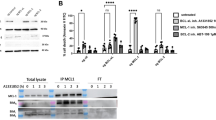
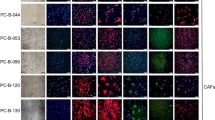
Summary
Cancer associated fibroblasts (CAFs) are the most abundant components of cancer-microenvironment. They play important roles in cancer initiation, progression, and metastasis. In addition, CAFs can confer drug-resistance to cancer cells. Considering their pro-tumorigenic roles, it is recommended to remove CAFs to prevent cancer recurrence after chemotherapy. Despite their clinical significance, few anti-CAF drugs have been developed. The objective of this study was to find a drug that could suppress the viability of patient-derived CAFs through repurposed screening of 51 drugs that were in clinical trials or received FDA approval. As a result, bortezomib (BTZ), carfilzomib (CFZ), and panobinostat (PST) were identified as anti-CAF drug candidates. It was confirmed that BTZ and PST could decrease the viability of various patients derived CAFs through inducing of caspase-3 mediated apoptosis. Interestingly, combination therapy with BTZ and PST showed better efficacy of inhibiting CAFs than single treatment. The synergistic effect between BTZ and PST on viability of CAFs was observed both in vitro CAF culture and in vivo mouse model. Furthermore, combination therapy with BTZ/PST and conventional anticancer compound docetaxel strongly inhibited tumor growth in xenografts of mouse breast cancer cells with mouse CAFs. In conclusion, our present study revealed that BTZ and PST could significantly reduce the viability of CAFs. Therefore, a combination therapy with BTZ/PST and anticancer drugs might be considered as a new rational for the development of anticancer therapy.






Similar content being viewed by others
References
Kalluri R (2016) The biology and function of fibroblasts in cancer. Nat Rev Cancer 16(9):582–598. https://doi.org/10.1038/nrc.2016.73
Balkwill FR, Capasso M, Hagemann T (2012) The tumor microenvironment at a glance. J Cell Sci 125(Pt 23):5591–5596. https://doi.org/10.1242/jcs.116392
Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL, Weinberg RA (2005) Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell 121(3):335–348. https://doi.org/10.1016/j.cell.2005.02.034
O'Connell JT, Sugimoto H, Cooke VG, MacDonald BA, Mehta AI, LeBleu VS, Dewar R, Rocha RM, Brentani RR, Resnick MB, Neilson EG, Zeisberg M, Kalluri R (2011) VEGF-A and tenascin-C produced by S100A4+ stromal cells are important for metastatic colonization. Proc Natl Acad Sci U S A 108(38):16002–16007. https://doi.org/10.1073/pnas.1109493108
Bergers G, Brekken R, McMahon G, TH V, Itoh T, Tamaki K, Tanzawa K, Thorpe P, Itohara S, Werb Z, Hanahan D (2000) Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol 2(10):737–744. https://doi.org/10.1038/35036374
Munoz-Galvan S, Gutierrez G, Perez M, Carnero A (2015) MAP17 (PDZKIP1) expression determines sensitivity to the proteasomal inhibitor Bortezomib by preventing Cytoprotective autophagy and NFkappaB activation in breast cancer. Mol Cancer Ther 14(6):1454–1465. https://doi.org/10.1158/1535-7163.MCT-14-1053
Oravecz-Wilson KI, Philips ST, Yilmaz OH, Ames HM, Li L, Crawford BD, Gauvin AM, Lucas PC, Sitwala K, Downing JR, Morrison SJ, Ross TS (2009) Persistence of leukemia-initiating cells in a conditional knockin model of an imatinib-responsive myeloproliferative disorder. Cancer Cell 16(2):137–148. https://doi.org/10.1016/j.ccr.2009.06.007
Orimo A, Tomioka Y, Shimizu Y, Sato M, Oigawa S, Kamata K, Nogi Y, Inoue S, Takahashi M, Hata T, Muramatsu M (2001) Cancer-associated myofibroblasts possess various factors to promote endometrial tumor progression. Clinical cancer research : an official journal of the American Association for Cancer Research 7(10):3097–3105
Erez N, Truitt M, Olson P, Arron ST, Hanahan D (2010) Cancer-associated fibroblasts are activated in incipient neoplasia to orchestrate tumor-promoting inflammation in an NF-kappaB-dependent manner. Cancer Cell 17(2):135–147. https://doi.org/10.1016/j.ccr.2009.12.041
Crawford Y, Kasman I, Yu L, Zhong C, Wu X, Modrusan Z, Kaminker J, Ferrara N (2009) PDGF-C mediates the angiogenic and tumorigenic properties of fibroblasts associated with tumors refractory to anti-VEGF treatment. Cancer Cell 15(1):21–34. https://doi.org/10.1016/j.ccr.2008.12.004
Bhowmick NA, Chytil A, Plieth D, Gorska AE, Dumont N, Shappell S, Washington MK, Neilson EG, Moses HL (2004) TGF-beta signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science 303(5659):848–851. https://doi.org/10.1126/science.1090922
Wang Z, Ma Q, Liu Q, Yu H, Zhao L, Shen S, Yao J (2008) Blockade of SDF-1/CXCR4 signalling inhibits pancreatic cancer progression in vitro via inactivation of canonical Wnt pathway. Br J Cancer 99(10):1695–1703. https://doi.org/10.1038/sj.bjc.6604745
Sun Y, Campisi J, Higano C, Beer TM, Porter P, Coleman I, True L, Nelson PS (2012) Treatment-induced damage to the tumor microenvironment promotes prostate cancer therapy resistance through WNT16B. Nat Med 18(9):1359–1368. https://doi.org/10.1038/nm.2890
Jung DW, Che ZM, Kim J, Kim K, Kim KY, Williams D (2010) Tumor-stromal crosstalk in invasion of oral squamous cell carcinoma: a pivotal role of CCL7. Int J Cancer 127(2):332–344. https://doi.org/10.1002/ijc.25060
Allinen M, Beroukhim R, Cai L, Brennan C, Lahti-Domenici J, Huang H, Porter D, Hu M, Chin L, Richardson A, Schnitt S, Sellers WR, Polyak K (2004) Molecular characterization of the tumor microenvironment in breast cancer. Cancer Cell 6(1):17–32. https://doi.org/10.1016/j.ccr.2004.06.010
Ohlund D, Elyada E, Tuveson D (2014) Fibroblast heterogeneity in the cancer wound. J Exp Med 211(8):1503–1523. https://doi.org/10.1084/jem.20140692
Domanska UM, Timmer-Bosscha H, Nagengast WB, Oude Munnink TH, Kruizinga RC, Ananias HJ, Kliphuis NM, Huls G, De Vries EG, de Jong IJ, Walenkamp AM (2012) CXCR4 inhibition with AMD3100 sensitizes prostate cancer to docetaxel chemotherapy. Neoplasia 14(8):709–718
Boire A, Covic L, Agarwal A, Jacques S, Sherifi S, Kuliopulos A (2005) PAR1 is a matrix metalloprotease-1 receptor that promotes invasion and tumorigenesis of breast cancer cells. Cell 120(3):303–313. https://doi.org/10.1016/j.cell.2004.12.018
Vosseler S, Lederle W, Airola K, Obermueller E, Fusenig NE, Mueller MM (2009) Distinct progression-associated expression of tumor and stromal MMPs in HaCaT skin SCCs correlates with onset of invasion. Int J Cancer 125(10):2296–2306. https://doi.org/10.1002/ijc.24589
Zigrino P, Kuhn I, Bauerle T, Zamek J, Fox JW, Neumann S, Licht A, Schorpp-Kistner M, Angel P, Mauch C (2009) Stromal expression of MMP-13 is required for melanoma invasion and metastasis. J Invest Dermatol 129(11):2686–2693. https://doi.org/10.1038/jid.2009.130
Lederle W, Hartenstein B, Meides A, Kunzelmann H, Werb Z, Angel P, Mueller MM (2010) MMP13 as a stromal mediator in controlling persistent angiogenesis in skin carcinoma. Carcinogenesis 31(7):1175–1184. https://doi.org/10.1093/carcin/bgp248
Lochter A, Galosy S, Muschler J, Freedman N, Werb Z, Bissell MJ (1997) Matrix metalloproteinase stromelysin-1 triggers a cascade of molecular alterations that leads to stable epithelial-to-mesenchymal conversion and a premalignant phenotype in mammary epithelial cells. J Cell Biol 139(7):1861–1872
Grum-Schwensen B, Klingelhofer J, Berg CH, El-Naaman C, Grigorian M, Lukanidin E, Ambartsumian N (2005) Suppression of tumor development and metastasis formation in mice lacking the S100A4(mts1) gene. Cancer Res 65(9):3772–3780. https://doi.org/10.1158/0008-5472.CAN-04-4510
Farmer P, Bonnefoi H, Anderle P, Cameron D, Wirapati P, Becette V, Andre S, Piccart M, Campone M, Brain E, Macgrogan G, Petit T, Jassem J, Bibeau F, Blot E, Bogaerts J, Aguet M, Bergh J, Iggo R, Delorenzi M (2009) A stroma-related gene signature predicts resistance to neoadjuvant chemotherapy in breast cancer. Nat Med 15(1):68–74. https://doi.org/10.1038/nm.1908
Meads MB, Gatenby RA, Dalton WS (2009) Environment-mediated drug resistance: a major contributor to minimal residual disease. Nat Rev Cancer 9(9):665–674. https://doi.org/10.1038/nrc2714
Heldin CH, Rubin K, Pietras K, Ostman A (2004) High interstitial fluid pressure - an obstacle in cancer therapy. Nat Rev Cancer 4(10):806–813. https://doi.org/10.1038/nrc1456
Straussman R, Morikawa T, Shee K, Barzily-Rokni M, Qian ZR, Du J, Davis A, Mongare MM, Gould J, Frederick DT, Cooper ZA, Chapman PB, Solit DB, Ribas A, Lo RS, Flaherty KT, Ogino S, Wargo JA, Golub TR (2012) Tumour micro-environment elicits innate resistance to RAF inhibitors through HGF secretion. Nature 487(7408):500–504. https://doi.org/10.1038/nature11183
Wilson TR, Fridlyand J, Yan Y, Penuel E, Burton L, Chan E, Peng J, Lin E, Wang Y, Sosman J, Ribas A, Li J, Moffat J, Sutherlin DP, Koeppen H, Merchant M, Neve R, Settleman J (2012) Widespread potential for growth-factor-driven resistance to anticancer kinase inhibitors. Nature 487(7408):505–509. https://doi.org/10.1038/nature11249
Liu J, Liao S, Diop-Frimpong B, Chen W, Goel S, Naxerova K, Ancukiewicz M, Boucher Y, Jain RK, Xu L (2012) TGF-beta blockade improves the distribution and efficacy of therapeutics in breast carcinoma by normalizing the tumor stroma. Proc Natl Acad Sci U S A 109(41):16618–16623. https://doi.org/10.1073/pnas.1117610109
Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, Madhu B, Goldgraben MA, Caldwell ME, Allard D, Frese KK, Denicola G, Feig C, Combs C, Winter SP, Ireland-Zecchini H, Reichelt S, Howat WJ, Chang A, Dhara M, Wang L, Ruckert F, Grutzmann R, Pilarsky C, Izeradjene K, Hingorani SR, Huang P, Davies SE, Plunkett W, Egorin M, Hruban RH, Whitebread N, McGovern K, Adams J, Iacobuzio-Donahue C, Griffiths J, Tuveson DA (2009) Inhibition of hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science 324(5933):1457–1461. https://doi.org/10.1126/science.1171362
Dufour A, Overall CM (2013) Missing the target: matrix metalloproteinase antitargets in inflammation and cancer. Trends Pharmacol Sci 34(4):233–242. https://doi.org/10.1016/j.tips.2013.02.004
Wen J, Matsumoto K, Taniura N, Tomioka D, Nakamura T (2004) Hepatic gene expression of NK4, an HGF-antagonist/angiogenesis inhibitor, suppresses liver metastasis and invasive growth of colon cancer in mice. Cancer Gene Ther 11(6):419–430. https://doi.org/10.1038/sj.cgt.7700705
Pietras K, Pahler J, Bergers G, Hanahan D (2008) Functions of paracrine PDGF signaling in the proangiogenic tumor stroma revealed by pharmacological targeting. PLoS Med 5(1):e19. https://doi.org/10.1371/journal.pmed.0050019
Santos AM, Jung J, Aziz N, Kissil JL, Pure E (2009) Targeting fibroblast activation protein inhibits tumor stromagenesis and growth in mice. J Clin Invest 119(12):3613–3625. https://doi.org/10.1172/JCI38988
Hofheinz RD, al-Batran SE, Hartmann F, Hartung G, Jager D, Renner C, Tanswell P, Kunz U, Amelsberg A, Kuthan H, Stehle G (2003) Stromal antigen targeting by a humanised monoclonal antibody: an early phase II trial of sibrotuzumab in patients with metastatic colorectal cancer. Onkologie 26(1):44–48. https://doi.org/10.1159/000069863
Lee KW, Yeo SY, Sung CO, Kim SH (2015) Twist1 is a key regulator of cancer-associated fibroblasts. Cancer Res 75(1):73–85. https://doi.org/10.1158/0008-5472.CAN-14-0350
Miyoshi H, Takahashi M, Gage FH, Verma IM (1997) Stable and efficient gene transfer into the retina using an HIV-based lentiviral vector. Proc Natl Acad Sci U S A 94(19):10319–10323
Basu A, Bodycombe NE, Cheah JH, Price EV, Liu K, Schaefer GI, Ebright RY, Stewart ML, Ito D, Wang S, Bracha AL, Liefeld T, Wawer M, Gilbert JC, Wilson AJ, Stransky N, Kryukov GV, Dancik V, Barretina J, Garraway LA, Hon CS, Munoz B, Bittker JA, Stockwell BR, Khabele D, Stern AM, Clemons PA, Shamji AF, Schreiber SL (2013) An interactive resource to identify cancer genetic and lineage dependencies targeted by small molecules. Cell 154(5):1151–1161. https://doi.org/10.1016/j.cell.2013.08.003
Chou TC (2010) Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res 70(2):440–446. https://doi.org/10.1158/0008-5472.CAN-09-1947
Bamias A, Pavlidis N (1998) Systemic chemotherapy in gastric cancer: where do we stand today? Oncologist 3(3):171–177
Longley DB, Harkin DP, Johnston PG (2003) 5-fluorouracil: mechanisms of action and clinical strategies. Nat Rev Cancer 3(5):330–338. https://doi.org/10.1038/nrc1074
Burnett JP, Lim G, Li Y, Shah RB, Lim R, Paholak HJ, McDermott SP, Sun L, Tsume Y, Bai S, Wicha MS, Sun D, Zhang T (2017) Sulforaphane enhances the anticancer activity of taxanes against triple negative breast cancer by killing cancer stem cells. Cancer Lett 394:52–64. https://doi.org/10.1016/j.canlet.2017.02.023
Alshaker H, Wang Q, Bohler T, Mills R, Winkler M, Arafat T, Kawano Y, Pchejetski D (2017) Combination of RAD001 (everolimus) and docetaxel reduces prostate and breast cancer cell VEGF production and tumour vascularisation independently of sphingosine-kinase-1. Sci Rep 7(1):3493. https://doi.org/10.1038/s41598-017-03728-3
San-Miguel JF, Einsele H, Moreau P (2016) The role of Panobinostat plus Bortezomib and dexamethasone in treating relapsed or relapsed and refractory multiple myeloma: a European perspective. Adv Ther 33(11):1896–1920. https://doi.org/10.1007/s12325-016-0413-7
Attal M, Lauwers-Cances V, Hulin C, Leleu X, Caillot D, Escoffre M, Arnulf B, Macro M, Belhadj K, Garderet L, Roussel M, Payen C, Mathiot C, Fermand JP, Meuleman N, Rollet S, Maglio ME, Zeytoonjian AA, Weller EA, Munshi N, Anderson KC, Richardson PG, Facon T, Avet-Loiseau H, Harousseau JL, Moreau P (2017) Lenalidomide, Bortezomib, and dexamethasone with transplantation for myeloma. N Engl J Med 376(14):1311–1320. https://doi.org/10.1056/NEJMoa1611750
Chhabra S (2017) Novel proteasome inhibitors and histone deacetylase inhibitors: progress in myeloma therapeutics. Pharmaceuticals (Basel) 10(2). https://doi.org/10.3390/ph10020040
Wang S, Wang L, Zhou Z, Deng Q, Li L, Zhang M, Liu L, Li Y (2017) Leucovorin enhances the anti-cancer effect of Bortezomib in colorectal cancer cells. Sci Rep 7(1):682. https://doi.org/10.1038/s41598-017-00839-9
Ishimoto T, Miyake K, Nandi T, Yashiro M, Onishi N, Huang KK, Lin SJ, Kalpana R, Tay ST, Suzuki Y, Cho BC, Kuroda D, Arima K, Izumi D, Iwatsuki M, Baba Y, Oki E, Watanabe M, Saya H, Hirakawa K, Baba H, Tan P (2017) Activation of transforming growth factor Beta 1 signaling in gastric cancer-associated fibroblasts increases their motility, via expression of rhomboid 5 homolog 2, and ability to induce invasiveness of gastric cancer cells. Gastroenterology 153(1):191–204 e116. https://doi.org/10.1053/j.gastro.2017.03.046
Zhang H, Yue J, Jiang Z, Zhou R, Xie R, Xu Y, Wu S (2017) CAF-secreted CXCL1 conferred radioresistance by regulating DNA damage response in a ROS-dependent manner in esophageal squamous cell carcinoma. Cell Death Dis 8(5):e2790. https://doi.org/10.1038/cddis.2017.180
Hanahan D, Coussens LM (2012) Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell 21(3):309–322. https://doi.org/10.1016/j.ccr.2012.02.022
Lee RH, Yoon N, Reneau JC, Prockop DJ (2012) Preactivation of human MSCs with TNF-alpha enhances tumor-suppressive activity. Cell Stem Cell 11(6):825–835. https://doi.org/10.1016/j.stem.2012.10.001
Rhim AD, Oberstein PE, Thomas DH, Mirek ET, Palermo CF, Sastra SA, Dekleva EN, Saunders T, Becerra CP, Tattersall IW, Westphalen CB, Kitajewski J, Fernandez-Barrena MG, Fernandez-Zapico ME, Iacobuzio-Donahue C, Olive KP, Stanger BZ (2014) Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell 25(6):735–747. https://doi.org/10.1016/j.ccr.2014.04.021
Itoh G, Chida S, Yanagihara K, Yashiro M, Aiba N, Tanaka M (2017) Cancer-associated fibroblasts induce cancer cell apoptosis that regulates invasion mode of tumours. Oncogene 36(31):4434–4444. https://doi.org/10.1038/onc.2017.49
Quante M, SP T, Tomita H, Gonda T, Wang SS, Takashi S, Baik GH, Shibata W, Diprete B, Betz KS, Friedman R, Varro A, Tycko B, Wang TC (2011) Bone marrow-derived myofibroblasts contribute to the mesenchymal stem cell niche and promote tumor growth. Cancer Cell 19(2):257–272. https://doi.org/10.1016/j.ccr.2011.01.020
Ludwig H, Khayat D, Giaccone G, Facon T (2005) Proteasome inhibition and its clinical prospects in the treatment of hematologic and solid malignancies. Cancer 104(9):1794–1807. https://doi.org/10.1002/cncr.21414
Shanker A, Brooks AD, Tristan CA, Wine JW, Elliott PJ, Yagita H, Takeda K, Smyth MJ, Murphy WJ, Sayers TJ (2008) Treating metastatic solid tumors with bortezomib and a tumor necrosis factor-related apoptosis-inducing ligand receptor agonist antibody. J Natl Cancer Inst 100(9):649–662. https://doi.org/10.1093/jnci/djn113
Brooks AD, Jacobsen KM, Li W, Shanker A, Sayers TJ (2010) Bortezomib sensitizes human renal cell carcinomas to TRAIL apoptosis through increased activation of caspase-8 in the death-inducing signaling complex. Molecular cancer research : MCR 8(5):729–738. https://doi.org/10.1158/1541-7786.MCR-10-0022
Gu Y, Bouwman P, Greco D, Saarela J, Yadav B, Jonkers J, Kuznetsov SG (2014) Suppression of BRCA1 sensitizes cells to proteasome inhibitors. Cell Death Dis 5:e1580. https://doi.org/10.1038/cddis.2014.537
Kao C, Chao A, Tsai CL, Chuang WC, Huang WP, Chen GC, Lin CY, Wang TH, Wang HS, Lai CH (2014) Bortezomib enhances cancer cell death by blocking the autophagic flux through stimulating ERK phosphorylation. Cell Death Dis 5:e1510. https://doi.org/10.1038/cddis.2014.468
Kretowski R, Borzym-Kluczyk M, Cechowska-Pasko M (2014) Efficient induction of apoptosis by proteasome inhibitor: bortezomib in the human breast cancer cell line MDA-MB-231. Mol Cell Biochem 389(1–2):177–185. https://doi.org/10.1007/s11010-013-1939-5
Lohberger B, Steinecker-Frohnwieser B, Stuendl N, Kaltenegger H, Leithner A, Rinner B (2016) The proteasome inhibitor Bortezomib affects chondrosarcoma cells via the mitochondria-caspase dependent pathway and enhances death receptor expression and autophagy. PLoS One 11(12):e0168193. https://doi.org/10.1371/journal.pone.0168193
Fineschi S, Reith W, Guerne PA, Dayer JM, Chizzolini C (2006) Proteasome blockade exerts an antifibrotic activity by coordinately down-regulating type I collagen and tissue inhibitor of metalloproteinase-1 and up-regulating metalloproteinase-1 production in human dermal fibroblasts. FASEB J 20(3):562–564. https://doi.org/10.1096/fj.05-4870fje
Fineschi S, Bongiovanni M, Donati Y, Djaafar S, Naso F, Goffin L, Argiroffo CB, Pache JC, Dayer JM, Ferrari-Lacraz S, Chizzolini C (2008) In vivo investigations on anti-fibrotic potential of proteasome inhibition in lung and skin fibrosis. Am J Respir Cell Mol Biol 39(4):458–465. https://doi.org/10.1165/rcmb.2007-0320OC
You BR, Park WH (2011) Proteasome inhibition by MG132 induces growth inhibition and death of human pulmonary fibroblast cells in a caspase-independent manner. Oncol Rep 25(6):1705–1712. https://doi.org/10.3892/or.2011.1211
Pujols L, Fernandez-Bertolin L, Fuentes-Prado M, Alobid I, Roca-Ferrer J, Agell N, Mullol J, Picado C (2012) Proteasome inhibition reduces proliferation, collagen expression, and inflammatory cytokine production in nasal mucosa and polyp fibroblasts. J Pharmacol Exp Ther 343(1):184–197. https://doi.org/10.1124/jpet.111.190710
Legesse-Miller A, Raitman I, Haley EM, Liao A, Sun LL, Wang DJ, Krishnan N, Lemons JM, Suh EJ, Johnson EL, Lund BA, Coller HA (2012) Quiescent fibroblasts are protected from proteasome inhibition-mediated toxicity. Mol Biol Cell 23(18):3566–3581. https://doi.org/10.1091/mbc.E12-03-0192
Hideshima T, Richardson PG, Anderson KC (2011) Mechanism of action of proteasome inhibitors and deacetylase inhibitors and the biological basis of synergy in multiple myeloma. Mol Cancer Ther 10(11):2034–2042. https://doi.org/10.1158/1535-7163.MCT-11-0433
Yu C, Friday BB, Yang L, Atadja P, Wigle D, Sarkaria J, Adjei AA (2008) Mitochondrial Bax translocation partially mediates synergistic cytotoxicity between histone deacetylase inhibitors and proteasome inhibitors in glioma cells. Neuro-Oncology 10(3):309–319. https://doi.org/10.1215/15228517-2007-063
Rao R, Nalluri S, Fiskus W, Savoie A, Buckley KM, Ha K, Balusu R, Joshi A, Coothankandaswamy V, Tao J, Sotomayor E, Atadja P, Bhalla KN (2010) Role of CAAT/enhancer binding protein homologous protein in panobinostat-mediated potentiation of bortezomib-induced lethal endoplasmic reticulum stress in mantle cell lymphoma cells. Clin Cancer Res 16(19):4742–4754. https://doi.org/10.1158/1078-0432.CCR-10-0529
Budman DR, Calabro A, Rosen L, Lesser M (2012) Identification of unique synergistic drug combinations associated with downexpression of survivin in a preclinical breast cancer model system. Anti-Cancer Drugs 23(3):272–279
Wang H, Cao Q, Dudek AZ (2012) Phase II study of panobinostat and bortezomib in patients with pancreatic cancer progressing on gemcitabine-based therapy. Anticancer Res 32(3):1027–1031
Sato A, Asano T, Isono M, Ito K (2014) Panobinostat synergizes with bortezomib to induce endoplasmic reticulum stress and ubiquitinated protein accumulation in renal cancer cells. BMC Urol 14:71. https://doi.org/10.1186/1471-2490-14-71
Tan D, Phipps C, Hwang WY, Tan SY, Yeap CH, Chan YH, Tay K, Lim ST, Lee YS, Kumar SG, Ng SC, Fadilah S, Kim WS, Goh YT (2015) Panobinostat in combination with bortezomib in patients with relapsed or refractory peripheral T-cell lymphoma: an open-label, multicentre phase 2 trial. Lancet Haematol 2(8):e326–e333. https://doi.org/10.1016/S2352-3026(15)00097-6
Teo EC, Valdez BC, Ji J, Li Y, Liu Y, Brammer JE, Hosing C, Nieto Y, Champlin RE, Andersson BS (2016) Synergistic cytotoxicity of busulfan, melphalan, gemcitabine, panobinostat, and bortezomib in lymphoma cells. Leuk Lymphoma 57(11):2644–2652. https://doi.org/10.3109/10428194.2016.1157871
Mondello P, Cuzzocrea S, Navarra M, Mian M (2017) Bone marrow micro-environment is a crucial player for myelomagenesis and disease progression. Oncotarget 8(12):20394–20409. https://doi.org/10.18632/oncotarget.14610
Perez LE, Parquet N, Meads M, Anasetti C, Dalton W (2010) Bortezomib restores stroma-mediated APO2L/TRAIL apoptosis resistance in multiple myeloma. Eur J Haematol 84(3):212–222. https://doi.org/10.1111/j.1600-0609.2009.01381.x
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MEST) (No. 2015R1A2A1A15054021) and a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI14C2517).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
Hak-Min Lee declares that he has no conflict of interest. Eunmyong Lee declares that she has no conflict of interest. So-Young Yeo declares that she has no conflict of interest. Sang Shin declares that he has no conflict of interest. Hyun-Kyu Park declares that he has no conflict of interest. Do-Hyun Nam declares that he has no conflict of interest. Seok-Hyung Kim declares that he has no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of Samsung Medical Center at Sungkyunkwan University, where the studies were conducted.
Electronic supplementary material
Supplementary Fig. 1
Combination treatment of bortezomib and panobinostat attenuates the growth of mouse breast cancer cells and CAFs. (a) Representative photograph of gross tumors from xenografts model with cancer cells alone or cancer cells with MMTV-CAF. Either 5 x 105of 168FARN cells alone or with 1.5 × 106 MMTV-CAFs were subcutaneously injected nude mice. (b) Tumor volumes in xenografts model of 168FARN mouse breast cancer cells alone or with MMTV-CAF at a ratio of 1:1 (n = 4 per group). Tumor volumes were measured using a caliper. Difference was evaluated by two- tailed student’s t-test. * P < 0.05. (c-d) Effect of either BTZ or PST alone or in combination treatment on 168FARN mouse breast cancer cells (c) or mouse MMTV-CAF cells (d). 168FARN cells or MMTV-CAF cells were plated and treated with BTZ (left panels), PST (middle panels), or both (right panels) for 2 days. Cell viability was calculated as a percentage to the control. Experiments were done in triplicates. Symbols represent mean ± SEM. (PPTX 324 kb)
Rights and permissions
About this article
Cite this article
Lee, HM., Lee, E., Yeo, SY. et al. Drug repurposing screening identifies bortezomib and panobinostat as drugs targeting cancer associated fibroblasts (CAFs) by synergistic induction of apoptosis. Invest New Drugs 36, 545–560 (2018). https://doi.org/10.1007/s10637-017-0547-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-017-0547-8