, Wang M, Najjar SS (2009) Arterial aging and subclinical arterial disease are fundamentally intertwined at macroscopic and molecular levels. Med Clin North Am 93:583–604 Table of Contents
Lakatta EG, Levy D (2003) Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: part I: aging arteries: a “set up” for vascular disease. Circulation 107:139–146
Orlandi A, Marcellini M, Spagnoli LG (2000) Aging influences development and progression of early aortic atherosclerotic lesions in cholesterol-fed rabbits. Arterioscler Thromb Vasc Biol 20:1123–1136
Anversa P, Palackal T, Sonnenblick EH, Olivetti G, Meggs LG, Capasso JM (1990) Myocyte cell loss and myocyte cellular hyperplasia in the hypertrophied aging rat heart. Circ Res 67:871–885
Cheng W, Reiss K, Li P, Chun MJ, Kajstura J, Olivetti G, Anversa P (1996) Aging does not affect the activation of the myocyte insulin-like growth factor-1 autocrine system after infarction and ventricular failure in Fischer 344 rats. Circ Res 78:536–546
Burlew BS (2004) Diastolic dysfunction in the elderly–the interstitial issue. Am J Geriatr Cardiol 13:29–38
Cheitlin MD (2003) Cardiovascular physiology-changes with aging. Am J Geriatr Cardiol 12:9–13
Gazoti Debessa CR, Mesiano Maifrino LB, Rodrigues de Souza R (2001) Age related changes of the collagen network of the human heart. Mech Ageing Dev 122:1049–1058
de Souza RR (2002) Aging of myocardial collagen. Biogerontology 3:325–335
Eghbali M, Eghbali M, Robinson TF, Seifter S, Blumenfeld OO (1989) Collagen accumulation in heart ventricles as a function of growth and aging. Cardiovasc Res 23:723–729
Orlandi A, Francesconi A, Marcellini M, Ferlosio A, Spagnoli LG (2004) Role of ageing and coronary atherosclerosis in the development of cardiac fibrosis in the rabbit. Cardiovasc Res 64:544–552
Lin J, Lopez EF, Jin Y, Van Remmen H, Bauch T, Han HC, Lindsey ML (2008) Age-related cardiac muscle sarcopenia: combining experimental and mathematical modeling to identify mechanisms. Exp Gerontol 43:296–306
Thomas DP, Zimmerman SD, Hansen TR, Martin DT, McCormick RJ (2000) Collagen gene expression in rat left ventricle: interactive effect of age and exercise training. J Appl Physiol 89:1462–1468
Iwanaga Y, Aoyama T, Kihara Y, Onozawa Y, Yoneda T, Sasayama S (2002) Excessive activation of matrix metalloproteinases coincides with left ventricular remodeling during transition from hypertrophy to heart failure in hypertensive rats. J Am Coll Cardiol 39:1384–1391
Janicki JS, Brower GL (2002) The role of myocardial fibrillar collagen in ventricular remodeling and function. J Card Fail 8:S319–S325
Baicu CF, Stroud JD, Livesay VA, Hapke E, Holder J, Spinale FG, Zile MR (2003) Changes in extracellular collagen matrix alter myocardial systolic performance. Am J Physiol Heart Circ Physiol 284:H122–H132
Wang J, Hoshijima M, Lam J, Zhou Z, Jokiel A, Dalton ND, Hultenby K, Ruiz-Lozano P, Ross J Jr, Tryggvason K, Chien KR (2006) Cardiomyopathy associated with microcirculation dysfunction in laminin alpha4 chain-deficient mice. J Biol Chem 281:213–220
Beltrami CA, Finato N, Rocco M, Feruglio GA, Puricelli C, Cigola E, Quaini F, Sonnenblick EH, Olivetti G, Anversa P (1994) Structural basis of end-stage failure in ischemic cardiomyopathy in humans. Circulation 89:151–163
Khan R, Sheppard R (2006) Fibrosis in heart disease: understanding the role of transforming growth factor-beta in cardiomyopathy, valvular disease and arrhythmia. Immunology 118:10–24
Dai DF, Santana LF, Vermulst M, Tomazela DM, Emond MJ, MacCoss MJ, Gollahon K, Martin GM, Loeb LA, Ladiges WC, Rabinovitch PS (2009) Overexpression of catalase targeted to mitochondria attenuates murine cardiac aging. Circulation 119:2789–2797
Basso N, Cini R, Pietrelli A, Ferder L, Terragno NA, Inserra F (2007) Protective effect of long-term angiotensin II inhibition. Am J Physiol Heart Circ Physiol 293:H1351–H1358
Benigni A, Corna D, Zoja C, Sonzogni A, Latini R, Salio M, Conti S, Rottoli D, Longaretti L et al (2009) Disruption of the Ang II type 1 receptor promotes longevity in mice. J Clin Invest 119:524–530
Billet S, Bardin S, Verp S, Baudrie V, Michaud A, Conchon S, Muffat-Joly M, Escoubet B, Souil E et al (2007) Gain-of-function mutant of angiotensin II receptor, type 1A, causes hypertension and cardiovascular fibrosis in mice. J Clin Invest 117:1914–1925
Sawada M, Carlson JC (1987) Changes in superoxide radical and lipid peroxide formation in the brain, heart and liver during the lifetime of the rat. Mech Ageing Dev 41:125–137
Siwik DA, Pagano PJ, Colucci WS (2001) Oxidative stress regulates collagen synthesis and matrix metalloproteinase activity in cardiac fibroblasts. Am J Physiol Cell Physiol 280:C53–C60
Cheng TH, Cheng PY, Shih NL, Chen IB, Wang DL, Chen JJ (2003) Involvement of reactive oxygen species in angiotensin II-induced endothelin-1 gene expression in rat cardiac fibroblasts. J Am Coll Cardiol 42:1845–1854
Frangogiannis NG (2004) Chemokines in the ischemic myocardium: from inflammation to fibrosis. Inflamm Res 53:585–595
Frangogiannis NG (2007) Chemokines in ischemia and reperfusion. Thromb Haemost 97:738–747
Frangogiannis NG, Dewald O, Xia Y, Ren G, Haudek S, Leucker T, Kraemer D, Taffet G, Rollins BJ, Entman ML (2007) Critical role of monocyte chemoattractant protein-1/CC chemokine ligand 2 in the pathogenesis of ischemic cardiomyopathy. Circulation 115:584–592
Dewald O, Zymek P, Winkelmann K, Koerting A, Ren G, Abou-Khamis T, Michael LH, Rollins BJ, Entman ML, Frangogiannis NG (2005) CCL2/Monocyte chemoattractant protein-1 regulates inflammatory responses critical to healing myocardial infarcts. Circ Res 96:881–889
Desmouliere A, Geinoz A, Gabbiani F, Gabbiani G (1993) Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol 122:103–111
Bujak M, Frangogiannis NG (2007) The role of TGF-beta signaling in myocardial infarction and cardiac remodeling. Cardiovasc Res 74:184–195
Brooks WW, Conrad CH (2000) Myocardial fibrosis in transforming growth factor beta(1)heterozygous mice. J Mol Cell Cardiol 32:187–195
Barcellos-Hoff MH, Dix TA (1996) Redox-mediated activation of latent transforming growth factor-beta 1. Mol Endocrinol 10:1077–1083
Park SK, Kim J, Seomun Y, Choi J, Kim DH, Han IO, Lee EH, Chung SK, Joo CK (2001) Hydrogen peroxide is a novel inducer of connective tissue growth factor. Biochem Biophys Res Commun 284:966–971
Lee AA, Dillmann WH, McCulloch AD, Villarreal FJ (1995) Angiotensin II stimulates the autocrine production of transforming growth factor-beta 1 in adult rat cardiac fibroblasts. J Mol Cell Cardiol 27:2347–2357
Campbell SE, Katwa LC (1997) Angiotensin II stimulated expression of transforming growth factor-beta1 in cardiac fibroblasts and myofibroblasts. J Mol Cell Cardiol 29:1947–1958
Saito K, Ishizaka N, Aizawa T, Sata M, Iso-o N, Noiri E, Mori I, Ohno M, Nagai R (2005) Iron chelation and a free radical scavenger suppress angiotensin II-induced upregulation of TGF-beta1 in the heart. Am J Physiol Heart Circ Physiol 288:H1836–H1843
Rosenkranz S (2004) TGF-beta1 and angiotensin networking in cardiac remodeling. Cardiovasc Res 63:423–432
Asif M, Egan J, Vasan S, Jyothirmayi GN, Masurekar MR, Lopez S, Williams C, Torres RL, Wagle D et al (2000) An advanced glycation endproduct cross-link breaker can reverse age-related increases in myocardial stiffness. Proc Natl Acad Sci USA 97:2809–2813
Shapiro BP, Owan TE, Mohammed SF, Meyer DM, Mills LD, Schalkwijk CG, Redfield MM (2008) Advanced glycation end products accumulate in vascular smooth muscle and modify vascular but not ventricular properties in elderly hypertensive canines. Circulation 118:1002–1010
Maggioni AP, Maseri A, Fresco C, Franzosi MG, Mauri F, Santoro E, Tognoni G (1993) Age-related increase in mortality among patients with first myocardial infarctions treated with thrombolysis. The investigators of the Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico (GISSI-2). N Engl J Med 329:1442–1448
St John Sutton M, Pfeffer MA, Moye L, Plappert T, Rouleau JL, Lamas G, Rouleau J, Parker JO, Arnold MO, Sussex B, Braunwald E (1997) Cardiovascular death and left ventricular remodeling two years after myocardial infarction: baseline predictors and impact of long-term use of captopril: information from the survival and ventricular enlargement (SAVE) trial. Circulation 96:3294–3299
Frangogiannis NG (2008) The immune system and cardiac repair. Pharmacol Res 58:88–111
Bujak M, Dobaczewski M, Gonzalez-Quesada C, Xia Y, Leucker T, Zymek P, Veeranna V, Tager AM, Luster AD, Frangogiannis NG (2009) Induction of the CXC chemokine interferon-gamma-inducible protein 10 regulates the reparative response following myocardial infarction. Circ Res 105:973–983
Dobaczewski M, Gonzalez-Quesada C, Frangogiannis NG (2009) The extracellular matrix as a modulator of the inflammatory and reparative response following myocardial infarction. J Mol Cell Cardiol 48(3):504–511
Jugdutt BI (2003) Ventricular remodeling after infarction and the extracellular collagen matrix: when is enough enough? Circulation 108:1395–1403
Frangogiannis NG (2006) The mechanistic basis of infarct healing. Antioxid Redox Signal 8:1907–1939
Frangogiannis NG, Ren G, Dewald O, Zymek P, Haudek S, Koerting A, Winkelmann K, Michael LH, Lawler J, Entman ML (2005) The critical role of endogenous Thrombospondin (TSP)-1 in preventing expansion of healing myocardial infarcts. Circulation 111:2935–2942
Cohn JN, Ferrari R, Sharpe N (2000) Cardiac remodeling–concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. Behalf of an international forum on cardiac remodeling. J Am Coll Cardiol 35:569–582
Bujak M, Kweon HJ, Chatila K, Li N, Taffet G, Frangogiannis NG (2008) Aging-related defects are associated with adverse cardiac remodeling in a mouse model of reperfused myocardial infarction. J Am Coll Cardiol 51:1384–1392
Swift ME, Burns AL, Gray KL, DiPietro LA (2001) Age-related alterations in the inflammatory response to dermal injury. J Invest Dermatol 117:1027–1035
Ding A, Hwang S, Schwab R (1994) Effect of aging on murine macrophages. Diminished response to IFN-gamma for enhanced oxidative metabolism. J Immunol 153:2146–2152
Flanders KC (2004) Smad3 as a mediator of the fibrotic response. Int J Exp Pathol 85:47–64
Bujak M, Ren G, Kweon HJ, Dobaczewski M, Reddy A, Taffet G, Wang XF, Frangogiannis NG (2007) Essential role of Smad3 in infarct healing and in the pathogenesis of cardiac remodeling. Circulation 116:2127–2138
Shivakumar K, Dostal DE, Boheler K, Baker KM, Lakatta EG (2003) Differential response of cardiac fibroblasts from young adult and senescent rats to ANG II. Am J Physiol Heart Circ Physiol 284:H1454–H1459
Wynn TA (2008) Cellular and molecular mechanisms of fibrosis. J Pathol 214:199–210
Jugdutt BI, Jelani A (2008) Aging and defective healing, adverse remodeling, and blunted post-conditioning in the reperfused wounded heart. J Am Coll Cardiol 51:1399–1403
Jugdutt BI (2008) Pleiotropic effects of cardiac drugs on healing post-MI. The good, bad, and ugly. Heart Fail Rev 13:439–452
Simpson PJ, Todd RF 3rd, Fantone JC, Mickelson JK, Griffin JD, Lucchesi BR (1988) Reduction of experimental canine myocardial reperfusion injury by a monoclonal antibody (anti-Mo1, anti-CD11b) that inhibits leukocyte adhesion. J Clin Invest 81:624–629
Frangogiannis NG (2006) Targeting the inflammatory response in healing myocardial infarcts. Curr Med Chem 13:1877–1893
Baran KW, Nguyen M, McKendall GR, Lambrew CT, Dykstra G, Palmeri ST, Gibbons RJ, Borzak S, Sobel BE, et al. (2001) Double-blind, randomized trial of an anti-CD18 antibody in conjunction with recombinant tissue plasminogen activator for acute myocardial infarction: limitation of myocardial infarction following thrombolysis in acute myocardial infarction (LIMIT AMI) study. Circulation 104:2778–2783
Cai D, Xaymardan M, Holm JM, Zheng J, Kizer JR, Edelberg JM (2003) Age-associated impairment in TNF-alpha cardioprotection from myocardial infarction. Am J Physiol Heart Circ Physiol 285:H463–H469
Xaymardan M, Zheng J, Duignan I, Chin A, Holm JM, Ballard VL, Edelberg JM (2004) Senescent impairment in synergistic cytokine pathways that provide rapid cardioprotection in the rat heart. J Exp Med 199:797–804
Lehrke S, Mazhari R, Durand DJ, Zheng M, Bedja D, Zimmet JM, Schuleri KH, Chi AS, Gabrielson KL, Hare JM (2006) Aging impairs the beneficial effect of granulocyte colony-stimulating factor and stem cell factor on post-myocardial infarction remodeling. Circ Res 99:553–560
Davis ME, Hsieh PC, Grodzinsky AJ, Lee RT (2005) Custom design of the cardiac microenvironment with biomaterials. Circ Res 97:8–15
Acknowledgments
Dr Frangogiannis’ laboratory is supported by NIH R01 HL-76246 and R01 HL-85440, the Alkek endowment and the Medallion Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, W., Frangogiannis, N.G. The role of inflammatory and fibrogenic pathways in heart failure associated with aging. Heart Fail Rev 15, 415–422 (2010). https://doi.org/10.1007/s10741-010-9161-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-010-9161-y