Abstract
Purpose
TSU-68 is a novel multiple tyrosine kinase inhibitor that inhibits vascular endothelial growth factor receptor-2, platelet-derived growth factor receptor and fibroblast growth factor receptor. TSU-68 demonstrated a strong anti-tumor effect against established human breast cancer xenografts in nude mice without apparent toxicity. We conducted a phase II study to evaluate the efficacy and safety of TSU-68 monotherapy in patients with metastatic breast cancer progressing despite prior treatment with an anthracycline-containing regimen and taxane.
Methods
TSU-68 was administered daily at a dose of 400 mg twice a day after meals in 20 patients. The primary endpoint was objective overall response rate according to the Response Evaluation Criteria in Solid Tumors guideline version 1.0. Secondary endpoints included clinical benefit rate (complete response, partial response and stable disease lasting for at least 24 weeks), exploratory assessments of change in mRNA levels of biological markers associated with angiogenesis in tumor tissue at the end of Cycle 1, and safety of TSU-68.
Results
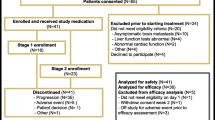
Twenty patients were enrolled into the study from October 2002 through April 2003. TSU-68 monotherapy produced objective overall response in none of the patients; however, clinical benefit was seen in 5 % of the patients. The mRNA levels of CD31, Flt-1 and Flk-1/KDR showed a decreasing trend in all 4 patients who provided additional written informed consent for collection of tumor tissue. However, no significant difference was observed in the change in mRNA level due to the small sample size. The most common adverse drug reaction (ADR) was tumor pain (60 %); hematological ADRs rarely occurred, and they were mild in severity. Only one patient experienced grade 2 rash and no patient experienced hypertension. No patients experienced a grade 4 ADR and no episode of death related to the study treatment occurred in the 20 patients.
Conclusions
TSU-68 monotherapy produced clinical benefit in only 5 % of the patients and did not produce objective overall response; however, the treatment was well tolerated. Further evaluation of the efficacy of TSU-68 will be worthwhile because the mRNA levels of CD31, Flt-1 and Flk-1/KDR decreased in 4 patients.


Similar content being viewed by others
References
Parkin DM, Bray F, Ferlay J et al (2005) Global cancer statistics, 2002. CA Cancer J Clin 55:74–108
Jemal A, Siegel R, Ward E et al (2008) Cancer Statistics, 2008. CA Cancer J Clin 58:71–96
Parkin DM, Bray FI, Devesa SS (2001) Cancer burden in the year 2000. The global picture. Eur J Cancer 37:S4–S66
Early Breast Cancer Trialists’ Collaborative Group (1998) Polychemotherapy for early breast cancer: an overview of the randomized trials. Lancet 352:930–942
Wood WC, Budman DR, Korzun AH et al (1994) Dose and dose intensity of adjuvant chemotherapy for stage II, node-positive breast carcinoma. N Engl J Med 330:1253–1259
Levine MN, Pritchard KI, Bramwell VH et al (2005) Randomized trial comparing cyclophosphamide, epirubicin, and fluorouracil with cyclophosphamide, methotrexate, and fluorouracil in premenopausal women with node-positive breast cancer: update of National Cancer Institute of Canada Clinical Trials Group Trial MA5. J Clin Oncol 23:5166–5170
Mamounas EP, Bryant J, Lembersky B et al (2005) Paclitaxel after doxorubicin plus cyclophosphamide as adjuvant chemotherapy for node-positive breast cancer: results from NSABP B-28. J Clin Oncol 23:3686–3696
Martin M, Pienkowski T, Mackey J et al (2005) Adjuvant docetaxel for node-positive breast cancer. N Engl J Med 352:2302–2313
Sparano JA, Wang M, Martino S et al (2008) Weekly paclitaxel in the adjuvant treatment of breast cancer. N Engl J Med 358:1663–1671
Folkman J (2007) Angiogenesis: an organizing principle for drug discovery? Nat Rev Drug Discov 6:273–286
Marty M, Pivot X (2008) The potential of anti-vascular endothelial growth factor therapy in metastatic breast cancer: clinical experience with anti-angiogenic agents, focusing on bevacizumab. Eur J Cancer 44:912–920
Benjamin LE, Golijanin D, Itin A et al (1999) Selective ablation of immature blood vessels in established human tumors follows vascular endothelial growth factor withdrawal. J Clin Invest 103:159–165
Abramsson A, Lindblom P, Betsholtz C (2003) Endothelial and nonendothelial sources of PDGF-B regulate pericyte recruitment and influence vascular pattern formation in tumors. J Clin Invest 112:1142–1151
Ostman A, Heldin CH (2007) PDGF receptors as targets in tumor treatment. Adv Cancer Res 97:247–274
Morikawa S, Baluk P, Kaidoh T et al (2002) Abnormalities in pericytes on blood vessels and endothelial sprouts in tumors. Am J Pathol 160:985–1000
Laird AD, Vajkoczy P, Shawver LK et al (2000) SU6668 is a potent antiangiogenic and antitumor agent that induces regression of established tumors. Cancer Res 60:4152–4160
Tokuyama J, Kubota T, Saikawa Y et al (2005) Tyrosine kinase inhibitor SU6668 inhibits peritoneal dissemination of gastric cancer via suppression of tumor angiogenesis. Anticancer Res 25(1A):17–22
Naumova E, Ubezio P, Garofalo A et al (2006) The vascular targeting property of paclitaxel is enhanced by SU6668, a receptor tyrosine kinase inhibitor, causing apoptosis of endothelial cells and inhibition of angiogenesis. Clin Cancer Res 12:1839–1849
Murakami H, Yamamoto N, Shimoyama T et al (2003) Phase I, pharmacokinetic, and biological studies of TSU-68, the oral vascular endothelial growth factor receptor tyrosine kinase inhibitor, administered after meals in patients with advanced solid tumors. Proc Am Soc Clin Oncol 22 (abstr 870)
Arai Y, Inaba Y, Yamamoto T et al (2010) A randomized phase II study of TSU-68 in patients (pts) with hepatocellular carcinoma (HCC) treated by transarterial chemoembolization (TACE). Proc Am Soc Clin Oncol 28 (abstr 4030)
Therasse P, Arbuck SG, Eisenhauer EA et al (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205–216
Common Toxicity Criteria v 2.0 (April 30, 1999), Cancer therapy evaluation program. National Cancer Institute, Bethesda
Fleming TR (1982) One-sample multiple testing procedure for phase II trials. Biometrics 38:143–151
Miller K, Wang M, Gralow J et al (2007) Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med 357:2666–2676
Miles D, Chan A, Romieu G et al (2008) Randomized, double-blind, placebo-controlled, phase III study of bevacizumab with docetaxel or docetaxel with placebo as first-line therapy for patients with locally recurrent or metastatic breast cancer: AVADO. J Clin Oncol 26 (abstr LBA1011)
Cobleigh MA, Langmuir VK, Sledge GW et al (2003) A phase I/II dose-escalation trial of bevacizumab in previously treated metastatic breast cancer. Semin Oncol 30:117–124
Miller KD, Chap LI, Holmes FA et al (2005) Randomized phase III trial of capecitabine compared with bevacizumab plus capecitabine in patients with previously treated metastatic breast cancer. J Clin Oncol 23:792–799
Carmeliet P (2005) Angiogenesis in life, disease and medicine. Nature 438:932–936
Casanovas O, Hicklin DJ, Bergers G et al (2005) Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer Cell 8:299–309
Mancuso MR, Davis R, Norberg SM et al (2006) Rapid vascular regrowth in tumors after reversal of VEGF inhibition. J Clin Invest 116:2610–2621
Xiong HQ, Herbst R, Faria SC et al (2004) A phase I surrogate endpoint study SU6668 in patients with solid tumors. Investig New Drug 22:459–466
Faivre S, Delbaldo C, Vera K et al (2006) Safety, pharmacokinetic, and antitumor activity of SU11248, a novel oral multitarget tyrosine kinase inhibitor, in patients with cancer. J Clin Oncol 24:25–35
Miller KD, Burstein HJ, Elias AD et al (2008) Phase II study of sunitinib malate, an oral multitargeted tyrosine kinase inhibitor, in patients with metastatic breast cancer previously treated with an anthracycline and a taxane. J Clin Oncol 26:1810–1816
Llovet JM, Ricci S, Mazzaferro V et al (2008) Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 359:378–390
Feldman DR, Baum MS, Ginsberg MS et al (2009) Phase I trial of bevacizumab plus escalated doses of sunitinib in patients with metastatic renal cell carcinoma. J Clin Oncol 27:1432–1439
Acknowledgments
We are also grateful to Yutaka Ariyoshi,Yuh Satata, and Tomohide Tamura for extramural review. We thank Junichi Yonezawa Toyomitsu Sato, Masahito Komuro, Kentaro Nagai, Hiroshi Nakayama, Yuji Takami, Itsumi Takaya, Kenzo Iizuka, and Kiyoshi Eshima for assistance in data collection and analysis.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Suzuki, Y., Saeki, T., Aogi, K. et al. A multicenter phase II study of TSU-68, a novel oral multiple tyrosine kinase inhibitor, in patients with metastatic breast cancer progressing despite prior treatment with an anthracycline-containing regimen and taxane. Int J Clin Oncol 18, 590–597 (2013). https://doi.org/10.1007/s10147-012-0421-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-012-0421-9