Abstract
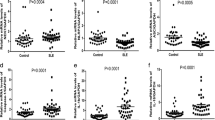
Tumor necrosis factor (TNF)-alpha is a pleiotropic cytokine. Systemic lupus erythematosus (SLE) is an autoimmune and inflammatory disease. However, until now, the expression and pathophysiological role of TNF adapters in SLE have been poorly understood. This study aims to investigate the expression of mRNA for the TNF adapter proteins including TNF receptor-associated death domain (TRADD) protein, Fas-associated death domain (FADD) protein, receptor-interacting protein 1 (RIP-1), and TNF receptor-associated factor-2 (TRAF-2) in peripheral blood mononuclear cells (PBMCs) from patients with SLE and to explore the relationship between the expression of these adapters and the SLE disease activity. PBMCs were isolated from the venous blood of 51 SLE patients and 17 healthy subjects. The expression of mRNA for TNF adapter molecules such as TRADD, FADD, RIP-1, and TRAF-2 in PBMCs were analyzed by semiquantitative reverse transcription-polymerase chain reaction (RT–PCR) and quantitative real-time RT–PCR. There were constitutive expressions of mRNA for TRADD, FADD, RIP-1, and TRAF-2 in PBMCs from healthy subjects. The expression of mRNA for all the adapter molecules significantly decreased in PBMCs from patients with SLE, which were 0.38-, 0.69-, 0.59-, and 0.55-fold, respectively, compared to those of control subjects (P < 0.05). The expression of Caspase 3 was significantly increased in SLE patients (P < 0.01); however, the expression of IL-1beta was not significantly different between SLE and control subjects. The expression of TRADD, FADD, RIP-1, and TRAF-2 in PBMCs from patients with SLE were negatively correlated with SLEDAI, the correlation coefficient of which was −0.285, −0.280, −0.307, and −0.298, respectively (P < 0.05). The expression of mRNA for TNF adapter molecules TRADD, FADD, RIP-1, and TRAF-2 decreased significantly in PBMCs from patients with SLE, and the expression of these adapters were negatively correlated with the SLE activity index. These abnormalities may be involved in the immunopathogenic injury mediated by the aberration TNF-alpha signaling pathway in SLE.




Similar content being viewed by others
References
Manson JJ, Isenberg DA (2003) The pathogenesis of systemic lupus erythematosus. Neth J Med 61:343–346
Jin O, Sun LY, Zhou KX et al (2005) Lymphocyte apoptosis and macrophage function: correlation with disease activity in systemic lupus erythematosus. Clin Rheumatol 24:107–110
Bijl M, Limburg PC, Kallenberg CG et al (2001) New insights into the pathogenesis of systemic lupus erythematosus (SLE): the role of apoptosis. Neth J Med 59:66–75
Gabler C, Kalden JR, Lorenz HM (2003) The putative role of apoptosis-modified histones for the induction of autoimmunity in systemic lupus erythematosus. Biochem Pharmacol 66:1441–1446
Lorenz HM, Herrmann M, Winkler T et al (2000) Role of apoptosis in autoimmunity. Apoptosis 5:443–449
Savill J, Fadok V (2000) Corpse clearance defines the meaning of cell death. Nature 407:784–788
Locksley RM, Killeen N, Lenardo MJ (2001) The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell 104:487–501
Véronique B, Michael K (2001) Signal transduction by tumor necrosis factor and its relatives. Trends Cell Biol 11:372–377
Wajant H, Pfizenmaier K, Scheurich P (2003) Tumor necrosis factor signaling. Cell Death Differ 10:45–65
Wiegmann K, Schutze S, Kampen E et al (1992) Human 55 kDa receptor for tumor necrosis factor coupled to signal transduction cascades. J Immunol 267:17997–18001
Hsu H, Xiong J, Goeddel DV (1995) The TNF receptor-1 associated protein TRADD signal cell death and NF-κB activation. Cell 81:495–504
Tartalia LA, Ayres TM, Wong GHW et al (1993) A novel domain within the 55 kDa TNF receptor signals cell death. Cell 74:845–853
Rothe M, Sarma V, Dixit VM et al (1995) TRAF-2 mediated activation of NF-κB by TNF receptor 2 and CD40. Science 269:1424–1427
Natoli G, Costanzo A, Lanni A et al (1997) Activation of SAPK/JNK by TNF receptor I through a non-cytotoxic TRAF-2 dependent pathway. Science 275:200–203
Liu ZG (2005) Molecular mechanism of TNF signaling and beyond. Cell Res 15:24–27
Aringer M, Feierl E, Steiner G et al (2002) Increased bioactive TNF in human systemic lupus erythematosus: associations with cell death. Lupus 11:102–108
Gabay C, Cakir N, Moral F et al (1997) Circulating levels of tumor necrosis factor soluble receptors in systemic lupus erythematosus are significantly higher than in other rheumatic diseases and correlate with disease activity. J Rheumatol 24:303–308
Aringer M, Zimmermann C, Graninger WB et al (2002) TNF is an essential mediator in lupus nephritis. Arthritis Rheum 46:3418–3419 (abstract)
Kontoyiannis D, Kollias G (2000) Accelerated autoimmunity and lupus nephritis in NZB mice with an engineered heterozygous deficiency in tumor necrosis factor. Eur J Immunol 30:2038–2047
Tan EM, Cohen AS, Fries JF et al (1982) The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 25:1271–1277
Bombardier C, Gladman DD, Urowitz MB et al (1992) Derivation of the SLEDAI. A disease activity index for lupus patients. The Committee on Prognosis Studies in SLE. Arthritis Rheum 35:630–640
Weening JJ, D’Agati VD, Schwartz MM et al (2004) The classification of glomerulonephritis in systemic lupus erythematosus revisited. J Am Soc Nephrol 15:241–250
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the \( 2^{{ - \Delta \Delta {\text{CT}}}} \) method. Methods 25:402–408
McDevitt H, Munson S, Ettinger R et al (2002) Multiple roles for tumor necrosis factor-α and lymphotoxinα/betain immunity and autoimmunity. Arthritis Res 4(Suppl 3):S141–S152
Aringer M, Smolen JS (2003) Complex cytokine effects in a complex autoimmune disease: tumor necrosis factor in systemic lupus erythematosus. Arthritis Res Ther 5:172–177
Rothe M, Sarma V, Dixit VM et al (1995) TRAF2-mediated activation of NF-κB by TNF receptor 2 and CD40. Science 269:1424–1427
Stanger BZ, Leder P, Lee TH et al (1995) RIP: a novel protein containing a death domain that interacts with Fas/APO-1 (CD95) in yeast and causes cell death. Cell 81:513–523
Chinnaiyan AM, O’Rourke K, Tewari M et al (1995) FADD, a novel death domain-containing protein, interacts with the death domain of Fas and initiates apoptosis. Cell 81:505–512
Baud V, Karin M (2001) Signal transduction by tumor necrosis factor and its relatives. Trends Cell Biol 9(11):372–377 (September)
Maas K, Chan S, Parker J et al (2002) Cutting edge: molecular portrait of human autoimmune disease. J Immunol 169:5–9
Emlen W, Niebur J, Kadera R (1994) Accelerated in vitro apoptosis of lymphocytes from patients with systemic lupus erythematosus. J Immunol 152:3685
Lorenz HM, Grunke M, Hiernymus T et al (1997) In vitro apoptosis and expression of apoptosis-related molecules in lymphocytes from patients with systemic lupus erythematosus and other autoimmune diseases. Arthritis Rheum 40:306
Rosen A, Casciola-Rosen L (2001) Clearing the way to mechanisms of autoimmunity. Nat Med 7:664
Balomenos D, Martinez AC (2000) Cell-cycle regulation in immunity, tolerance and autoimmunity. Immunol Today 21:551
Jin O, Sun LY, Zhou KX et al (2005) Lymphocyte apoptosis and macrophage function: correlation with disease activity in systemic lupus erythematosus. Clin Rheumatol 24:107–110
Devitt A, Pierce S, Oldreive C et al (2003) CD14-dependent clearance of apoptotic cells by human macrophages: the role of phosphatidylserine. Cell Death Differ 10:371–382
Gabler C, Kalden JR, Lorenz HM (2003) The putative role of apoptosis-modified histones for the induction of autoimmunity in systemic lupus erythematosus. Biochem Pharmacol 66:1441–1446
Gordon C, Salmon M (2001) Update on systemic lupus erythematosus: autoantibodies and apoptosis. Clin Med 1:10–14
Lemay S, Mao C, Singh AK (1996) Cytokine gene expression in the MRL/lpr model of lupus nephritis. Kidney Int 50:85–93
Donnelly, RP, Levine J et al (1990) Aberrant regulation of interleukin 1 expression in macrophages from young autoimmune-prone mice. J Immunol 145:3231
Maczynska I, Millo B, Ratajczak-Stefanska V et al (2006) Proinflammatory cytokine (IL-1beta, IL-6, IL-12, IL-18 and TNF-alpha) levels in sera of patients with subacute cutaneous lupus erythematosus (SCLE). Immunol Lett 102(1):79–82, 15
Fan H, Longacre A, Meng F, Patel V, Hsiao K et al (2004) Cytokine dysregulation induced by apoptotic cells is a shared characteristic of macrophages from nonobese diabetic and systemic lupus erythematosus-prone mice. J Immunol 172(8):4834–4843, 15
Koh JS, Wang Z, Levine JS (2000) Cytokine dysregulation induced by apoptotic cells is a shared characteristic of murine lupus. J Immunol 165(8):4190–4201
Acknowledgements
We gratefully thank all the patients, healthy volunteers, and all the medical staff who generously collaborated with this research. We are grateful to Prof. Youyan Yang, Hanshi Xu, and the staff of the Division of Department of Rheumatology, First Affiliated Hospital of Sun Yat-sen University for their assistance with the sample collection. This work is supported by the Chinese National Natural Science Research Grant (Grant no. 30271668 and 30572290) to Prof. Xiao Yang.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhu, L., Yang, X., Chen, W. et al. Decreased expressions of the TNF-alpha signaling adapters in peripheral blood mononuclear cells (PBMCs) are correlated with disease activity in patients with systemic lupus erythematosus. Clin Rheumatol 26, 1481–1489 (2007). https://doi.org/10.1007/s10067-006-0531-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-006-0531-8