Abstract
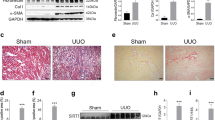
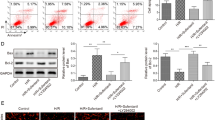
We have found that Fas/FasL-mediated “extrinsic” pathway promoted cell apoptosis induced by renal ischemic injury. This study is to elucidate the upstream mechanism regulating FasL-induced extrinsic pathway during renal ischemia/reperfusion. Results demonstrated that when SIRT2 was activated by renal ischemia/reperfusion, activated SIRT2 could bind to and deacetylate FOXO3a, promoting FOXO3a nuclear translocation which resulted in an increase of nuclear FOXO3a along with FasL expression and activation of caspase8 and caspase3, triggering cell apoptosis during renal ischemia/reperfusion. The administration of SIRT2 inhibitor AGK2 prior to renal ischemia decreased significantly the number of apoptotic renal tubular cells and alleviated ultrastructure injury. These results indicate that inhibition of FOXO3a deacetylation might be a promising therapeutic approach for renal ischemia /reperfusion injury.







Similar content being viewed by others
References
Gobe G, Willgoss D, Hogg N, Schoch E, Endre Z (1999) Cell survival or death in renal tubular epithelium after ischemia–reperfusion injury. Kidney Int 56:1299–1304
Wang Y, Ji HX, Xing SH, Pei DS, Guan QH (2007) SP600125, a selective JNK inhibitor, protects ischemic renal injury via suppressing the extrinsic pathways of apoptosis. Life Sci 80:2067–2075
Havasi A, Borkan SC (2011) Apoptosis and acute kidney injury. Kidney Int 80:29–40
Fadeel B, Orrenius S (2005) Apoptosis: a basic biological phenomenon with wide ranging implications in human disease. J Int Med 258:479–517
Kim R, Emi M, Tanabe K, Murakami S, Uchida Y, Arihiro K (2006) Regulation and interplay of apoptotic and non-apoptotic cell death. J Pathol 208:319–326
Zhuang S, Kinsey GR, Yan Y, Han J, Schnellmann RG (2008) Extracellular signal-regulated kinase activation mediates mitochondrial dysfunction and necrosis induced by hydrogen peroxide in renal proximal tubular cells. J Pharmacol Exp Ther 325: 732–740
Wang Y, Pei DS, Ji HX, Xing SH (2008) Protective effect of a standardized Ginkgo extract (Ginaton) on renal ischemia/reperfusion injury via suppressing the activation of JNK signal pathway. Phytomedicine 15: 923–931
Wang Y, Ji HX, Zheng JN, Pei DS, Hu SQ, Qiu SL (2009) Protective effect of selenite on renal ischemia/reperfusion injury through inhibiting ASK1-MKK3-p38 signal pathway. Redox Report 14:243–250
Faris M, Latinis KM, Kempiak SJ, Koretzky GA, Nel A (1998) Stress-induced Fas ligand expression in T cells is mediated through a MEK kinase 1-regulated response element in the Fas ligand promoter. Mol Cell Biol 18:5414–5424
He P, Zhou G, Qu D, Zhang B, Wang Y, Li D (2013) HBx inhibits proliferation and induces apoptosis via Fas/FasL upregulation in rat renal tubular epithelial cells. J Nephrol 26:1033–1041
Wang Q, Zhang QG, Wu DN, Yin XH, Zhang GY (2007) Neuroprotection of selenite against ischemic brain injury through negatively regulating early activation of ASK1/JNK cascade via activation of PI3K/AKT pathway. Acta Pharmacol Sin 28:19–27
Guan QH, Pei DS, Liu XM, Wang XT, Xu TL, Zhang GY (2006) Neuroprotection against ischemic brain injury by SP600125 via suppressing the extrinsic and intrinsic pathways of apoptosis. Brain Res 1092:36–46
Carter ME, Brunet A (2007) FOXO transcription factors. Curr Biol 17:R113–R114
Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME (1999) Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 96: 857–868
Barthélémy C, Henderson CE, Pettmann B (2004) Foxo3a induces motoneuron death through the Fas pathway in cooperation with JNK. BMC Neurosci 5:48
Yang JY, Xia W, Hu MC (2006) Ionizing radiation activates expression of FOXO3a, Fas ligand, and Bim, and induces cell apoptosis. Int J Oncol 29:643–648
Wang F, Nguyen M, Qin FX, Tong Q (2007) SIRT2 deacetylates FOXO3a in response to oxidative stress and caloric restriction. Aging Cell 6:505–514
Zhang XB, Tang N, Hadden TJ, Rishi AK (2011) Akt, FoxO and regulation of apoptosis. Biochim Biophys Acta 1813:1978–1986
Fu Z, Tindall DJ (2008) FOXOs, cancer and regulation of apoptosis. Oncogene 27:2312–2319
Fukunaga K, Ishigami T, Kawano T (2005) Transcriptional regulation of neuronal genes and its effect on neural functions: expression and function of forkhead transcription factors in neurons. J Pharmacol Sci 98: 205–211
Gilley J, Coffer PJ, Ham J (2003) FOXO transcription factors directly activate bim gene expression and promote apoptosis in sympathetic neurons. J Cell Biol 162:613–622
Hasegawa K, Kawahara T, Fujiwara K et al (2012) Necdin controls Foxo1 acetylation in hypothalamic arcuate neurons to modulate the thyroid axis. J Neurosci 32:5562–5572
Bahia PK, Pugh V, Hoyland K, Hensley V, Rattray M, Williams RJ (2012) Neuroprotective effects of phenolic antioxidant tBHQ associate with inhibition of FoxO3a nuclear translocation and activity. J Neurochem 123:182–191
Sundaresan NR, Gupta M, Kim G, Rajamohan SB, Isbatan A, Gupta MP (2009) Sirt3 blocks the cardiac hypertrophic response by augmenting Foxo3a-dependent antioxidant defense mechanisms in mice. J Clin Invest 119:2758–2771
Essers MA, Weijzen S, de Vries-Smits AM, Saarloos I, de Ruiter ND, Bos JL, Burgering BM (2004) FOXO transcription factor activation by oxidative stress mediated by the small GTPase Ral and JNK. EMBO J 23:4802–4812
Verdin E, Hirschey MD, Finley LWS, Haigis MC (2010) Sirtuin regulation of mitochondria: energy production, apoptosis, and signaling. Trends Biochem Sci 35:669–675
North BJ, Marshall BL, Borra MT, Denu JM, Verdin E (2003) The human Sir2 ortholog, SIRT2, is an NAD+-dependent tubulin deacetylase. Mol Cell 11:437–444
Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL et al (2004) Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science 303:2011–2015
Narayan N, Lee IH, Borenstein R, Sun J, Wong R et al (2012) The NAD-dependent deacetylase SIRT2 is required for programmed necrosis. Nature 492:199–204
Outeiro TF, Kontopoulos E, Altmann SM, Kufareva I, Strathearn KE et al (2007) Sirtuin 2 inhibitors rescue alpha-synuclein-mediated toxicity in models of Parkinson’s disease. Science 317:516–519
Donmez G, Outeiro TF (2013) SIRT1 and SIRT2: emerging targets in neurodegeneration. EMBO Mol Med 5:344–352
Liu NM, Tian J, Wang WW, Han GF, Cheng J, Huang J, Zhang JY (2013) Effect of erythropoietin on mesenchymal stem cell differentiation and secretion in vitro in an acute kidney injury microenvironment. Genet Mol Res 12:6477–6487
Michan S, Sinclair D (2007) Sirtuins in mammals: insights into their biological function. Biochem J 404:1–13
Fan H, Yang HC, You L, Wang YY, He WJ, Hao CM (2013) The histone deacetylase, SIRT1, contributes to the resistance of young mice to ischemia/reperfusion-induced acute kidney injury. Kidney Int 83:404–413
Daitoku H, Hatta M, Matsuzaki H, Aratani S, Ohshima T, Miyagishi M, Nakajima T, Fukamizu A (2004) Silent information regulator 2 potentiates Foxo1-mediated transcription through its deacetylase activity. Proc Natl Acad Sci 101: 10042–10047
Sessler T, Healy S, Samali A, Szegezdi E (2013) Structural determinants of DISC function: new insights into death receptor-mediated apoptosis signalling. Pharmacol Ther 140:186–199
Price PM, Hodeify R (2012) A possible mechanism of renal cell death after ischemia/reperfusion. Kidney Int 81:720–721
Anderson MJ, Viars CS, Czekay S, Cavenee WK, Arden KC (1998) Cloning and characterization of three human forkhead genes that comprise an FKHR-like gene subfamily. Genomics 47:187–199
Wang J, Liu S, Yin Y, Li M, Wang B, Yang L, Jiang Y (2015) FOXO3-mediated up-regulation of Bim contributes to rhein-induced cancer cell apoptosis. Apoptosis 20:399–409
Biggs WH III, Meisenhelder J, Hunter T, Cavenee WK, Arden KC (1999) Protein kinase B/Akt-mediated phosphorylation promotes nuclear exclusion of the winged helix transcription factor FKHR1. Proc Natl Acad Sci USA 96:7421–7426
Brownawell AM, Kops GJ, Macara IG, Burgering BM (2001) Inhibition of nuclear import by protein kinase B (Akt) regulates the subcellular distribution and activity of the forkhead transcription factor AFX. Mol Cell Biol 21:3534–3546
Van Der Heide LP, Hoekman MF, Smidt MP (2004) The ins and outs of FoxO shuttling: mechanisms of FoxO translocation and transcriptional regulation. Biochem J 380:297–309
Fukuoka M, Daitoku H, Hatta M, Matsuzaki H, Umemura S, Fukamizu A (2003) Negative regulation of forkhead transcription factor AFX (Foxo4) by CBP-induced acetylation. Int J Mol Med 12:503–508
Matsuzaki H, Daitoku H, Hatta M, Aoyama H, Yoshimochi K, Fukamizu A (2005) Acetylation of Foxo1 alters its DNA-binding ability and sensitivity to phosphorylation. Proc Natl Acad Sci USA 102:11278–11283
Bertaggia E, Coletto L, Sandri M (2012) Posttranslational modifications control FoxO3 activity during denervation. Am J Physiol Cell Physiol 302:C587–C596
Nakamura Y, Ogura M, Tanaka D, Inagaki N (2008) Localization of mouse mitochondrial SIRT proteins: shift of SIRT3 to nucleus by co-expression with SIRT5. Biochem Biophys Res Commun 366:174–179
Scher MB, Vaquero A, Reinberg D (2007) SirT3 is a nuclear NAD+-dependent histone deacetylase that translocates to the mitochondria upon cellular stress. Genes Dev 21:920–928
Acknowledgements
We are grateful to Dr. Mckaig, Donald R from the Rhode Island hospital, affiliated Hospital of Brown University USA, for proofreading. We thank Prof. Dong Hongyan, Department of Neurobiology of Xuzhou Medical College, for Ultramicroscopic Morphological observation of renal cell apoptosis. This work was supported by a grant from the Project of the National natural Science Foundation (No.81271267), the Project of the Natural Science Foundation of Jiangsu province (Nos. BK2010171 and BK20161179),and the Project of the Natural Science Foundation of Xuzhou (Nos. XZZD1338 and KC15SH047).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Wang, Y., Mu, Y., Zhou, X. et al. SIRT2-mediated FOXO3a deacetylation drives its nuclear translocation triggering FasL-induced cell apoptosis during renal ischemia reperfusion. Apoptosis 22, 519–530 (2017). https://doi.org/10.1007/s10495-016-1341-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-016-1341-3