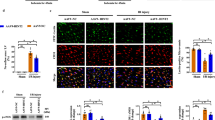
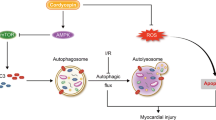
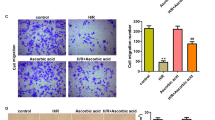
Abstract
Microvascular damage is a key pathological change in myocardial ischemia/reperfusion (I/R) injury. Using a rat model of myocardial I/R, our current study has provided the first evidence that nicotinamide adenine dinucleotide (NAD+) administration can significantly attenuate myocardial I/R-induced microvascular damage, including reduced regional blood perfusion, decreased microvessel density and integrity, and coronary microvascular endothelial cells (CMECs) injury. In studies with primary cultured CMECs under hypoxia/reoxygenation (HR) and a rat model of I/R, our results suggested that the protective effect of NAD+ on CMECs exposed to HR or I/R is at least partially mediated by the NAD+-induced restoration of autophagic flux, especially lysosomal autophagy: NAD+ treatment markedly induced transcription factor EB (TFEB) activation and attenuated lysosomal dysfunction in the I/R or HR-exposed cells. Collectively, our study has provided the first in vivo and in vitro evidence that NAD+ significantly rescued the impaired autophagic flux and cell apoptosis that was induced by I/R in rat CMECs, which is mediated in part through the action of TFEB-mediated lysosomal autophagy.








Similar content being viewed by others
References
Bhuiyan MS, Pattison JS, Osinska H, James J, Gulick J, McLendon PM, Hill JA, Sadoshima J, Robbins J (2013) Enhanced autophagy ameliorates cardiac proteinopathy. J Clin Invest 12312:5284–5297. https://doi.org/10.1172/jci70877
Botker HE, Hausenloy D, Andreadou I, Antonucci S, Boengler K, Davidson SM, Deshwal S, Devaux Y, Di Lisa F, Di Sante M, Efentakis P, Femmino S, Garcia-Dorado D, Giricz Z, Ibanez B, Iliodromitis E, Kaludercic N, Kleinbongard P, Neuhauser M, Ovize M, Pagliaro P, Rahbek-Schmidt M, Ruiz-Meana M, Schluter KD, Schulz R, Skyschally A, Wilder C, Yellon DM, Ferdinandy P, Heusch G (2018) Practical guidelines for rigor and reproducibility in preclinical and clinical studies on cardioprotection. Basic Res Cardiol 1135:39. https://doi.org/10.1007/s00395-018-0696-8
Cui D, Sun D, Wang X, Yi L, Kulikowicz E, Reyes M, Zhu J, Yang ZJ, Jiang W, Koehler RC (2017) Impaired autophagosome clearance contributes to neuronal death in a piglet model of neonatal hypoxic-ischemic encephalopathy. Cell Death Dis 87:e2919. https://doi.org/10.1038/cddis.2017.318
Das A, Huang GX, Bonkowski MS, Longchamp A, Li C, Schultz MB, Kim LJ, Osborne B, Joshi S, Lu Y, Trevino-Villarreal JH, Kang MJ, Hung TT, Lee B, Williams EO, Igarashi M, Mitchell JR, Wu LE, Turner N, Arany Z, Guarente L, Sinclair DA (2018) Impairment of an endothelial NAD(+)-H2S signaling network is a reversible cause of vascular aging. Cell 1731:74–89 e20. https://doi.org/10.1016/j.cell.2018.02.008
Davidson SM, Ferdinandy P, Andreadou I, Botker HE, Heusch G, Ibanez B, Ovize M, Schulz R, Yellon DM, Hausenloy DJ, Garcia-Dorado D (2019) Multitarget strategies to reduce myocardial ischemia/reperfusion injury: JACC review topic of the week. J Am Coll Cardiol 731:89–99. https://doi.org/10.1016/j.jacc.2018.09.086
de Waha S, Patel MR, Granger CB, Ohman EM, Maehara A, Eitel I, Ben-Yehuda O, Jenkins P, Thiele H, Stone GW (2017) Relationship between microvascular obstruction and adverse events following primary percutaneous coronary intervention for ST-segment elevation myocardial infarction: an individual patient data pooled analysis from seven randomized trials. Eur Heart J 3847:3502–3510. https://doi.org/10.1093/eurheartj/ehx414
Fan Y, Lu H, Liang W, Garcia-Barrio MT, Guo Y, Zhang J, Zhu T, Hao Y, Zhang J, Chen YE (2018) Endothelial TFEB (transcription factor eb) positively regulates postischemic angiogenesis. Circ Res 1227:945–957. https://doi.org/10.1161/circresaha.118.312672
Galaup A, Gomez E, Souktani R, Durand M, Cazes A, Monnot C, Teillon J, Le Jan S, Bouleti C, Briois G, Philippe J, Pons S, Martin V, Assaly R, Bonnin P, Ratajczak P, Janin A, Thurston G, Valenzuela DM, Murphy AJ, Yancopoulos GD, Tissier R, Berdeaux A, Ghaleh B, Germain S (2012) Protection against myocardial infarction and no-reflow through preservation of vascular integrity by angiopoietin-like 4. Circulation 1251:140–149. https://doi.org/10.1161/CIRCULATIONAHA.111.049072
Gedik N, Thielmann M, Kottenberg E, Peters J, Jakob H, Heusch G, Kleinbongard P (2014) No evidence for activated autophagy in left ventricular myocardium at early reperfusion with protection by remote ischemic preconditioning in patients undergoing coronary artery bypass grafting. PLoS ONE 95:e96567. https://doi.org/10.1371/journal.pone.0096567
Godar RJ, Ma X, Liu H, Murphy JT, Weinheimer CJ, Kovacs A, Crosby SD, Saftig P, Diwan A (2015) Repetitive stimulation of autophagy-lysosome machinery by intermittent fasting preconditions the myocardium to ischemia-reperfusion injury. Autophagy 119:1537–1560. https://doi.org/10.1080/15548627.2015.1063768
Granger DN, Kvietys PR (2017) Reperfusion therapy—what's with the obstructed, leaky and broken capillaries? Pathophysiology 244:213–228. https://doi.org/10.1016/j.pathophys.2017.09.003
Han X, Tai H, Wang X, Wang Z, Zhou J, Wei X, Ding Y, Gong H, Mo C, Zhang J, Qin J, Ma Y, Huang N, Xiang R, Xiao H (2016) AMPK activation protects cells from oxidative stress-induced senescence via autophagic flux restoration and intracellular NAD(+) elevation. Aging Cell 153:416–427. https://doi.org/10.1111/acel.12446
Heusch G, Kleinbongard P, Skyschally A, Levkau B, Schulz R, Erbel R (2012) The coronary circulation in cardioprotection: more than just one confounder. Cardiovasc Res 942:237–245. https://doi.org/10.1093/cvr/cvr271
Heusch G (2015) Molecular basis of cardioprotection: signal transduction in ischemic pre-, post-, and remote conditioning. Circ Res 1164:674–699. https://doi.org/10.1161/circresaha.116.305348
Heusch G (2016) The coronary circulation as a target of cardioprotection. Circ Res 11810:1643–1658. https://doi.org/10.1161/circresaha.116.308640
Heusch G, Gersh BJ (2017) The pathophysiology of acute myocardial infarction and strategies of protection beyond reperfusion: a continual challenge. Eur Heart J 3811:774–784. https://doi.org/10.1093/eurheartj/ehw224
Heusch G (2019) Coronary microvascular obstruction: the new frontier in cardioprotection. Basic Res Cardiol 1146:45. https://doi.org/10.1007/s00395-019-0756-8
Heusch G, Kleinbongard P, Rassaf T (2019) Cardioprotection beyond infarct size reduction. Circ Res 1245:679–680. https://doi.org/10.1161/circresaha.119.314679
Hosseini L, Vafaee MS, Badalzadeh R (2019) Melatonin and nicotinamide mononucleotide attenuate myocardial ischemia/reperfusion injury via modulation of mitochondrial function and hemodynamic parameters in aged rats. J Cardiovasc Pharmacol Ther. https://doi.org/10.1177/1074248419882002
Jahania SM, Sengstock D, Vaitkevicius P, Andres A, Ito BR, Gottlieb RA, Mentzer RM Jr (2013) Activation of the homeostatic intracellular repair response during cardiac surgery. J Am Coll Surg 2164:719–726. https://doi.org/10.1016/j.jamcollsurg.2012.12.034(discussion 726-719)
Kadlec AO, Beyer AM, Ait-Aissa K, Gutterman DD (2016) Mitochondrial signaling in the vascular endothelium: beyond reactive oxygen species. Basic Res Cardiol 1113:26. https://doi.org/10.1007/s00395-016-0546-5
Kleinbongard P, Heusch G (2015) Extracellular signalling molecules in the ischaemic/reperfused heart—druggable and translatable for cardioprotection? Br J Pharmacol 1728:2010–2025. https://doi.org/10.1111/bph.12902
Lee Y, Kwon I, Jang Y, Song W, Cosio-Lima LM, Roltsch MH (2017) Potential signaling pathways of acute endurance exercise-induced cardiac autophagy and mitophagy and its possible role in cardioprotection. J Physiol Sci 676:639–654. https://doi.org/10.1007/s12576-017-0555-7
Li Y, Liang P, Jiang B, Tang Y, Liu X, Liu M, Sun H, Chen C, Hao H, Liu Z, Xiao X (2020) CARD9 promotes autophagy in cardiomyocytes in myocardial ischemia/reperfusion injury via interacting with Rubicon directly. Basic Res Cardiol 1153:29. https://doi.org/10.1007/s00395-020-0790-6
Lindsey ML, Bolli R, Canty JM Jr, Du XJ, Frangogiannis NG, Frantz S, Gourdie RG, Holmes JW, Jones SP, Kloner RA, Lefer DJ, Liao R, Murphy E, Ping P, Przyklenk K, Recchia FA, Schwartz Longacre L, Ripplinger CM, Van Eyk JE, Heusch G (2018) Guidelines for experimental models of myocardial ischemia and infarction. Am J Physiol Heart Circ Physiol 3144:H812–H838. https://doi.org/10.1152/ajpheart.00335.2017
Liu Y, Xue X, Zhang H, Che X, Luo J, Wang P, Xu J, Xing Z, Yuan L, Liu Y, Fu X, Su D, Sun S, Zhang H, Wu C, Yang J (2018) Neuronal-targeted TFEB rescues dysfunction of the autophagy-lysosomal pathway and alleviates ischemic injury in permanent cerebral ischemia. Autophagy. https://doi.org/10.1080/15548627.2018.1531196
Ma X, Liu H, Foyil SR, Godar RJ, Weinheimer CJ, Diwan A (2012) Autophagy is impaired in cardiac ischemia-reperfusion injury. Autophagy 89:1394–1396. https://doi.org/10.4161/auto.21036
Ma X, Liu H, Foyil SR, Godar RJ, Weinheimer CJ, Hill JA, Diwan A (2012) Impaired autophagosome clearance contributes to cardiomyocyte death in ischemia/reperfusion injury. Circulation 12525:3170–3181. https://doi.org/10.1161/circulationaha.111.041814
Medina DL, Di Paola S, Peluso I, Armani A, De Stefani D, Venditti R, Montefusco S, Scotto-Rosato A, Prezioso C, Forrester A, Settembre C, Wang W, Gao Q, Xu H, Sandri M, Rizzuto R, De Matteis MA, Ballabio A (2015) Lysosomal calcium signalling regulates autophagy through calcineurin and TFEB. Nat Cell Biol 173:288–299. https://doi.org/10.1038/ncb3114
Nadtochiy SM, Wang YT, Nehrke K, Munger J, Brookes PS (2018) Cardioprotection by nicotinamide mononucleotide (NMN): Involvement of glycolysis and acidic pH. J Mol Cell Cardiol 121:155–162. https://doi.org/10.1016/j.yjmcc.2018.06.007
Niccoli G, Montone RA, Ibanez B, Thiele H, Crea F, Heusch G, Bulluck H, Hausenloy DJ, Berry C, Stiermaier T, Camici PG, Eitel I (2019) Optimized treatment of ST-elevation myocardial infarction. Circ Res 1252:245–258. https://doi.org/10.1161/circresaha.119.315344
Reffelmann T, Kloner RA (2002) Microvascular reperfusion injury: rapid expansion of anatomic no reflow during reperfusion in the rabbit. Am J Physiol Heart Circ Physiol 2833:H1099–1107. https://doi.org/10.1152/ajpheart.00270.2002
Settembre C, Di Malta C, Polito VA, Garcia Arencibia M, Vetrini F, Erdin S, Erdin SU, Huynh T, Medina D, Colella P, Sardiello M, Rubinsztein DC, Ballabio A (2011) TFEB links autophagy to lysosomal biogenesis. Science 3326036:1429–1433. https://doi.org/10.1126/science.1204592
Wang P, Guan YF, Du H, Zhai QW, Su DF, Miao CY (2012) Induction of autophagy contributes to the neuroprotection of nicotinamide phosphoribosyltransferase in cerebral ischemia. Autophagy 81:77–87. https://doi.org/10.4161/auto.8.1.18274
Xie M, Kong Y, Tan W, May H, Battiprolu PK, Pedrozo Z, Wang ZV, Morales C, Luo X, Cho G, Jiang N, Jessen ME, Warner JJ, Lavandero S, Gillette TG, Turer AT, Hill JA (2014) Histone deacetylase inhibition blunts ischemia/reperfusion injury by inducing cardiomyocyte autophagy. Circulation 12910:1139–1151. https://doi.org/10.1161/circulationaha.113.002416
Xie Y, Jiang D, Xiao J, Fu C, Zhang Z, Ye Z, Zhang X (2018) Ischemic preconditioning attenuates ischemia/reperfusion-induced kidney injury by activating autophagy via the SGK1 signaling pathway. Cell Death Dis 93:338. https://doi.org/10.1038/s41419-018-0358-7
Yamamoto T, Byun J, Zhai P, Ikeda Y, Oka S, Sadoshima J (2014) Nicotinamide mononucleotide, an intermediate of NAD+ synthesis, protects the heart from ischemia and reperfusion. PLoS ONE 96:e98972. https://doi.org/10.1371/journal.pone.0098972
Zhang DX, Zhang JP, Hu JY, Huang YS (2016) The potential regulatory roles of NAD(+) and its metabolism in autophagy. Metabolism 654:454–462. https://doi.org/10.1016/j.metabol.2015.11.010
Zhang H, Ge S, He K, Zhao X, Wu Y, Shao Y, Wu X (2019) FoxO1 inhibits autophagosome-lysosome fusion leading to endothelial autophagic-apoptosis in diabetes. Cardiovasc Res. https://doi.org/10.1093/cvr/cvz014
Zhang Y, Xu M, Xia M, Li X, Boini KM, Wang M, Gulbins E, Ratz PH, Li PL (2014) Defective autophagosome trafficking contributes to impaired autophagic flux in coronary arterial myocytes lacking CD38 gene. Cardiovasc Res 1021:68–78. https://doi.org/10.1093/cvr/cvu011
Zhang Y, Wang B, Fu X, Guan S, Han W, Zhang J, Gan Q, Fang W, Ying W, Qu X (2016) Exogenous NAD(+) administration significantly protects against myocardial ischemia/reperfusion injury in rat model. Am J Transl Res 88:3342–3350
Zhou H, Wang J, Zhu P, Zhu H, Toan S, Hu S, Ren J, Chen Y (2018) NR4A1 aggravates the cardiac microvascular ischemia reperfusion injury through suppressing FUNDC1-mediated mitophagy and promoting Mff-required mitochondrial fission by CK2alpha. Basic Res Cardiol 1134:23. https://doi.org/10.1007/s00395-018-0682-1
Acknowledgements
The authors would like to acknowledge the financial support by a National Natural Science Foundation of China (81770420 and 61533016 to X.Q.), a Center of Geriatric Coronary Artery Disease, a Major Research Grant from the Scientific Committee of Shanghai Municipality (16JC1400500 and 16JC1400502 to W.Y.), and a Major Special Program Grant of Shanghai Municipality (2017SHZDZX01 to W.Y.), Research Fund for the Scientific and Technical Project of Huadong Hospital (2019JC024 to Z.Y.J.)
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Zhang, YJ., Zhang, M., Zhao, X. et al. NAD+ administration decreases microvascular damage following cardiac ischemia/reperfusion by restoring autophagic flux. Basic Res Cardiol 115, 57 (2020). https://doi.org/10.1007/s00395-020-0817-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00395-020-0817-z