Abstract
Objectives
To study the occurrence of relapse of herpes simplex encephalitis (HSE) and to find out whether soluble activity markers in cerebrospinal fluid (CSF) indicate direct viral or immune– mediated events.
Methods
A consecutive series of 32 adult survivors of HSE were followed to determine the incidence of clinical relapse of HSE. Four patients had neurological deterioration interpreted as relapsing HSE. Four non–relapsing HSE cases were selected as matched controls. Fiftynine batched, paired CSF and serum samples from the eight HSE patients were analysed for soluble activity markers, predominantly cytokines and mediators (interferon– γ, soluble CD8, tumour necrosis factor–α, and interleukin–10), amount of HSV–DNA and markers of glial and neuronal destruction (neurofilament protein, glial fibrillary acidic protein, S–100–β, and neuron specific enolase).
Results
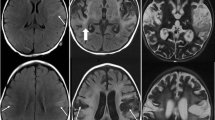
Relapse of HSE was diagnosed in 3 of 26 (12 %) acyclovir–treated patients (5 episodes during 6.1 years of followup) and in 1 of 6 vidarabine–recipients. All relapses occurred from 1 to 4 months after acute HSE, except for a second relapse after 3.3 years in one patient. Computer tomography at relapses revealed few abnormalities apart from those found during the primary disease. Intravenous acyclovir and corticosteroids were given for 7–21 days in all the relapse patients. All relapse patients seemed to recover to the pre–relapse condition. HSV–DNA was demonstrated in CSF in all patients during the acute stage but not in any of 13 CSF samples taken during relapse phases. The HSV viral load during the acute stage of HSE was not higher or of longer duration in the relapsing patients than in the non–relapsing HSE controls. The levels of sCD8 were increased in nearly all CSF samples tested with peaks of sCD8 at one month of acute HSE. In all episodes of relapse, sCD8 peaks were detected during the first week at high levels. CSF levels of neuron–specific enolase, S–100 and glial fibrillary acidic protein were markedly lower at relapse than at the acute stage of HSV–1 encephalitis.
Conclusion
The lack of demonstrable HSV DNA in CSF, the lack of acute CSF signs and the lack of signs of neural and glia cells destruction indicate that a direct viral cytotoxicity is not the major pathogenic mechanism in relapse. Instead, the pronounced CSF proinflammatory immunological response and the relative lack of CSF anti–inflammatory cytokine IL–10 response suggest immunologically–mediated pathogenicity.
Similar content being viewed by others
References
Sköldenberg B, Forsgren M, Alestig K, Bergström T, et al. (1984) Acyclovir versus vidarabine in herpes simplex encephalitis. Randomised multicentre study in consecutive Swedish patients. Lancet ii:707–711
Whitley RJ, Soong SJ, Hirsch MS, et al. (1981) Herpes simplex encephalitis. N Engl J Med 304:313–318
Koenig H, Rabinowitz SG, Day E, Miller V (1979) Post–infectious encephalomyelitis after successful treatment of herpes simplex encephalitis with adenine arabinoside. Ultrastructural observations. N Engl J Med 300:1089–1093
Davis LE, McLaren LC (1983) Relapsing herpes simplex encephalitis following antiviral therapy. Ann Neurol 13:192–195
Dix RD, Baringer JR, Panitch HS, Rosenberg SH, et al. (1983) Recurrent herpes simplex encephalitis: Recovery of virus after ara–A treatment. Ann Neurol 13:196–200
Yamada S, Kameyama T, Nagaya S, Hashizume Y, Yoshida M (2003) Relapsing herpes simplex encephalitis: pathological confirmation of viral reactivation. J Neurol Neurosurg Psychiatry 74:262–264
Abramson JS, Roach SE, Levy HB (1984) Post–infectious encephalopathy after treatment of herpes simplex encephalitis with acyclovir. Ped Infect Dis 3:146–147
Wang HS, Kuo MF, Huang SC, Chou ML (1994) Choreoathetosis as an initial sign of relapsing of herpes simplex encephalitis. Pediatric Neurol 11:341–344
Barthez–Carpentier MA, Rozenberg F, Dussaix E, et al. (1995) Relapse of herpes simplex encephalitis. J Child Neurol 10:363–368
De Tiège XM, Rozenberg F, Des Portes V, Lobut JB, Lebon P, Ponsot G, Héron B (2003) Herpes simplex encephalitis relapses in children. Differentiation of two neurologic entities. Neurology 61:241–243
Aurelius A, Andersson A, Forsgren M, Sköldenberg B, Strannegård Ö (1994) Cytokine and other markers of intrathecal immune response in patients with herpes simplex encephalitis. J Inf Dis 170:678–681
Aurelius A, Forsgren M, Sköldenberg B, Strannegård Ö (1993) Persistent intrathecal immune activation in patients with herpes simplex encephalitis. J Inf Dis 168:1248–1252
Glimåker M, Kragsbjerg P, Forsgren M, Olcén P (1993) TNF–α in cerebrospinal fluid from patients with meningitis of different etiology. J Infect Dis 167:882–889
Günther G, Haglund M, Lindquist L, Forsgren M, Sköldenberg B (1996) Intrathecal production of neopterin and β–2 microglobulin in Tick–borne encephalitis (TBE) compared to meningo–encephalitis of other etiology. Scand J Inf Dis 28:131–138
Rosengren LE, Karlsson J–E, Karlsson J–O, Persson LI, Wikkelso C (1996) Patients with amyotrophic lateral sclerosis and other neurodegenerative diseases have increased levels of neurofilament protein in CSF. J Neurochem 67:2013–2018
Mokuno K, Kato K, Kawai K, Matsuoka Y, Yanagi T, Sobue I (1983) Neuronspecific enolase and S–100 protein levels in cerebrospinal fluid of patients with various neurological diseases. J Neurol Sci 60:443–451
Hay E, Royds JA, Davies–Jones GA, Lewtas NA, Timperley WR, Taylor CB (1984) Cerebrospinal fluid enolase in stroke. J Neurol Neurosurg Psychiatry 47:724–729
Li Y, Wang X, Yang Z (1995) Neuronspecific enolase in patients with acute ischemic stroke and related dementia. Chin Med J (Engl) 108:221–223
Aurell A, Rosengren LE, Karlsson B, Haglid KG (1991) Determination of S–100 and glial fibrillary acidic protein concentrations in cerebrospinal fluid after brain infarction. Stroke 22:1254–1258
Studahl M, Rosengren L, Gunther G, Hagberg L (2000) Difference in pathogenesis between herpes simplex virus type 1 encephalitis and tick–borne encephalitis demonstrated by means of cerebrospinal fluid markers of glial and neuronal destruction. J Neurol 247:636–642
Aurelius E, Johansson B, Sköldenberg B, Staland Å, Forsgren M (1991) Rapid diagnosis of herpes simplex encephalitis by nested polymerase chain reaction assay of cerebrospinal fluid. Lancet 337:189–192
Aurelius E, Forsgren M, Skoog E, Sköldenberg B (1989) Serodiagnosis of herpes simplex encephalitis by antibody capture enzyme–linked immunosorbent assay. Serodiagn Immunother Infect Dis 3:249–258
Schloss L, van Loon AM, Cinque P, et al. (2003) An international external quality assessment of nucleic acid amplification of herpes simplex virus. J Clin Virol 28:175–185
Hjalmarsson A, Aurelius E, Glimåker M, Hart J, Kraut M, Sköldenberg B (2005) Contralateral, subacute relapse of herpes simplex encephalitis with severe sequels. In manuscript
Ito Y, Kimura H, Yabuta Y, Ando Y, Murakami T, Shiomi M, Morishima T (2000) Exacerbation of herpes simplex encephalitis after successful treatment with acyclovir. Clin Infect Dis 30:185–187
Barthez–Carpentier MA, Rozenberg F, Dussaix E, et al. (1995) Relapse of herpes simplex encephalitis. J Child Neurol 10:363–368
Hargrave DR, Webb DW (1998) Movement disorder in association with herpes simplex encephalitis in children: a review. Dev Med Child Neurol 40:640–642
Yamada S, Kameyama T, Nagaya S, Hashizume Y, Yoshida M (2003) Relapsing herpes simplex encephalitis: pathological confirmation of viral reactivation. J Neurol Neurosurg Psychiatry 7:262–264
Billiau A (1996) Interferon–γ. Biology and role in pathogenesis. Adv Immunology 62:61–129
Lebon P, Boutin B, Dulac O, Ponsot G, Arthuis M (1988) Interferon–γ in acute and subacute encephalitis. BMJ 296: 9–11
Frei K, Leist TP, Meager A, et al. (1988) Production of B–cell stimulatory factor and interferon γ in the central nervous system during viral meningitis and encephalitis. Evaluation in a murine model infection and in patients. J Exp Med 168:449–453
Fiorentino DF, Zlotnik A, Mosmann TR, O'Garra A (1991) IL–10 inhibits cytokine production by activated macrophages. J Immunol 147:3815–3822
Abbott RJ, Bolderson I, Gruer PJK (1987) Immunoreactive IFN–γ in CSF in neurological disorders. J Neurol Neurosurg Psychiatry 50:882–885
Griffin DE, Ward BJ, Jauregui E, Johnson RT, Vaisberg A (1990) Immune activation during measles: Interferon–γ and neopterin in plasma and cerebrospinal fluid in complicated and uncomplicated disease. J Inf Dis 161:449–453
Fong TA, Mosmann TR (1990) Alloreactive murine CD8+ T cell clones secrete the Th1 pattern of cytokines. J Immunol 144:1744–1752
McCarron RM, Wang L, Racke MK, McFarlin DE, Spatz M (1993) Cytokine– regulated adhesion between encephalitogenic T lymphocytes and cerebrovascular endothelial cells. J Neuroimmunol 43:23–30
Florquin S, Amraoui Z, Abramowicz D, Goldman M (1994) Systemic release and protective role of IL–10 in staphylococcal enterotoxin B–induced shock in mice. J Immunol 153:2618–2623
Chomarat P, Rissoan MC, Banchereau J, Miossec P (1993) Interferon gamma inhibits interleukin 10 production by monocytes. J Exp Med 177:523–527
Sabri F, De Milito A, Pirskanen R, Elovaara I, Hagberg L, Cinque P, Price R, Chiodi F (2001) Elevated levels of soluble Fas and Fas ligand in cerebrospinal fluid of patients with AIDS dementia complex. J Neuroimmunol 114:197–206
Ohsako S, Hara M, Harigai M, Fukasawa C, Kashiwazaki S (1994) Expression and function of Fas antigen and bcl–2 in human systemic lupus erythematosus lymphocytes. Clin Immunol Immunopathol 73:109–114
Ciusani E, Frigerio S, Gelati M, Corsini E, Dufour A, Nespolo A, La Mantia L, Milanese C, Massa G, Salmaggi A (1998) Soluble Fas (Apo–1) levels in cerebrospinal fluid of multiple sclerosis patients. J Neuroimmunol 82:5–12
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sköldenberg, ., Aurelius, E., Hjalmarsson, A. et al. Incidence and pathogenesis of clinical relapse after herpes simplex encephalitis in adults. J Neurol 253, 163–170 (2006). https://doi.org/10.1007/s00415-005-0941-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-005-0941-6