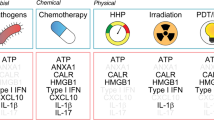
Abstract
The success of some chemo- and radiotherapeutic regimens relies on the induction of immunogenic tumor cell death and on the induction of an anticancer immune response. Cells succumbing to immunogenic cell death undergo specific changes in their surface characteristics and release pro-immunogenic factors according to a defined spatiotemporal pattern. This stimulates antigen presenting cells such as dendritic cells to efficiently take up tumor antigens, process them, and cross-prime cytotoxic T lymphocytes, thus eliciting a tumor-specific cognate immune response. Such a response can also target therapy-resistant tumor (stem) cells, thereby leading, at least in some instances, to tumor eradication. In this review, we shed some light on the molecular identity of the factors that are required for cell death to be perceived as immunogenic. We discuss the intriguing observations that the most abundant endoplasmic reticulum protein, calreticulin, the most abundant intracellular metabolite, ATP, and the most abundant non-histone chromatin-binding protein, HMGB1, can determine whether cell death is immunogenic as they appear on the surface or in the microenvironment of dying cells.



Similar content being viewed by others
Abbreviations
- APC:
-
Antigen presenting cell
- CRT:
-
Calreticulin
- DAMP:
-
Danger-associated molecular pattern
- DC:
-
Dendritic cell
- eIF2α:
-
Eukaryotic translation initiation factor 2α
- EIF2AK3:
-
eIF2α kinase 3
- ER:
-
Endoplasmic reticulum
- ENTPD1:
-
Ectonucleoside triphosphate diphosphohydrolase
- Gb3:
-
Globotriaosylceramide
- HMGB1:
-
High-mobility group box 1
- HSP:
-
Heat shock protein
- ICD:
-
Immunogenic cell death
- IFN-γ:
-
Interferon-γ
- IL-1β:
-
Interleukin-1β
- LPS:
-
Lipopolysaccharide
- MHC:
-
Major histocompatibility complex
- MYD88:
-
Myeloid differentiation primary response protein 88
- NLRP3:
-
NLR family, pyrin domain containing 3
- NO:
-
Nitric oxide
- oxLDLs:
-
Oxidized low-density lipoproteins
- PAMP:
-
Pathogen-associated molecular pattern
- PS:
-
Phosphatidylserine
- PYCARD:
-
PYD and CARD domain containing
- RNAi:
-
RNA interference
- SIRPα:
-
Signal-regulatory protein α
- SNARE:
-
SNAP and NSF attachment receptors
- SNCEE:
-
S-nitroso-l-cysteine ethyl esther
- SPA:
-
Surfactant protein A
- TLR:
-
Toll-like receptor
- WT:
-
Wild type
References
Zitvogel, L., Apetoh, L., Ghiringhelli, F., & Kroemer, G. (2008). Immunological aspects of cancer chemotherapy. Nat Rev Immunol, 8(1), 59–73.
Savill, J., & Fadok, V. (2000). Corpse clearance defines the meaning of cell death. Nature, 407(6805), 784–788.
Matzinger, P. (2002). The danger model: a renewed sense of self. Science, 296(5566), 301–305.
Kroemer, G., Galluzzi, L., Vandenabeele, P., Abrams, J., Alnemri, E. S., Baehrecke, E. H., et al. (2009). Classification of cell death: recommendations of the nomenclature committee on cell death 2009. Cell Death Differ, 16(1), 3–11.
Zou, W. (2006). Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol, 6(4), 295–307.
Rubio, M. T., Ittelet, D., Raymond, E., Blay, J. Y., Bernard, J., & Chouaib, S. (2004). The immunosuppressive effect of vincristine on allostimulatory potential of human dendritic cells interferes with their function and survival. Int J Oncol, 25(2), 407–412.
Zitvogel, L., Apetoh, L., Ghiringhelli, F., Andre, F., Tesniere, A., & Kroemer, G. (2008). The anticancer immune response: indispensable for therapeutic success? J Clin Invest, 118(6), 1991–2001.
Casares, N., Pequignot, M. O., Tesniere, A., Ghiringhelli, F., Roux, S., Chaput, N., et al. (2005). Caspase-dependent immunogenicity of doxorubicin-induced tumor cell death. J Exp Med, 202(12), 1691–1701.
Obeid, M., Tesniere, A., Ghiringhelli, F., Fimia, G. M., Apetoh, L., Perfettini, J. L., et al. (2007). Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med, 13(1), 54–61.
Jung, S., Unutmaz, D., Wong, P., Sano, G., De los Santos, K., Sparwasser, T., et al. (2002). In vivo depletion of cd11c+ dendritic cells abrogates priming of cd8+ t cells by exogenous cell-associated antigens. Immunity, 17(2), 211–220.
Apetoh, L., Ghiringhelli, F., Tesniere, A., Obeid, M., Ortiz, C., Criollo, A., et al. (2007). Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med, 13(9), 1050–1059.
Apetoh, L., Ghiringhelli, F., Tesniere, A., Criollo, A., Ortiz, C., Lidereau, R., et al. (2007). The interaction between hmgb1 and tlr4 dictates the outcome of anticancer chemotherapy and radiotherapy. Immunol Rev, 220, 47–59.
Ghiringhelli, F., Apetoh, L., Tesniere, A., Aymeric, L., Ma, Y., Ortiz, C., et al. (2009). Activation of the nlrp3 inflammasome in dendritic cells induces il-1beta-dependent adaptive immunity against tumors. Nat Med, 15(10), 1170–1178.
Banchereau, J., & Steinman, R. M. (1998). Dendritic cells and the control of immunity. Nature, 392(6673), 245–252.
Albert, M. L., Sauter, B., & Bhardwaj, N. (1998). Dendritic cells acquire antigen from apoptotic cells and induce class i-restricted ctls. Nature, 392(6671), 86–89.
Green, D. R., Ferguson, T., Zitvogel, L., & Kroemer, G. (2009). Immunogenic and tolerogenic cell death. Nat Rev Immunol, 9(5), 353–363.
Grivennikov, S. I., Greten, F. R., & Karin, M. (2010). Immunity, inflammation, and cancer. Cell, 140(6), 883–899.
Zitvogel, L., Kepp, O., & Kroemer, G. (2010). Decoding cell death signals in inflammation and immunity. Cell, 140(6), 798–804.
Zitvogel, L., Mayordomo, J. I., Tjandrawan, T., DeLeo, A. B., Clarke, M. R., Lotze, M. T., et al. (1996). Therapy of murine tumors with tumor peptide-pulsed dendritic cells: dependence on T cells, B7 costimulation, and T helper cell 1-associated cytokines. J Exp Med, 183(1), 87–97.
Spisek, R., Charalambous, A., Mazumder, A., Vesole, D. H., Jagannath, S., & Dhodapkar, M. V. (2007). Bortezomib enhances dendritic cell (DC)-mediated induction of immunity to human myeloma via exposure of cell surface heat shock protein 90 on dying tumor cells: therapeutic implications. Blood, 109(11), 4839–4845.
Mukhopadhaya, A., Mendecki, J., Dong, X., Liu, L., Kalnicki, S., Garg, M., et al. (2007). Localized hyperthermia combined with intratumoral dendritic cells induces systemic antitumor immunity. Cancer Res, 67(16), 7798–7806.
Didelot, C., Lanneau, D., Brunet, M., Joly, A. L., De Thonel, A., Chiosis, G., et al. (2007). Anti-cancer therapeutic approaches based on intracellular and extracellular heat shock proteins. Curr Med Chem, 14(27), 2839–2847.
Locher, C., Rusakiewicz, S., Tesniere, A., Ghiringhelli, F., Apetoh, L., Kroemer, G., et al. (2009). Witch hunt against tumor cells enhanced by dendritic cells. Ann NY Acad Sci, 1174, 51–60.
Gardai, S. J., McPhillips, K. A., Frasch, S. C., Janssen, W. J., Starefeldt, A., Murphy-Ullrich, J. E., et al. (2005). Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of lrp on the phagocyte. Cell, 123(2), 321–334.
Kepp, O., Gdoura, A., Martins, I., Panaretakis, T., Schlemmer, F., Tesniere, A., et al. (2010). Lysyl tRNA synthetase is required for the translocation of calreticulin to the cell surface in immunogenic death. Cell Cycle, 9(15), 3072–3077.
Krause, K. H., & Michalak, M. (1997). Calreticulin. Cell, 88(4), 439–443.
Michalak, M., Corbett, E. F., Mesaeli, N., Nakamura, K., & Opas, M. (1999). Calreticulin: one protein, one gene, many functions. Biochem J, 344(Pt 2), 281–292.
Johnson, S., Michalak, M., Opas, M., & Eggleton, P. (2001). The ins and outs of calreticulin: from the er lumen to the extracellular space. Trends Cell Biol, 11(3), 122–129.
Bedard, K., Szabo, E., Michalak, M., & Opas, M. (2005). Cellular functions of endoplasmic reticulum chaperones calreticulin, calnexin, and erp57. Int Rev Cytol, 245, 91–121.
Panaretakis, T., Kepp, O., Brockmeier, U., Tesniere, A., Bjorklund, A. C., Chapman, D. C., et al. (2009). Mechanisms of pre-apoptotic calreticulin exposure in immunogenic cell death. EMBO J, 28(5), 578–590.
Breckenridge, D. G., Stojanovic, M., Marcellus, R. C., & Shore, G. C. (2003). Caspase cleavage product of bap31 induces mitochondrial fission through endoplasmic reticulum calcium signals, enhancing cytochrome c release to the cytosol. J Cell Biol, 160(7), 1115–1127.
Kroemer, G., Galluzzi, L., & Brenner, C. (2007). Mitochondrial membrane permeabilization in cell death. Physiol Rev, 87(1), 99–163.
Kepp, O., Senovilla, L., Galluzzi, L., Panaretakis, T., Tesniere, A., Schlemmer, F., et al. (2009). Viral subversion of immunogenic cell death. Cell Cycle, 8(6), 860–869.
Panaretakis, T., Joza, N., Modjtahedi, N., Tesniere, A., Vitale, I., Durchschlag, M., et al. (2008). The co-translocation of erp57 and calreticulin determines the immunogenicity of cell death. Cell Death Differ, 15(9), 1499–1509.
Fadok, V. A., Voelker, D. R., Campbell, P. A., Cohen, J. J., Bratton, D. L., & Henson, P. M. (1992). Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J Immunol, 148(7), 2207–2216.
Tyurina, Y. Y., Basova, L. V., Konduru, N. V., Tyurin, V. A., Potapovich, A. I., Cai, P., et al. (2007). Nitrosative stress inhibits the aminophospholipid translocase resulting in phosphatidylserine externalization and macrophage engulfment: implications for the resolution of inflammation. J Biol Chem, 282(11), 8498–8509.
Tarr, J. M., Young, P. J., Morse, R., Shaw, D. J., Haigh, R., Petrov, P. G., et al. (2010). A mechanism of release of calreticulin from cells during apoptosis. J Mol Biol, 401(5), 799–812.
Latour, S., Tanaka, H., Demeure, C., Mateo, V., Rubio, M., Brown, E. J., et al. (2001). Bidirectional negative regulation of human T and dendritic cells by cd47 and its cognate receptor signal-regulator protein-alpha: down-regulation of IL-12 responsiveness and inhibition of dendritic cell activation. J Immunol, 167(5), 2547–2554.
Oldenborg, P. A., Gresham, H. D., & Lindberg, F. P. (2001). Cd47-signal regulatory protein alpha (sirpalpha) regulates fcgamma and complement receptor-mediated phagocytosis. J Exp Med, 193(7), 855–862.
Castelli, C., Ciupitu, A. M., Rini, F., Rivoltini, L., Mazzocchi, A., Kiessling, R., et al. (2001). Human heat shock protein 70 peptide complexes specifically activate antimelanoma T cells. Cancer Res, 61(1), 222–227.
Gehrmann, M., Liebisch, G., Schmitz, G., Anderson, R., Steinem, C., De Maio, A., et al. (2008). Tumor-specific Hsp70 plasma membrane localization is enabled by the glycosphingolipid Gb3. PLoS ONE, 3(4), e1925.
Chen, T., Guo, J., Han, C., Yang, M., & Cao, X. (2009). Heat shock protein 70, released from heat-stressed tumor cells, initiates antitumor immunity by inducing tumor cell chemokine production and activating dendritic cells via TLR4 pathway. J Immunol, 182(3), 1449–1459.
Delamarre, L., Couture, R., Mellman, I., & Trombetta, E. S. (2006). Enhancing immunogenicity by limiting susceptibility to lysosomal proteolysis. J Exp Med, 203(9), 2049–2055.
Shiratsuchi, A., Watanabe, I., Takeuchi, O., Akira, S., & Nakanishi, Y. (2004). Inhibitory effect of Toll-like receptor 4 on fusion between phagosomes and endosomes/lysosomes in macrophages. J Immunol, 172(4), 2039–2047.
Tesniere, A., Schlemmer, F., Boige, V., Kepp, O., Martins, I., Ghiringhelli, F., et al. (2010). Immunogenic death of colon cancer cells treated with oxaliplatin. Oncogene, 29(4), 482–491.
Scaffidi, P., Misteli, T., & Bianchi, M. E. (2002). Release of chromatin protein hmgb1 by necrotic cells triggers inflammation. Nature, 418(6894), 191–195.
Bell, C. W., Jiang, W., Reich, C. F., III, & Pisetsky, D. S. (2006). The extracellular release of hmgb1 during apoptotic cell death. Am J Physiol Cell Physiol, 291(6), C1318–1325.
Andersson, U., Wang, H., Palmblad, K., Aveberger, A. C., Bloom, O., Erlandsson-Harris, H., et al. (2000). High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. J Exp Med, 192(4), 565–570.
Wang, H., Bloom, O., Zhang, M., Vishnubhakat, J. M., Ombrellino, M., Che, J., et al. (1999). HMG-1 as a late mediator of endotoxin lethality in mice. Science, 285(5425), 248–251.
Bonaldi, T., Talamo, F., Scaffidi, P., Ferrera, D., Porto, A., Bachi, A., et al. (2003). Monocytic cells hyperacetylate chromatin protein HMGB1 to redirect it towards secretion. EMBO J, 22(20), 5551–5560.
Gardella, S., Andrei, C., Ferrera, D., Lotti, L. V., Torrisi, M. R., Bianchi, M. E., et al. (2002). The nuclear protein HMGB1 is secreted by monocytes via a non-classical, vesicle-mediated secretory pathway. EMBO Rep, 3(10), 995–1001.
Sancho, D., Joffre, O. P., Keller, A. M., Rogers, N. C., Martinez, D., Hernanz-Falcon, P., et al. (2009). Identification of a dendritic cell receptor that couples sensing of necrosis to immunity. Nature, 458(7240), 899–903.
Shankaran, V., Ikeda, H., Bruce, A. T., White, J. M., Swanson, P. E., Old, L. J., et al. (2001). IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature, 410(6832), 1107–1111.
Schroder, K., & Tschopp, J. (2010). The inflammasomes. Cell, 140(6), 821–832.
Franchi, L., Eigenbrod, T., Munoz-Planillo, R., & Nunez, G. (2009). The inflammasome: a caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat Immunol, 10(3), 241–247.
Latz, E. (2010). The inflammasomes: mechanisms of activation and function. Curr Opin Immunol, 22(1), 28–33.
Martins, I., Tesniere, A., Kepp, O., Michaud, M., Schlemmer, F., Senovilla, L., et al. (2009). Chemotherapy induces ATP release from tumor cells. Cell Cycle, 8(22), 3723–3728.
Ferrari, D., Pizzirani, C., Adinolfi, E., Lemoli, R. M., Curti, A., Idzko, M., et al. (2006). The P2X7 receptor: a key player in IL-1 processing and release. J Immunol, 176(7), 3877–3883.
Elliott, M. R., Chekeni, F. B., Trampont, P. C., Lazarowski, E. R., Kadl, A., Walk, S. F., et al. (2009). Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature, 461(7261), 282–286.
Stout, C. E., Costantin, J. L., Naus, C. C., & Charles, A. C. (2002). Intercellular calcium signaling in astrocytes via ATP release through connexin hemichannels. J Biol Chem, 277(12), 10482–10488.
Zhang, Z., Chen, G., Zhou, W., Song, A., Xu, T., Luo, Q., et al. (2007). Regulated ATP release from astrocytes through lysosome exocytosis. Nat Cell Biol, 9(8), 945–953.
Acknowledgments
GK is supported by the Ligue Nationale contre le Cancer (Equipes labellisée), Agence Nationale pour la Recherche (ANR), European Commission (Apo-Sys, ChemoRes, ApopTrain), Fondation pour la Recherche Médicale (FRM), Institut National du Cancer (INCa), and Cancéropôle Ile-de-France. IM is supported by the Ligue Nationale contre le Cancer, OK by AICR, LG by Apo-Sys and FS by FRM.
Author information
Authors and Affiliations
Corresponding author
Additional information
Oliver Kepp and Lorenzo Galluzzi contributed equally to this work.
Rights and permissions
About this article
Cite this article
Kepp, O., Galluzzi, L., Martins, I. et al. Molecular determinants of immunogenic cell death elicited by anticancer chemotherapy. Cancer Metastasis Rev 30, 61–69 (2011). https://doi.org/10.1007/s10555-011-9273-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10555-011-9273-4