Abstract
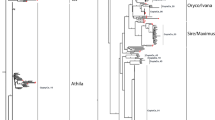
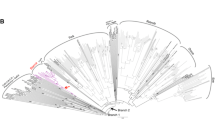
In this paper we describe a pair of novel Ty3/gypsy retrotransposons isolated from the dioecious plant Silene latifolia, consisting of a non-autonomous element Retand-1 (3.7 kb) and its autonomous partner Retand-2 (11.1 kb). These two elements have highly similar long terminal repeat (LTR) sequences but differ in the presence of the typical retroelement coding regions (gag-pol genes), most of which are missing in Retand-1. Moreover, Retand-2 contains two additional open reading frames in antisense orientation localized between the pol gene and right LTR. Retand transcripts were detected in all organs tested (leaves, flower buds and roots) which, together with the high sequence similarity of LTRs in individual elements, indicates their recent transpositional activity. The autonomous elements are similarly abundant (2,700 copies) as non-autonomous ones (2,100 copies) in S. latifolia genome. Retand elements are also present in other Silene species, mostly in subtelomeric heterochromatin regions of all chromosomes. The only exception is the subtelomere of the short arm of the Y chromosome in S. latifolia which is known to lack the terminal heterochromatin. An interesting feature of the Retand elements is the presence of a tandem repeat sequence, which is more amplified in the non-autonomous Retand-1.





Similar content being viewed by others
References
Altshul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402
Ananiev EV, Phillips RL, Rines HW (1998) Complex structure of knob DNA chromosome 9: retrotransposon invasion into heterochromatin. Genetics 149:2025–2037
Balint-Kurti PJ, Clendennen SK, Dolezelova M, Valarik M, Dolezel J, Beetham PR, May GD (2000) Identification and chromosomal localization of the monkey retrotransposon in Musa sp. Mol Gen Genet 263:908–915
Bennetzen JL (1996) The contributions of retroelements to plant genome organization, function and evolution. Trends Microbiol 4:347–353
Brodie R, Roper RL, Upton C (2004) JDotter: a Java interface to multiple doplots generated by dotter. Bioinformatics 20:279–281
Buzek J, Koutnikova H, Houben A, Riha K, Janousek B, Siroky J, Grant S, Vyskot B (1997) Isolation and characterization of X chromosome-derived DNA sequences from a dioecious plant Melandrium album. Chromosome Res 5:57–65
Chavanne F, Zhang D-X, Liaud M-F, Cerff R (1998) Structure and evolution of Cyclops: a novel giant retrotransposon of the Ty3/Gypsy family highly amplified in pea and other legume species. Plant Mol Biol 37:363–375
Cheng ZJ, Murata M (2003) A centromeric tandem repeat family originating from a part of Ty3/gypsy-retroelement in wheat and its relatives. Genetics 164:665–672
Dong F, Miller JT, Jackson S, Wang G-L, Ronald PC, Jiang J (1998) Rice (Oryza sativa) centromeric regions consist of complex DNA. Proc Natl Acad Sci USA 95:8135–8140
Feschotte C, Jiang N, Wessler SR (2002) Plant transposable elements: where genetics meets genomics. Nat Rev Genet 3:329–341
Fransz PF, Armstrong A, de Jong JH, Parnell LD, van Drunen G, Dean C, Zabel P, Bisseling T, Jones GH (2000) Integrated cytogenetic map of chromosome arm 4S of A. thaliana: structural organization of heterochromatic knob and centromere region. Cell 100:367–376
Fukui K-N, Suzuki G, Lagudah ES, Rahman S, Appels R, Yamamoto M, Mukai Y (2001) Physical arrangement of retrotransopson-related repeats in centromeric region in wheat. Plant Cell Physiol 42:189–196
Havecker ER, Gao X, Voytas DF (2004) The diversity of LTR retrotransposons. Genome Biol 5:225
Hull R (2001) Classifying reverse transcribing elements: a proposal and a challenge to the ICTV. Arch Virol 146:2255–2261
Jiang N, Bao Z, Temnykh S, Cheng Z, Jiang J, Wing RA, McCouch SR, Wesler SR (2002a) Dasheng: a recently amplified nonautonomous long terminal repeat element that is a major component of pericentromeric regions in rice. Genetics 161:1293–1305
Jiang N, Jordan IK, Wessler SR (2002b) Dasheng and RIRE2. A nonautonomous long terminal repeat element and its putative autonomous partner in the rice genome. Plant Physiol 130:1697–1705
Kalendar R, Tanskanen J, Immonen S, Nevo E, Schulman AH (2000) Genome evolution of wild barley (Hordeum spontaneum) by BARE-1 retrotransposon dynamics in response to sharp microclimatic divergence. Proc Natl Acad Sci USA 97:6603–6607
Kalendar R, Vicient CM, Peleg O, Anamthawat-Jonsson K, Bolshoy A, Schulman AH (2004) Large retrotransposon derivates: abundant, conserved, but nonautonomous retroelements of barley and related genomes. Genetics 166:1437–1450
Kumar A, Bennetzen JL (1999) Plant retrotransposons. Annu Rev Genet 33:479–532
Langdon T, Seago C, Jones RN, Ougham H, Thomas H, Forster JW, Jenkins G (2000) De novo evolution of satellite DNA on the rye B chromosome. Genetics 154:869–884
Lankenau S, Corces VG, Lankenau DH (1994) The Drosophila micropia retrotransposon encodes a testis specific antisense RNA complementary to reverse transcriptase. Mol Cell Biol 14:1764–1775
Lengerova M, Moore RC, Grant SR, Vyskot B (2003) The sex chromosomes of Silene latifolia revisited and revised. Genetics 165:935–938
Lengerova M, Kejnovsky E, Hobza R, Macas J, Grant SR, Vyskot B (2004) Multicolor FISH mapping of the dioecious model plant, Silene latifolia. Theor Appl Genet 108:1193–1199
Lowe TM, Eddy SR (1997) tRNAscan-SE: a program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res 25:955–964
Macas J, Navratilova A, Meszaros T (2003) Sequence subfamilies of satellite repeats related to rDNA intergenic spacer are differentially amplified on Vicia sativa chromosomes. Chromosoma 112:152–158
Marchler-Bauer A, Anderson JB, DeWeese-Scott C, Fedorova ND, Geer LY, He S, Hurwitz DI et al (2003) CDD: a curated Entrez database of conserved domain alignments. Nucleic Acids Res 31:383–387
Martinez-Izquierdo JA, Garcia-Martinez J, Vicient CM (1997) What makes Grande1 retrotransposon different? Genetica 100:15–28
Matsunaga S, Yagisawa F, Yamamoto M, Uchida W, Nakao S, Kawano S (2002) LTR retrotransposons in the dioecious plant Silene latifolia. Genome 45:745–751
Miller JT, Jackson SA, Nasuda S, Gill BS, Wing RA, Jiang J (1998) Cloning and characterization of a centromere-specific repetitive DNA element from Sorghum bicolor. Theor Appl Genet 96:832–839
Monfort A, Vicient CM, Raz R, Puigdomenech P, Martinez-Izquierdo J (1995). Molecular analysis of a putative transposable retroelement from the Zea genus with internal clusters of tandem repeats. DNA Res 2:255–261
Neumann P, Pozarkova D, Macas J (2003) Highly abundant pea LTR retrotransposon Ogre is constitutively transcribed and partially spliced. Plant Mol Biol 53:399–410
Neumann P, Pozarkova D, Koblizkova A, Macas J (2005) PIGY, a new plant envelope-class LTR retrotransposon. Mol Gen Genomics 273:43–53
Ohtsubo H, Kumekawa N, Ohtsubo E (1999) RIRE2, a novel gypsy-type retrotransposon from rice. Genes Genet Syst 74:83–91
Pardue ML, Danilevskaya ON, Lowenhaupt K, Slot F, Traverse KL (1996) Drosophila telomeres: new view on chromosome evolution. Trend Genet 12:48–52
Pasero P, Sjakste N, Blettry C, Got C, Marilley M (1993) Long-range organization and sequence-directed curvature of Xenopus laevis satellite 1 DNA. Nucleic Acids Res 21:4703–4710
Pearce SR, Harrison G, Li D, Heslop-Harrison JS, Kumar A, Flavell AJ (1996a) The Ty1-copia group retrotransposons in Vicia species: copy number, sequence heterogeneity and chromosomal localisation. Mol Gen Genet 250:305–315
Pearce SR, Pich U, Harrison G, Heslop-Harrison JS, Flavell AJ, Schubert I, Kumar A (1996b) The Ty1-copia group retrotransposons of Allium cepa are distributed throughout the chromosomes but are enriched in the terminal heterochromatin. Chromosome Res 4:365–371
Pearson WR, Lipman DJ (1988) Improved tools for biological sequence comparison. Proc Natl Acad Sci USA 85:2444–2448
Peterson-Burch BD, Wright DA, Laten HM, Voytas DF (2000) Retroviruses in plants? Trends Genet 16:151–152
Presting GG, Malysheva L, Fuchs J, Schubert I (1998) A Ty3/gypsy retrotransposon-like sequences localize to the centromeric regions of cereal chromosomes. Plant J 16:721–728
Rossi MS, Pesce CG, Reig OA, Kornblihtt AR, Zorzopulos J (1993) Retroviral-like features in the monomer of the major satellite DNA from the South American rodents of the genus Ctenomys. DNA Seq 3:379–381
SanMiguel P, Bennetzen J (1998) Evidence that recent increase in maize genome size was caused by the massive amplification of intergene retrotransposons. Ann Bot 82:37–44
SanMiguel P, Tikhonov A, Jin YK, Motchoulskaia N, Zakharov D, Melake-Behan A, Springer PS, Edwards KJ, Lee M, Avramova Z, Bennetzen JL (1996) Nested retrotransposons in the intergenic regions of the maize genome. Science 273:765–769
Sanz-Alferez S, SanMiguel P, Jin Y-K, Springer PS, Bennetzen JL (2003) Structure and evolution of the Cinful retrotransposon family of maize. Genome 46:745–752
Siroky J, Lysak MA, Dolezel J, Kejnovsky E, Vyskot B (2001) Heterogeneity of rDNA distribution and genome size in Silene spp. Chromosome Res 9:387–393
Staden R (1996) The Staden sequence analysis package. Mol Biotechnol 5:233–241
Suoniemi A, Tanskanen J, Schulman AH (1998) Gypsy like retrotransposons are widespread in the plant kingdom. Plant J 13:699–705
Tek AL, Song J, Macas J, Jiang J (2005) Sobo, a recently amplified satellite repeat of potato, and its implications for the origin of tandemly repeated sequences. Genetics 170:1231–1238
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Unfried K, Schiebel K, Hemleben V (1991) Subrepeats of rDNA intergenic spacer present as prominent independent satellite DNA in Vigna radiata but not in Vigna angularis. Gene 99:63–68
Vicient CM, Suoniemi A, Anamthawat-Jonsson K, Tanskanen J, Beharav A, Nevo E, Schulman AH (1999) Retrotransposon BARE-1 and its role in genome evolution in the genus Hordeum. Plant Cell 11:1769–1784
Zaki EA (2003) Plant retroviruses: structure, evolution and future application. Afr J Biotechnol 2:136–139
Zuker M (2003) Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res 31:3406–3415
Acknowledgments
This work was supported by the Czech Science Foundation (grant nos. 204/05/2097, 204/05/H505 and 204/04/1207) and AVOZ50510513 from the Academy of Sciences of the Czech Republic.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M.-A. Grandbastien
Nucleotide sequence data reported are available in the DDBJ/EMBL/GenBank databases under the accession numbers DQ023669 (Retand-2) and DQ023670 (Retand-1).
Rights and permissions
About this article
Cite this article
Kejnovsky, E., Kubat, Z., Macas, J. et al. Retand: a novel family of gypsy-like retrotransposons harboring an amplified tandem repeat. Mol Genet Genomics 276, 254–263 (2006). https://doi.org/10.1007/s00438-006-0140-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00438-006-0140-x