Abstract
Little is known of the dynamics of centromeric DNA in polyploid plants. We report the sequences of two centromere-associated bacterial artificial chromosome clones from a Triticum boeoticum library. Both autonomous and non-autonomous wheat centromeric retrotransposons (CRWs) were identified, both being closely associated with the centromeres of wheat. Fiber-fluorescence in situ hybridization and chromatin immunoprecipitation analysis showed that wheat centromeric retrotransposons (CRWs) represent a dominant component of the wheat centromere and are associated with centromere function. CRW copy number showed variation among different genomes: the D genome chromosomes contained fewer copies than either the A or B genome chromosomes. The frequency of lengthy continuous CRW arrays was higher than that in either rice or maize. The dynamics of CRWs and other retrotransposons at centromeric and pericentromeric regions during diploid speciation and polyploidization of wheat and its related species are discussed.







Similar content being viewed by others
References
Aragón-Alcaide L, Miller T, Schwatzacher T, Reader S, Moore G (1996) A cereal centromeric sequence. Chromosoma 105:261–268
Bennetzen JL (2000) Transposable element contributions to plant gene and genome evolution. Plant Mol Biol 42:251–269
Chen F, Zhang X, Xia G, Jia J (2002) Construction and characterization of a bacterial artificial chromosome library for Triticum boeoticum. Acta Bot Sinica 44:451–456
Cheng ZJ, Murata M (2003) A centromeric tandem repeat family originating from a part of Ty3/gypsy-retroelement in wheat and its relatives. Genetics 164:665–672
Cheng Z, Dong F, Langdon T, Ouyang S, Buell CR, Gu M, Blattner FR, Jiang J (2002) Functional rice centromeres are marked by a satellite repeat and a centromere-specific retrotransposon. Plant Cell 14:1691–1704
Copenhaver GP, Nickel K, Kuromori T, Benito MI, Kaul S, Lin X, Bevan M, Murphy G, Harris B, Parnell LD, McCombie WR, Martienssen RA, Marra M, Preuss D (1999) Genetic definition and sequence analysis of Arabidopsis centromeres. Science 286:2468–2474
Dong F, Miller JT, Jackson SA, Wang GL, Pamela C, Ronald PC, Jiang J (1998) Rice (Oryza sativa) centromeric regions consist of complex DNA. Proc Natl Acad Sci USA 95:8135–8140
Gaut BS, Morton BR, McCaig BC, Clegg MT (1996) Substitutions rate comparisons between grasses and palm: synonymous rate differences at the nucleargene Adh parallel rate differences at the plastid gene rbcL. Proc Natl Acad Sci USA 93:10274–10279
Gu YQ, Coleman-Derr D, Kong X, Anderson OD (2004) Rapid genome evolution revealed by comparative sequence analysis of orthologous regions from four Triticeae genomes. Plant Physiol 135:459–470
Hall AE, Kettler GC, Preuss D (2006) Dynamic evolution at pericentromeres. Genome Res 16:355–364
Houben A, Schubert I (2003) DNA and proteins of plant centromeres. Curr Opin Plant Biol 6:554–560
Houben A, Schroeder-Reiter E, Nagaki K, Nasuda S, Wanner G, Murata M, Endo TR (2007) CENH3 interacts with the centromeric retrotransposon cereba and GC-rich satellites and locates to centromeric substructures in barley. Chromosoma 116:275–283
Hudakova S, Michalek W, Presting GG, ten Hoopen R, dos Santos K, Jasencakova Z, Schubert I (2001) Sequence organization of barley centromeres. Nucleic Acids Res 29:5029–5035
Ito H, Nasuda S, Endo TR (2004) A direct repeat sequence associated with the centromeric retrotransposons in wheat. Genome 47:747–756
Ito H, Miura A, Takashima K, Kakutani T (2007) Ecotype-specific and chromosome-specific expansion of variant centromeric satellites in Arabidopsis thaliana. Mol Genet Genomics 277:23–30
Jackson SA, Wang ML, Goodman HM, Jiang J (1998) Application of fiber-FISH in physical mapping of Arabidopsis thaliana. Genome 41:566–572
Jiang J, Gill BS (1994) Different species-specific chromosome translocations in Triticum timopheevii and T. turgidum support the diphyletic origin of polyploid wheats. Chromosome Res 2:59–64
Jiang J, Gill BS, Wang GL, Ronald PC, Ward DC (1995) Metaphase and interphase fluorescence in situ hybridization mapping of the rice genome with bacterial artificial chromosomes. Proc Natl Acad Sci USA 92:4487–4491
Jiang J, Nasuda S, Dong F, Scherrer CW, Woo SS, Wingi RA, Gill BS, Ward DC (1996) A conserved repetitive DNA element located in the centromeres of cereal chromosomes. Proc Natl Acad Sci USA 93:14210–14213
Jiang J, Birchler JA, Parrott WA, Dawe RK (2003) A molecular view of plant centromeres. Trends Plant Sci 18:570–575
Jin W, Melo JR, Nagaki K, Talbert PB, Henikoff S, Dawe RK, Jiang J (2004) Maize centromeres: organization and functional adaptation in the genetic background of oat. Plant Cell 16:571–581
Kalendar R, Vicient CM, Peleg O, Anamthawat-Jonsson K, Bolshoy A, Schulman AH (2004) Large retrotransposon derivatives: abundant, conserved but non-autonomous retroelements of barley and related genomes. Genetics 166:1437–1450
Kawabe A, Charlesworth D (2007) Patterns of DNA variation among three centromere satellite families in Arabidopsis halleri and A. lyrata. J Mol Evol 64:237–247
Kishii M, Tsujimoto H (2002) Genus-specific localization of the TaiI family of tandem-repetitive sequences in either the centromeric or subtelomeric regions in Triticeae species (Poaceae) and its evolution in wheat. Genome 45:946–955
Kishii M, Nagaki K, Tsujimoto H (2001) A tandem repetitive sequence located in the centromeric region of common wheat (Triticum aestivum) chromosomes. Chromosome Res 9:417–428
Kong XY, Gu YQ, You FM, Dubcovsky J, Anderson OD (2004) Dynamics of the evolution orthologous and paralogous portions of a complex locus region in two genomes of allopolyploid wheat. Plant Mol Biol 54:55–69
Kumar A, Bennetzen JL (1999) Plant retrotransposons. Annu Rev Genet 33:479–532
Langdon T, Seago C, Mende M, Leggett M, Thomas H, Forster JW, Thomas H, Jones RN, Jenkins G (2000) Retrotransposon evolution in diverse plant genomes. Genetics 156:313–325
Lee HR, Zhang W, Langdon T, Jin W, Yan H, Cheng Z, Jiang J (2005) Chromatin immunoprecipitation cloning reveals rapid evolutionary patterns of centromeric DNA in Oryza species. Proc Natl Acad Sci USA 102:11793–11798
Levy AA, Feldman M (2002) The impact of polyploidy on grass genome evolution. Plant Physiol 130:1587–1593
Li W, Zhang P, Fellers JP, Friebe B, Gill BS (2004) Sequence composition, organization, and evolution of the core Triticeae genome. Plant J 40:500–511
Lim KB, Yang TJ, Hwang YJ, Kim JS, Park JY, Kwon SJ, Kim J, Choi BS, Lim MH, Jin M, Kim HI, de Jong H, Bancroft I, Lim Y, Park BS (2007) Characterization of the centromere and peri-centromere retrotransposons in Brassica rapa and their distribution in related Brassica species. Plant J 47:173–183
Liu Z, Yue W, Dong YS, Zhang XY (2006) Identification and preliminary analysis of several centromere-associated bacterial artificial chromosome clones from a diploid wheat (Triticum boeoticum Boiss.) library. J Integrat Plant Biol 48:348–358
Ma J, Wing RA, Bennetzen JL, Jackson SA (2007a) Plant centromere organization: a dynamic structure with conserved functions. Trends Genet 23:134–139
Ma J, Wing RA, Bennetzen JL, Jackson SA (2007b) Evolutionary history and positional shift of a rice centromere. Genetics 177:1217–1220
McFadden ES, Sears ER (1944) The artificial synthesis of Triticum spelta. Rec Genet Soc Am 13:26–27
McIntyre CL, Pereira S, Moran LB, Appels R (1990) New Secale cereale (rye) DNA derivatives for the detection of rye chromosome segments in wheat. Genome 33:635–640
Miller JT, Dong F, Jackson SA, Song J, Jiang J (1998) Retrotransposon-related DNA sequences in the centromeres of grass chromosomes. Genetics 150:1615–1623
Mukai Y, Nakahara Y, Yamamoto M (1993) Simultaneous discrimination of the three genomes in hexaploid wheat by multicolor fluorescence in situ hybridization using total genomic and highly repeated DNA probes. Genome 36:489–494
Nagaki K, Murata M (2005) Characteriztion of CENH3 and centromere-associated DNA sequences in sugarcane. Chromosome Res 13:195–203
Nagaki K, Song J, Stupar RM, Parokonny AS, Yuan Q, Ouyang S, Liu J, Hsiao J, Jones KM, Dawe RK, Buell CR, Jiang J (2003a) Molecular and cytological analyses of large tracks of centromeric DNA reveal the structure and evolutionary dynamics of maize centromeres. Genetics 163:759–770
Nagaki K, Talbert PB, Zhong CX, Dawe RK, Henikoff S, Jiang J (2003b) Chromatin immunoprecipitation reveals that the 180-bp satellite repeat is the key functional DNA element of Arabidopsis thaliana centromeres. Genetics 163:1221–1225
Nagaki K, Cheng Z, Ouyang S, Talbert PB, Kim M, Jones KM, Henikoff S, Buell CR, Jiang J (2004) Sequencing of a rice centromere uncovers active genes. Nat Genet 36:138–145
Nagaki K, Neumann P, Zhang D, Ouyang S, Buell CR, Cheng Z, Jiang J (2005) Structure, divergence, and distribution of the CRR centromeric retrotransposon family in rice. Mol Biol Evol 22:845–855
Paux E, Roger D, Badaeva E, Gay G, Bernard M, Sourdille P, Feuillet C (2006) Characterizing the composition and evolution of homoeologous genomes in hexaploid wheat through BAC-end sequencing on chromosome 3B. Plant J 48:463–474
Presting GG, Malysheva L, Fuchs J, Schubert I (1998) A Ty3/gypsy retrotransposon-like sequence localizes to the centromeric regions of cereal chromosomes. Plant J 16:721–728
Rayburn AL, Gill BS (1986) Molecular identification of the D-genome chromosomes of wheat. J Hered 77:253–255
Reed KC, Mann DA (1985) Rapid transfer of DNA from agarose gels to nylon membranes. Nucleic Acids Res 13:7202–7221
Saffery R, Sumer H, Hassan S, Wong LH, Craig JM, Todokoro K, Anderson M, Stafford A, Choo KH (2003) Transcription within a functional human centromere. Mol Cell 12:509–516
SanMiguel PJ, Ramakrishna W, Bennetzen JL, Busso CS, Dubcovsky J (2002) Transposable elements, genes and recombination in a 215-kb contig from wheat chromosome 5Am. Funct Integr Genomics 2:70–80
Sharp PJ, Kreis M, Shewry PR, Gale MD (1988) Location of β-amylase sequences in wheat and its relatives. Theor Appl Genet 75:286–290
Yan H, Ito H, Nobuta K, Ouyang S, Jin W, Tian S, Lu C, Venu RC, Wang GL, Green PJ, Wing RA, Buell CR, Meyers BC, Jiang J (2005) Genomic and genetic characterization of rice Cen3 reveals extensive transcription and evolutionary implications of a complex centromere. Plant Cell 18:2123–2133
Zhang XY, Dong YS, Wang RR-C (1996) Characterization of genomes and chromosomes in partial amphiploids of the hybrid Triticum aestivum × Thinopyrum ponticum by in situ hybridization, isozyme analysis and RAPD. Genome 39:1062–1071
Zhang P, Li W, Friebe B, Gill BS (2004a) Simultaneous painting of three genomes in hexaploid wheat by BAC-FISH. Genome 47:979–987
Zhang P, Li W, Fellers J, Friebe B, Gill BS (2004b) BAC-FISH in wheat identifies chromosome landmarks consisting of different types of transposable elements. Chromosoma 112:288–299
Zhong CX, Marshall JB, Topp C, Mroczek R, Kato A, Nagaki K, Birchler JA, Jiang J, Dawe RK (2002) Centromeric retroelements and satellites interact with maize kinetochore protein CENH3. Plant Cell 14:2825–2836
Acknowledgements
The authors are grateful to Dr. J. Jiang (University of Wisconsin, Madison, USA) for providing the RCS1 plasmid and valuable comments on the manuscript, to Dr. S. Henikoff (Fred Hutchinson Cancer Research Center) for the rice CENH3 antibody, to Dr. C. Feuillet (INRA, Clermont-Ferrand, France) for providing the 3B centromere-associated BAC clones, to J. Wu (ICS, CAAS) for help with the BAC sequencing and bioinformatic analysis, and to Z. Cheng and L. Mao (ICS, CAAS) for valuable discussion. They also thank www.smartenglish.co.uk for linguistic advice in the preparation of this manuscript. This research was supported by the Natural Science Foundation of China (39870494, 30771208).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Communicated by I. Schubert
Zhao Liu and Wei Yue made an equal contribution to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material
Fig. S1
CRW LTRs in BAC clone TbBAC30 (5′→3′ the direction of the LTR, asterisks the inverted repeat at the terminals of the LTR) (PPT 36.0 KB)
Fig. S2
Chromosome localization of the CR2–1 sequence (part of the LTR region of an autonomous CRW) in hexaploid wheat. a Biotin-labeled probe (red). b Probed with digoxingenin-11-dUTP-labeled pAs1 (yellow/green) and biotin-16-dUTP-labeled pSc119.2 (red). The signals on the A genome centromeres are stronger than those on the B or D genome centromeres. Bars 10 μm (GIF 4.94 MB)
Fig. S3
In situ hybridization in hexaploid wheat cv. Chinese Spring with amplicons derived from A. speltoides (a) or A. tauschii (b) and centromeric-associated BAC clones derived either from cv. Chinese Spring chromosome 3B (c) or A. tauschii (d) as probes. LTRm primers were used for PCR (listed in Table S4). The BAC used in c was 3B-078-D03 (centromeric signals: green) and in d was 5C01 (centromeric signals: red). Digoxingenin-11-dUTP-labeled pAs1 (yellow/green) and biotin-16-dUTP-labeled pSc119.2 (red) were used simultaneously to distinguish the chromosomes of the B and D genomes. The signals on D genome centromeres are weaker than those on A or B genome centromeres. Bars 10 μm (GIF 8.45 MB)
Fig. S4
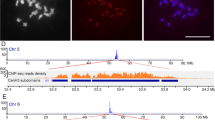
Representative quantitative real-time PCR as used in ChIP analysis, for determining the relative fold enrichment (RFE) of CENH3-associated sequences in the bound fraction over the mock control. All samples showed obvious enrichment of precipitated DNA, except for Erika and 5.8S. The red and green curves correspond to the two mock DNA samples (pellet), whereas the blue and yellow curves correspond to the two bound DNAs (pellet). (a) 365–1c, (b) 5′-UTR, (c) gag, (d) RT, (e) INT, (f) non-autonomous CRW LTR, (g) Erika, (h) 5.8S (CK). The cycle threshold (CT) was taken with the baseline of fluorescence intensity being manually set at a value between 0.01 and 0.05. (PPT 103 KB)
Fig. S5
Southern hybridization profiles of Triticeae species, following probing with three retrotransposon sequences. (a) Wgel_TbBAC5–1p, (b) Erika_TbBAC5–1, (c) Sukkula_TbBAC5–1 with the probes of C04–1, G10, and Sukkula (see Fig. 1a and Table S4). Genome symbols are used to identify the lanes after gel electrophoresis (Cu: A. umbrellulata, Au: T. urartu, Ab: T. monococcum subsp. aegilopoides, S: A. speltoides var. ligustica, D: A. tauschii, AB: T. orientale, AG: T. araraticum, ABD: T. aestivum). The genomic DNA was fully digested by HindIII (a, b) or BamHI (c). The signals in the five species containing the A genome are significantly stronger than those in the other species. The polyploid wheats thus presumably inherited the hybridization patterns of their diploid ancestors. (PPt 241 KB)
Fig. S6
In situ hybridization in four Triticum species, generated by probing with Erika_TbBAC5–1. (a) T. urartu, (b) T. dicoccoides, (c) T. araraticum, (d) T. aestivum. The probe sub-clone G10, encompassing a partial LTR and a partial coding region of Erika_TbBAC5–1 (marked in Fig. 1a), was labeled with digoxingenin-11-dUTP and detected with anti-digoxigenin-fluorescein (yellow/green). Chromosomes were counter-stained with propidium iodide (PI) in Vectashield for anti-digoxigenin-fluorescein detection. Dispersed signals were distributed on the arms of the A genome chromosomes and almost absent in the centromeres, showing that Erika_TbBAC5–1 is non-centromeric sequence-specific (or abundant) in the A genome (arrows in b-d translocations between the A and B or G genome chromosomes). Bars 10 μm (GIF 76.6 KB)
Table S1
Non-centromere-specific retrotransposons present in BAC clone TbBAC30 (DOC 33.0 KB)
Table S2
Incomplete CRWs present in BAC clones TbBAC5 and TbBAC30 (DOC 32.5 KB)
Table S3
The CRW, PrBS (primer-binding site), and PPT (polypurine tract) present in BAC clones TbBAC5 and TbBAC30 (DOC 31.5 KB)
Table S4
Probes used for Southern hybridization, FISH, and fiber-FISH (DOC 64.0 KB)
Table S5
Primers used for ChIP analyses (no a non-autonomous) (DOC 38.0 KB)
Table S6
Quantification of real-time PCR products in ChIP analyses (no a non-autonomous) (DOC 45.0 KB)
Rights and permissions
About this article
Cite this article
Liu, Z., Yue, W., Li, D. et al. Structure and dynamics of retrotransposons at wheat centromeres and pericentromeres. Chromosoma 117, 445–456 (2008). https://doi.org/10.1007/s00412-008-0161-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00412-008-0161-9