Abstract
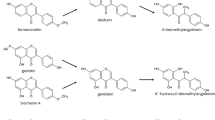
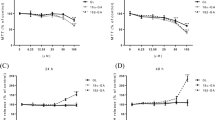
The purposes of this study were to determine the effect of structural change on the intestinal disposition of isoflavones and to elucidate the mechanisms responsible for transport of phase II isoflavone conjugates. Transport and metabolism of six isoflavones (i.e., genistein, daidzein, glycitein, formononetin, biochanin A, and prunetin) were studied in the human intestinal Caco-2 model and mature Caco-2 cell lysate. Glucuronides were the main metabolites in intact Caco-2 cells for all isoflavones except prunetin, which was mainly sulfated. In addition, the 7-hydroxy group was the main site for glucuronidation whereas the 4′-hydroxy group was only one of the possible sites for sulfation. Glucuronidated isoflavones (except biochanin A) were preferably excreted to the basolateral side, whereas sulfated metabolites (except genistein and glycitein) were mainly excreted into the apical side. Polarized excretion of most isoflavone conjugates was inhibited by the multidrug resistance-related protein (MRP) inhibitor leukotriene C4 (0.1 μM) and the organic anion transporter (OAT) inhibitor estrone sulfate (10 μM). When formation and excretion rates of isoflavones were determined simultaneously, the results showed that formation served as the rate-limiting step for all isoflavone conjugates (both glucuronides and sulfates) except for genistein glucuronide, which had comparable excretion and formation rates. In conclusion, the intestinal disposition of isoflavones was structurally dependent, polarized, and mediated by MRP and OAT. Formation generally served as the rate-limiting step in the cellular excretion of conjugated isoflavones in the Caco-2 cell culture model.






Similar content being viewed by others
Abbreviations
- HBSS:
-
Hank’s balanced salt solution
- LTC4 :
-
Leukotriene C4
- MRP:
-
Multidrug resistance related protein
- OAT:
-
Organic anion transporter
- PAPS:
-
3′-Phosphoadenosine 5′-phosphosulfate
- SULT:
-
Sulfotransferases
- UDPGA:
-
Uridine diphosphoglucuronic acid
- UGT:
-
Uridine diphosphate-5′-glucuronosyltransferase or UDP-glucuronosyltransferase
References
Birt DF, Hendrich S, Wang W (2001) Dietary agents in cancer prevention: flavonoids and isoflavonoids. Pharmacol Ther 90:157–177
Bock KW, Eckle T, Ouzzine M, Fournel-Gigleux S (2000) Coordinate induction by antioxidants of UDP-glucuronosyltransferase UGT1A6 and the apical conjugate export pump MRP2 (multidrug resistance protein 2) in Caco-2 cells. Biochem Pharmacol 59:467–470
Bock-Hennig BS, Kohle C, Nill K, Bock KW (2002) Influence of t-butylhydroquinone and beta-naphthoflavone on formation and transport of 4-methylumbelliferone glucuronide in Caco-2/TC-7 cell monolayers. Biochem Pharmacol 63:123–128
Busby MG, Jeffcoat AR, Bloedon LT, Koch MA, Black T, Dix KJ, Heizer WD, Thomas BF, Hill JM, Crowell JA, Zeisel SH (2002) Clinical characteristics and pharmacokinetics of purified soy isoflavones: single-dose administration to healthy men. Am J Clin Nutr 75:126–136
Chen J, Lin H, Hu M (2003) Metabolism of flavonoids via enteric recycling: role of intestinal disposition. J Pharmacol Exp Ther 304:1228–1235
Coldham NG, Zhang AQ, Key P, Sauer MJ (2003) Absolute bioavailability of [14C] genistein in the rat; plasma pharmacokinetics of parent compound, genistein glucuronide and total radioactivity. Eur J Drug Metab Pharmacokinet 27:249–258
Hirohashi T, Suzuki H, Chu XY, Tamai I, Tsuji A, Sugiyama Y (2000) Function and expression of multidrug resistance-associated protein family in human colon adenocarcinoma cells (Caco-2). J Pharmacol Exp Ther 292:265–270
Hu M, Chen J, Tran D, Zhu Y, Leonardo G (1994) The Caco-2 cell monolayers as an intestinal metabolism model: metabolism of dipeptide Phe-Pro. J Drug Target 2:79–89
Hu M, Chen J, Zhu Y, Dantzig AH, Stratford RE Jr, Kuhfeld MT (1994) Mechanism and kinetics of transcellular transport of a new β-lactam antibiotic loracarbef across a human intestinal epithelial model system (Caco-2). Pharm Res 11:1405–1413
Hu M, Li Y, Penman BW, Huang SM, Thummel K, Crespi CL (1999) Morphological and metabolic characterization of Caco-2 cells expressing high levels of cDNA-derived cytochrome P4503A4. Pharm Res 16:1352–1359
Hu M, Chen J, Lin H (2003) Metabolism of flavonoids via enteric recycling: mechanistic studies of disposition of apigenin in the Caco-2 cell culture model. J Pharmacol Exp Ther 307:314–321
King RA, Bursill DB (1998) Plasma and urinary kinetics of the isoflavones daidzein and genistein after a single soy meal in humans. Am J Clin Nutr 67:867–872
King RA, Broadbent JL, Head RJ (1996) Absorption and excretion of the soy isoflavone genistein in rats. J Nutr 126:176–182
Kurzer M, Xu X (1997) Dietary phytoestrogens. Annu Rev Nutr 17:353–381
Liu Y, Hu M (2002) Absorption and metabolism of flavonoids in the Caco-2 cell culture model and a perfused rat intestinal model. Drug Metab Dispos 30:370–377
O’Leary KA, Day AJ, Needs PW, Mellon FA, O’Brien NM, Williamson G (2003) Metabolism of quercetin-7- and quercetin-3-glucuronides by an in vitro hepatic model: the role of human beta-glucuronidase, sulfotransferase, catechol-O-methyltransferase and multi-resistant protein 2 (MRP2) in flavonoid metabolism. Biochem Pharmacol 65:479–491
Sekine T, Cha SH, Endou H (2000) The multispecific organic anion transporter (OAT) family. Pflugers Arch 440:337–350
Setchell KD, Brown NM, Desai P, Zimmer-Nechemias L, Wolfe BE, Brashear WT, Kirschner AS, Cassidy A, Heubi JE (2001) Bioavailability of pure isoflavones in healthy humans and analysis of commercial soy isoflavone supplements. J Nutr 131:1362S–1375S
Setchell KD, Faughnan MS, Avades T, Zimmer-Nechemias L, Brown NM, Wolfe BE, Brashear WT, Desai P, Oldfield MF, Botting NP, Cassidy A (2003) Comparing the pharmacokinetics of daidzein and genistein with the use of 13C-labeled tracers in premenopausal women. Am J Clin Nutr 77:411–419
Shelnutt SR, Cimino CO, Wiggins PA, Ronis MJ, Badger TM (2002) Pharmacokinetics of the glucuronide and sulfate conjugates of genistein and daidzein in men and women after consumption of a soy beverage. Am J Clin Nutr 76:588–594
Vaidyanathan JB, Walle T (2001) Transport and metabolism of the tea flavonoid (−)-epicatechin by the human intestinal cell line Caco-2. Pharm Res 18:1420–1425
Walgren RA, Lin JT, Kinne RK, Walle T (2000) Cellular uptake of dietary flavonoid quercetin 4′-β-glucoside by sodium-dependent glucose transporter SGLT1. J Pharmacol Exp Ther 294:837–843
Walle UK, Galijatovic A, Walle T (1999) Transport of the flavonoid chrysin and its conjugated metabolites by the human intestinal cell line Caco-2. Biochem Pharmacol 58:431–438
Yang CS, Landau JM, Huang MT, Newmark HL (2001) Inhibition of carcinogenesis by dietary polyphenolic compounds. Annu Rev Nutr 21:381–406
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, J., Lin, H. & Hu, M. Absorption and metabolism of genistein and its five isoflavone analogs in the human intestinal Caco-2 model. Cancer Chemother Pharmacol 55, 159–169 (2005). https://doi.org/10.1007/s00280-004-0842-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-004-0842-x