Abstract
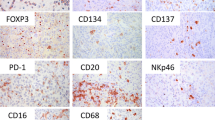
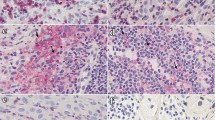
Various clinical and experimental observations detected an immunological host defense in cutaneous melanoma. In order to investigate the prognostic value of leukocyte effector mechanisms, we examined the presence of different subsets of leukocytes in tumor samples of 58 patients diagnosed with primary cutaneous melanoma. The presence of T lymphocytes, cytotoxic T lymphocytes, B lymphocytes, CD16+ cells and macrophages was correlated to Breslow depth. A significantly higher amount of several subsets of leukocytes was found in samples with a more progressed tumor stage and survival analysis demonstrated that a higher amount of T lymphocytes and CD16+ cells was associated with a short survival. The amount of FOXP3+ regulatory T lymphocytes did not correlate with survival, nevertheless, it correlated with the amount of total infiltrate. In contrast, analysis of the expression of CD69, a marker for activated lymphocytes, demonstrated that patients with a higher amount of CD69+ lymphocytes had a better survival. In addition, a new parameter for aggressiveness of melanoma, tumor cell plasticity [i.e., the presence of periodic acid Schiff’s (PAS) reagent positive loops], also predicted short survival and a trend of a higher amount of tumor infiltrating leukocytes in tumors with PAS positive loops was observed. These findings demonstrate that leukocyte infiltration and the presence of PAS loops is a sign of tumor aggressiveness and may have prognostic value.





Similar content being viewed by others
References
Ramirez-Montagut T, et al (2003) Immunity to melanoma: unraveling the relation of tumor immunity and autoimmunity. Oncogene 22(20):3180–3187
Chen Q, Wang WC, Evans SS (2003) Tumor microvasculature as a barrier to antitumor immunity. Cancer Immunol Immunother 52(11):670–679
Vesalainen S, et al (1994) Histological grade, perineural infiltration, tumour-infiltrating lymphocytes and apoptosis as determinants of long-term prognosis in prostatic adenocarcinoma. Eur J Cancer 30A(12):1797–1803
Marrogi AJ, et al (1997) Study of tumor infiltrating lymphocytes and transforming growth factor-beta as prognostic factors in breast carcinoma. Int J Cancer 74(5):492–501
Chao HT, et al (1999) Lymphocyte-infiltrated FIGO Stage IIB squamous cell carcinoma of the cervix is a prominent factor for disease-free survival. Eur J Gynaecol Oncol 20(2):136–140
Naito Y, et al (1998) CD8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Res 58(16):3491–3494
Schumacher K, et al (2001) Prognostic significance of activated CD8(+) T cell infiltrations within esophageal carcinomas. Cancer Res 61(10):3932–3936
Cho Y, et al (2003) CD4+ and CD8+ T cells cooperate to improve prognosis of patients with esophageal squamous cell carcinoma. Cancer Res 63(7):1555–1559
Zhang L, et al (2003) Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med 348(3):203–213
Sato E, et al (2005) Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci USA 102(51):18538–18543
Badoual C, et al (2006) Prognostic value of tumor-infiltrating CD4+ T-cell subpopulations in head and neck cancers. Clin Cancer Res 12(2):465–472
Hiraoka K, et al (2006) Concurrent infiltration by CD8+ T cells and CD4+ T cells is a favourable prognostic factor in non-small-cell lung carcinoma. Br J Cancer 94(2):275–280
Hussein MR (2005) Tumour-infiltrating lymphocytes and melanoma tumorigenesis: an insight. Br J Dermatol 153(1):18–21
Clark WH Jr, et al (1989) Model predicting survival in stage I melanoma based on tumor progression. J Natl Cancer Inst 81(24):1893–1904
Clemente CG, et al (1996) Prognostic value of tumor infiltrating lymphocytes in the vertical growth phase of primary cutaneous melanoma. Cancer 77(7):1303–1310
Thorn M, et al (1994) Trends in tumour characteristics and survival of malignant melanoma 1960–84: a population-based study in Sweden. Br J Cancer 70(4):743–748
Barnhill RL, et al (1996) Predicting five-year outcome for patients with cutaneous melanoma in a population-based study. Cancer 78(3):427–432
van der Schaft DW, et al (2005) Tumor cell plasticity in Ewing sarcoma, an alternative circulatory system stimulated by hypoxia. Cancer Res 65(24):11520–11528
Hillen F, et al (2006) Proliferating endothelial cells, but not microvessel density, is a prognostic parameter in human cutaneous melanoma. Melanoma Res 16:453–457
Warso MA, et al (2001) Prognostic significance of periodic acid-Schiff-positive patterns in primary cutaneous melanoma. Clin Cancer Res 7(3):473–477
Hendrix MJ, et al (2003) Vasculogenic mimicry and tumour-cell plasticity: lessons from melanoma. Nat Rev Cancer 3(6):411–421
Thies A, et al (2001) PAS-positive loops and networks as a prognostic indicator in cutaneous malignant melanoma. J Pathol 195(5):537–542
Levi F, et al (2004) Cancer mortality in Europe, 1995–1999, and an overview of trends since 1960. Int J Cancer 110(2):155–169
Carlson JA, et al (2003) Malignant melanoma 2003: predisposition, diagnosis, prognosis, and staging. Am J Clin Pathol 120(Suppl):S101–S127
Eberlein TJ, Rosenstein M, Rosenberg SA (1982) Regression of a disseminated syngeneic solid tumor by systemic transfer of lymphoid cells expanded in interleukin 2. J Exp Med 156(2):385–397
Rosenberg SA, Spiess P, Lafreniere R (1986) A new approach to the adoptive immunotherapy of cancer with tumor-infiltrating lymphocytes. Science 233(4770):1318–1321
Overwijk WW, et al (1998) gp100/pmel 17 is a murine tumor rejection antigen: induction of “self”-reactive, tumoricidal T cells using high-affinity, altered peptide ligand. J Exp Med 188(2):277–286
Dudley ME, et al (2002) Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science 298(5594):850–854
Yee C, et al (2002) Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: in vivo persistence, migration, and antitumor effect of transferred T cells. Proc Natl Acad Sci USA 99(25):16168–16173
Dudley ME, et al (2005) Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol 23(10):2346–2357
Morgan RA, et al (2006) Cancer regression in patients after transfer of genetically engineered lymphocytes. Science 314(5796):126–129
Hussein MR, et al (2006) Immunohistological characterisation of tumour infiltrating lymphocytes in melanocytic skin lesions. J Clin Pathol 59(3):316–324
Brocker EB, et al (1988) Inflammatory cell infiltrates in human melanoma at different stages of tumor progression. Int J Cancer 41(4):562–567
Piras F, et al (2005) The predictive value of CD8, CD4, CD68, and human leukocyte antigen-D-related cells in the prognosis of cutaneous malignant melanoma with vertical growth phase. Cancer 104(6):1246–1254
Jorkov AS, et al (2003) Immune response in blood and tumour tissue in patients with metastatic malignant melanoma treated with IL-2, IFN alpha and histamine dihydrochloride. Anticancer Res 23(1B):537–542
Jovic V, et al (2001) Impaired perforin-dependent NK cell cytotoxicity and proliferative activity of peripheral blood T cells is associated with metastatic melanoma. Tumori 87(5):324–329
Mantovani A, et al (1992) The origin and function of tumor-associated macrophages. Immunol Today 13(7):265–270
Bingle L, Brown NJ, Lewis CE (2002) The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. J Pathol 196(3):254–265
Dirkx AEM, et al (2006) Monocyte/macrophage infiltration in tumors: modulators of angiogenesis. J Leukoc Biol 80(6):1183–1196
Bennett SR, et al (1998) B cells directly tolerize CD8(+) T cells. J Exp Med 188(11):1977–1983
Perricone MA, et al (2004) Enhanced efficacy of melanoma vaccines in the absence of B lymphocytes. J Immunother 27(4):273–281
Shah S, et al (2005) Increased rejection of primary tumors in mice lacking B cells: inhibition of anti-tumor CTL and TH1 cytokine responses by B cells. Int J Cancer 117(4):574–586
Lapointe R, et al (2003) CD40-stimulated B lymphocytes pulsed with tumor antigens are effective antigen-presenting cells that can generate specific T cells. Cancer Res 63(11):2836–2843
Martinez-Escribano JA, et al (2003) Changes in the number of CD80(+), CD86(+), and CD28(+) peripheral blood lymphocytes have prognostic value in melanoma patients. Hum Immunol 64(8):796–801
Aklilu M, et al (2004) Depletion of normal B cells with rituximab as an adjunct to IL-2 therapy for renal cell carcinoma and melanoma. Ann Oncol 15(7):1109–1114
Massague J (1990) The transforming growth factor-beta family. Annu Rev Cell Biol 6:597–641
Fiorentino DF, et al (1991) IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J Immunol 146(10):3444–3451
Ladanyi A, et al (2004) T-cell activation marker expression on tumor-infiltrating lymphocytes as prognostic factor in cutaneous malignant melanoma. Clin Cancer Res 10(2):521–530
Rubinstein N, et al (2004) Targeted inhibition of galectin-1 gene expression in tumor cells results in heightened T cell-mediated rejection; A potential mechanism of tumor-immune privilege. Cancer Cell 5(3):241–251
Le QT, et al (2005) Galectin-1: a link between tumor hypoxia and tumor immune privilege. J Clin Oncol 23(35):8932–8941
Pawelec G (2004) Tumour escape from the immune response. Cancer Immunol Immunother 53(10):843
Hori S, Nomura T, Sakaguchi S (2003) Control of regulatory T cell development by the transcription factor Foxp3. Science 299(5609):1057–1061
Woo EY, et al (2001) Regulatory CD4(+) CD25(+) T cells in tumors from patients with early-stage non-small cell lung cancer and late-stage ovarian cancer. Cancer Res 61(12):4766–4772
Woo EY, et al (2002) Cutting edge: regulatory T cells from lung cancer patients directly inhibit autologous T cell proliferation. J Immunol 168(9):4272–4276
Sasada T, et al (2003) CD4+CD25+ regulatory T cells in patients with gastrointestinal malignancies: possible involvement of regulatory T cells in disease progression. Cancer 98(5):1089–1099
Wolf AM, et al (2003) Increase of regulatory T cells in the peripheral blood of cancer patients. Clin Cancer Res 9(2):606–612
Viguier M, et al (2004) Foxp3 expressing CD4+CD25(high) regulatory T cells are overrepresented in human metastatic melanoma lymph nodes and inhibit the function of infiltrating T cells. J Immunol 173(2):1444–1453
Alvaro T, et al (2005) Outcome in Hodgkin’s lymphoma can be predicted from the presence of accompanying cytotoxic and regulatory T cells. Clin Cancer Res 11(4):1467–1473
Wolf D, et al (2005) The expression of the regulatory T cell-specific forkhead box transcription factor FoxP3 is associated with poor prognosis in ovarian cancer. Clin Cancer Res 11(23):8326–8331
Hiraoka N, et al (2006) Prevalence of FOXP3+ regulatory T cells increases during the progression of pancreatic ductal adenocarcinoma and its premalignant lesions. Clin Cancer Res 12(18):5423–5434
Bates GJ, et al (2006) Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. J Clin Oncol 24(34):5373–5380
Appay V, et al (2006) New generation vaccine induces effective melanoma-specific CD8+ T cells in the circulation but not in the tumor site. J Immunol 177(3):1670–1678
Ahmadzadeh M, Rosenberg SA (2006) IL-2 administration increases CD4+ CD25(hi) Foxp3+ regulatory T cells in cancer patients. Blood 107(6):2409–2414
Nair S, et al (2007) Vaccination against the forkhead family transcription factor Foxp3 enhances tumor immunity. Cancer Res 67(1):371–380
Cambiaggi C, et al (1992) Constitutive expression of CD69 in interspecies T-cell hybrids and locus assignment to human chromosome 12. Immunogenetics 36(2):117–120
Healy CG, et al (1998) Impaired expression and function of signal-transducing zeta chains in peripheral T cells and natural killer cells in patients with prostate cancer. Cytometry 32(2):109–119
Koch M, et al (2006) Tumor infiltrating T lymphocytes in colorectal cancer: tumor-selective activation and cytotoxic activity in situ. Ann Surg 244(6):986–992; discussion 992-3
Slingluff CL Jr, et al (1988) Lethal “thin” malignant melanoma. Identifying patients at risk. Ann Surg 208(2):150–161
Blessing K, McLaren KM (1992) Histological regression in primary cutaneous melanoma: recognition, prevalence and significance. Histopathology 20(4):315–322
Trau H, et al (1983) Metastases of thin melanomas. Cancer 51(3):553–556
Wanebo HJ, Cooper PH, Hagar RW (1985) Thin (less than or equal to 1 mm) melanomas of the extremities are biologically favorable lesions not influenced by regression. Ann Surg 201(4):499–504
Shaw HM, et al (1987) Thin malignant melanomas and recurrence potential. Arch Surg 122(10):1147–1150
Fontaine D, et al (2003) Partial regression of primary cutaneous melanoma: is there an association with sub-clinical sentinel lymph node metastasis? Am J Dermatopathol 25(5):371–376
Molema G, Griffioen AW (1998) Rocking the foundations of solid tumor growth by attacking the tumor’s blood supply. Immunol Today 19(9):392–394
Maniotis AJ, et al (1999) Vascular channel formation by human melanoma cells in vivo and in vitro: vasculogenic mimicry. Am J Pathol 155(3):739–752
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hillen, F., Baeten, C.I.M., van de Winkel, A. et al. Leukocyte infiltration and tumor cell plasticity are parameters of aggressiveness in primary cutaneous melanoma. Cancer Immunol Immunother 57, 97–106 (2008). https://doi.org/10.1007/s00262-007-0353-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-007-0353-9