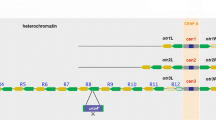
Abstract
Constitutive heterochromatin is composed mainly of repetitive elements and represents the typical inert chromatin structure in eukaryotic cells. Approximately half of the mammalian genome is made of repeat sequences, such as satellite DNA, telomeric DNA, and transposable elements. As essential genes are not present in these regions, most of these repeat sequences were considered as junk DNA in the past. However, it is now clear that these regions are essential for chromosome stability and the silencing of neighboring genes. Genetic and biochemical studies have revealed that histone methylation at H3K9 and its recognition by heterochromatin protein 1 represent the fundamental mechanism by which heterochromatin forms. Although this molecular mechanism is highly conserved from yeast to human cells, its detailed epigenetic regulation is more complex and dynamic for each distinct constitutive heterochromatin structure in higher eukaryotes. It can also vary according to the developmental stage. Chromatin immunoprecipitation followed by sequencing (ChIP-seq) analysis is a powerful tool to investigate the epigenetic regulation of eukaryote genomes, but non-unique reads are usually discarded during standard ChIP-seq data alignment to reference genome databases. Therefore, specific methods to obtain global epigenetic information concerning repetitive elements are needed. In this review, we focus on such approaches and we summarize the latest molecular models for distinct constitutive heterochromatin types in mammals.



Similar content being viewed by others
Abbreviations
- ADD:
-
ATRX-DNMT3-DNMTT3L
- ALT:
-
Alternative lengthening of telomeres
- ATRX:
-
α-Thalassemia/mental retardation X-linked
- BEND3:
-
BEN domain containing 3
- DAXX:
-
Death domain-associated protein
- ERV:
-
Endogenous retrovirus
- HUSH:
-
Human-silencing hub
- HP1:
-
Heterochromatin protein 1
- IAP:
-
Intracisternal A particle
- KAP1:
-
KRAB-ZFP-associated protein 1
- KRAB-ZFP:
-
Krüppel-associated box domain-zinc finger proteins
- LINE:
-
Long-interspersed nucleotide element
- LTR:
-
Long terminal repeat
- Mommes:
-
Modifiers of murine metastable epialleles
- NuRD:
-
Nucleosome remodeling and deacetylase complex
- ORC:
-
Origin recognition complex
- Pc:
-
Polycomb
- PEV:
-
Position effect variegation
- PICh:
-
Proteomics of isolated chromatin segment
- piRNA:
-
PIWI-interacting RNA
- PIWI:
-
P-element-induced wimpy testis
- POT1:
-
Protection of telomeres 1
- RAP1:
-
Repressor and activator protein 1
- SAHF:
-
Senescence-associated heterochromatic foci
- SETDB1:
-
SET domain bifurcated 1
- Su(var):
-
Suppressor of variegation
- TERRA:
-
Telomere repeat-containing RNA
- TIN2:
-
TRF1interacting nuclear protein 2
- TPP1:
-
POT1- and TIN2-interacting protein
- TRF1:
-
Telomeric repeat-binding factor 1
References
Aravin A et al (2006) A novel class of small RNAs bind to MILI protein in mouse testes. Nature 442(7099):203–207
Azzalin CM et al (2007) Telomeric repeat containing RNA and RNA surveillance factors at mammalian chromosome ends. Science 318(5851):798–801
Bannister AJ et al (2001) Selective recognition of methylated lysine 9 on histone H3 by the HP1 chromo domain. Nature 410(6824):120–124
Bartke T et al (2010) Nucleosome-interacting proteins regulated by DNA and histone methylation. Cell 143(3):470–484
Blewitt ME et al (2005) An N-ethyl-N-nitrosourea screen for genes involved in variegation in the mouse. Proc Natl Acad Sci U S A 102(21):7629–7634
Brower-Toland B et al (2007) Drosophila PIWI associates with chromatin and interacts directly with HP1a. Genes Dev 21(18):2300–2311
Bulut-Karslioglu A et al (2012) A transcription factor-based mechanism for mouse heterochromatin formation. Nat Struct Mol Biol 19(10):1023–1030
Bulut-Karslioglu A et al (2014) Suv39h-dependent H3K9me3 marks intact retrotransposons and silences LINE elements in mouse embryonic stem cells. Mol Cell 55(2):277–290
Cesare AJ, Reddel RR (2010) Alternative lengthening of telomeres: models, mechanisms and implications. Nat Rev Genet 11(5):319–330
Davis CM et al (1989) Activation and demethylation of the intracisternal a particle genes by 5-azacytidine. Cell Differ Dev 27(2):83–93
Daxinger L et al (2013) An ENU mutagenesis screen identifies novel and known genes involved in epigenetic processes in the mouse. Genome Biol 14(9):R96
Day DS et al (2010) Estimating enrichment of repetitive elements from high-throughput sequence data. Genome Biol 11(6):R69
Dejardin J (2015) Switching between epigenetic states at pericentromeric heterochromatin. Trends Genet 31(11):661–672
Dejardin J, Kingston RE (2009) Purification of proteins associated with specific genomic loci. Cell 136(1):175–186
Deng W, Lin H (2002) Miwi, a murine homolog of piwi, encodes a cytoplasmic protein essential for spermatogenesis. Dev Cell 2(6):819–830
Deng Z et al (2009) TERRA RNA binding to TRF2 facilitates heterochromatin formation and ORC recruitment at telomeres. Mol Cell 35(4):403–413
Dodge JE et al (2004) Histone H3-K9 methyltransferase ESET is essential for early development. Mol Cell Biol 24(6):2478–2486
Elgin SC, Reuter G (2013) Position-effect variegation, heterochromatin formation, and gene silencing in drosophila. Cold Spring Harb Perspect Biol 5(8):a017780
Elsasser SJ et al (2012) DAXX envelops a histone H3.3-H4 dimer for H3.3-specific recognition. Nature 491(7425):560–565
Elsasser SJ et al (2015) Histone H3.3 is required for endogenous retroviral element silencing in embryonic stem cells. Nature 522(7555):240–244
Engelen E et al (2015) Proteins that bind regulatory regions identified by histone modification chromatin immunoprecipitations and mass spectrometry. Nat Commun 6:7155
Eymery A et al (2016) The methyltransferase Setdb1 is essential for meiosis and mitosis in mouse oocytes and early embryos. Development 143(15):2767–2779
Garcia-Cao M et al (2004) Epigenetic regulation of telomere length in mammalian cells by the Suv39h1 and Suv39h2 histone methyltransferases. Nat Genet 36(1):94–99
Gaspar-Maia A et al (2011) Open chromatin in pluripotency and reprogramming. Nat Rev Mol Cell Biol 12(1):36–47
Goldberg AD et al (2010) Distinct factors control histone variant H3.3 localization at specific genomic regions. Cell 140(5):678–691
Gonzalo S et al (2006) DNA methyltransferases control telomere length and telomere recombination in mammalian cells. Nat Cell Biol 8(4):416–424
Greer EL, Shi Y (2012) Histone methylation: a dynamic mark in health, disease and inheritance. Nat Rev Genet 13(5):343–357
Gregory TR (2005) Synergy between sequence and size in large-scale genomics. Nat Rev Genet 6(9):699–708
He Q et al (2015) The Daxx/Atrx complex protects tandem repetitive elements during DNA hypomethylation by promoting H3K9 trimethylation. Cell Stem Cell 17(3):273–286
Heaphy CM et al (2011) Altered telomeres in tumors with ATRX and DAXX mutations. Science 333(6041):425
Ide S, Dejardin J (2015) End-targeting proteomics of isolated chromatin segments of a mammalian ribosomal RNA gene promoter. Nat Commun 6:6674
Ishizu H et al (2012) Biology of PIWI-interacting RNAs: new insights into biogenesis and function inside and outside of germlines. Genes Dev 26(21):2361–2373
Iwasaki YW et al (2016) Piwi modulates chromatin accessibility by regulating multiple factors including histone H1 to repress transposons. Mol Cell 63(3):408–419
Iwase S et al (2011) ATRX ADD domain links an atypical histone methylation recognition mechanism to human mental-retardation syndrome. Nat Struct Mol Biol 18(7):769–776
Jackson-Grusby L et al (2001) Loss of genomic methylation causes p53-dependent apoptosis and epigenetic deregulation. Nat Genet 27(1):31–39
James TC et al (1989) Distribution patterns of HP1, a heterochromatin-associated nonhistone chromosomal protein of drosophila. Eur J Cell Biol 50(1):170–180
Jost KL et al (2012) Heterochromatin and gene positioning: inside, outside, any side? Chromosoma 121(6):555–563
Karimi MM et al (2011) DNA methylation and SETDB1/H3K9me3 regulate predominantly distinct sets of genes, retroelements, and chimeric transcripts in mESCs. Cell Stem Cell 8(6):676–687
Kipling D et al (1991) Mouse minor satellite DNA genetically maps to the centromere and is physically linked to the proximal telomere. Genomics 11(2):235–241
Lachner M et al (2001) Methylation of histone H3 lysine 9 creates a binding site for HP1 proteins. Nature 410(6824):116–120
Liu S et al (2014) Setdb1 is required for germline development and silencing of H3K9me3-marked endogenous retroviruses in primordial germ cells. Genes Dev 28(18):2041–2055
Martens JH et al (2005) The profile of repeat-associated histone lysine methylation states in the mouse epigenome. EMBO J 24(4):800–812
Marzec P et al (2015) Nuclear-receptor-mediated telomere insertion leads to genome instability in ALT cancers. Cell 160(5):913–927
Matsui T et al (2010) Proviral silencing in embryonic stem cells requires the histone methyltransferase ESET. Nature 464(7290):927–931
Mefford HC, Trask BJ (2002) The complex structure and dynamic evolution of human subtelomeres. Nat Rev Genet 3(2):91–102
Meshorer E, Misteli T (2006) Chromatin in pluripotent embryonic stem cells and differentiation. Nat Rev Mol Cell Biol 7(7):540–546
Meshorer E et al (2006) Hyperdynamic plasticity of chromatin proteins in pluripotent embryonic stem cells. Dev Cell 10(1):105–116
Moyzis RK et al (1988) A highly conserved repetitive DNA sequence, (TTAGGG)n, present at the telomeres of human chromosomes. Proc Natl Acad Sci U S A 85(18):6622–6626
Muller HJ (1930) Types of visible variations induced by X-rays in Drosophila. J Genet 22:299–334
Nakayama J et al (2001) Role of histone H3 lysine 9 methylation in epigenetic control of heterochromatin assembly. Science 292(5514):110–113
Narita M et al (2003) Rb-mediated heterochromatin formation and silencing of E2F target genes during cellular senescence. Cell 113(6):703–716
Nishibuchi G, Nakayama J (2014) Biochemical and structural properties of heterochromatin protein 1: understanding its role in chromatin assembly. J Biochem 156(1):11–20
O’Sullivan RJ, Karlseder J (2010) Telomeres: protecting chromosomes against genome instability. Nat Rev Mol Cell Biol 11(3):171–181
Padeken J et al (2015) Repeat DNA in genome organization and stability. Curr Opin Genet Dev 31:12–19
Peters AH et al (2001) Loss of the Suv39h histone methyltransferases impairs mammalian heterochromatin and genome stability. Cell 107(3):323–337
Rea S et al (2000) Regulation of chromatin structure by site-specific histone H3 methyltransferases. Nature 406(6796):593–599
Riethman H (2008) Human telomere structure and biology. Annu Rev Genomics Hum Genet 9:1–19
Rowe HM et al (2010) KAP1 controls endogenous retroviruses in embryonic stem cells. Nature 463(7278):237–240
Sadaie M et al (2013) Redistribution of the Lamin B1 genomic binding profile affects rearrangement of heterochromatic domains and SAHF formation during senescence. Genes Dev 27(16):1800–1808
Saksouk N et al (2014) Redundant mechanisms to form silent chromatin at pericentromeric regions rely on BEND3 and DNA methylation. Mol Cell 56(4):580–594
Saksouk N et al (2015) Constitutive heterochromatin formation and transcription in mammals. Epigenetics Chromatin 8:3
Santos F et al (2005) Dynamic chromatin modifications characterise the first cell cycle in mouse embryos. Dev Biol 280(1):225–236
Schotta G et al (2004) A silencing pathway to induce H3-K9 and H4-K20 trimethylation at constitutive heterochromatin. Genes Dev 18(11):1251–1262
Schueler MG, Sullivan BA (2006) Structural and functional dynamics of human centromeric chromatin. Annu Rev Genomics Hum Genet 7:301–313
Solovei I et al (2009) Nuclear architecture of rod photoreceptor cells adapts to vision in mammalian evolution. Cell 137(2):356–368
Solovei I et al (2013) LBR and Lamin a/C sequentially tether peripheral heterochromatin and inversely regulate differentiation. Cell 152(3):584–598
Tchasovnikarova IA et al (2015) GENE SILENCING. Epigenetic silencing by the HUSH complex mediates position-effect variegation in human cells. Science 348(6242):1481–1485
Treangen TJ, Salzberg SL (2012) Repetitive DNA and next-generation sequencing: computational challenges and solutions. Nat Rev Genet 13(1):36–46
Trojer P, Reinberg D (2007) Facultative heterochromatin: is there a distinctive molecular signature? Mol Cell 28(1):1–13
Tsumura A et al (2006) Maintenance of self-renewal ability of mouse embryonic stem cells in the absence of DNA methyltransferases Dnmt1, Dnmt3a and Dnmt3b. Genes Cells 11(7):805–814
Udugama M et al (2015) Histone variant H3.3 provides the heterochromatic H3 lysine 9 tri-methylation mark at telomeres. Nucleic Acids Res 43(21):10227–10237
Vermeulen M et al (2010) Quantitative interaction proteomics and genome-wide profiling of epigenetic histone marks and their readers. Cell 142(6):967–980
Vissel B, Choo KH (1989) Mouse major (gamma) satellite DNA is highly conserved and organized into extremely long tandem arrays: implications for recombination between nonhomologous chromosomes. Genomics 5(3):407–414
Wang CI et al (2013) Chromatin proteins captured by ChIP-mass spectrometry are linked to dosage compensation in Drosophila. Nat Struct Mol Biol 20(2):202–209
Waye JS, Willard HF (1986) Structure, organization, and sequence of alpha satellite DNA from human chromosome 17: evidence for evolution by unequal crossing-over and an ancestral pentamer repeat shared with the human X chromosome. Mol Cell Biol 6(9):3156–3165
Wierer M, Mann M (2016) Proteomics to study DNA-bound and chromatin-associated gene regulatory complexes. Hum Mol Genet 25(R2):R106–R114
Wu SC, Zhang Y (2010) Active DNA demethylation: many roads lead to Rome. Nat Rev Mol Cell Biol 11(9):607–620
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editors: Nick Gilbert and Davide Marenduzzo
Rights and permissions
About this article
Cite this article
Nishibuchi, G., Déjardin, J. The molecular basis of the organization of repetitive DNA-containing constitutive heterochromatin in mammals. Chromosome Res 25, 77–87 (2017). https://doi.org/10.1007/s10577-016-9547-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10577-016-9547-3