Abstract
Purpose
To investigate the molecular mechanisms underlying the variable standard uptake value (SUV) of 18F-fluorodeoxyglucose positron emission tomography-computed tomography (18F-FDG PET-CT) imaging in hepatocellular carcinoma (HCC) and whether hypoxia-induced glucose transporter expression contributes to the progression of HCC and the rate of glycolysis in HCC cells.
Materials and methods
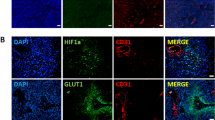
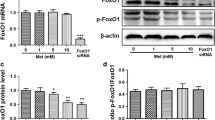
Sixteen HCC specimens obtained from patients who underwent pre-treatment staging with 18F-FDG PET-CT imaging were divided into high maximum SUV (SUVmax > 8) and low SUVmax (SUVmax < 5) groups and employed for whole-genome gene expression profiling using GeneChip Human Genome U133 Plus 2.0 Arrays. The relationship between SUVmax and the expression of glucose transporters 1 and 3 (GLUT1 and GLUT3) was further validated using immunohistochemical analysis. The expression of GLUT1 and GLUT3 in different HCC cells under hypoxia and normoxia conditions were monitored by quantitative reverse transcription PCR (RT-qPCR). Glycolysis and FDG uptake by HCC cells were measured using the Seahorse XF glycolysis stress test and 18F-FDG PET-CT imaging. The effect of GLUT1 and GLUT3 on glucose uptake in HCC cells was examined using the fluorescent D-glucose analog 2-[N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl) amino]-2-deoxy-d-glucose (2-NBDG) followed by detection of fluorescence produced by the cells using flow cytometry.
Results
Glucose transporters are differentially expressed between samples from HCC patients with high and low SUVmax. In particular, over-expression of GLUT1 and GLUT3 in high SUVmax patients was correlated with high glucose uptake and overall survival. The expression of GLUT1 and GLUT3 was significantly induced by hypoxia in different HCC cells. High expression of GLUT1 and GLUT3 in HCC cells were correlated with high rates of glycolysis and 18F-FDG uptake. Therefore, our data suggested that hypoxia-induced glucose transporters expression could result in the variations of 18F-FDG PET-CT imaging and progression of HCC, contributing to more aggressive disease phenotypes like large tumor size, recurrence, and poor survival.
Conclusion
Over-expression of GLUT1 and GLUT3 significantly increase glucose uptake in HCC cells. Hypoxia-induced glucose transporters expression may therefore be a contributing variable in 18F-FDG PET-CT imaging and progression in HCC.






Similar content being viewed by others
References
Rudalska R, Dauch D, Longerich T, McJunkin K, Wuestefeld T, Kang T-W, et al. In vivo RNAi screening identifies a mechanism of sorafenib resistance in liver cancer. Nat Med. 2014;20:1138.
Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–32.
Fu Y, Ong L-C, Ranganath SH, Zheng L, Kee I, Zhan W, et al. A dual tracer 18F-FCH/18F-FDG PET imaging of an orthotopic brain tumor xenograft model. PLoS One. 2016;11:e0148123.
Y-i K, Paeng JC, Cheon GJ, Suh K-S, Lee DS, Chung J-K, et al. Prediction of posttransplantation recurrence of hepatocellular carcinoma using metabolic and volumetric indices of 18F-FDG PET/CT. J Nucl Med. 2016;57:1045–51.
Sung PS, Park HL, Yang K, Hwang S, Song MJ, Jang JW, et al. 18 F–fluorodeoxyglucose uptake of hepatocellular carcinoma as a prognostic predictor in patients with sorafenib treatment. Eur J Nucl Med Mol Imaging. 2018;45:384–91.
Na SJ, Oh JK, Hyun SH, Lee JW, Hong IK, Song B-I, et al. 18F-FDG PET/CT can predict survival of advanced hepatocellular carcinoma patients: a multicenter retrospective cohort study. J Nucl Med. 2017;58:730–6.
Lin C-Y, Liao C-W, Chu L-Y, Yen K-Y, Jeng L-B, Hsu C-N, et al. Predictive value of 18F-FDG PET/CT for vascular invasion in patients with hepatocellular carcinoma before liver transplantation. Clin Nucl Med. 2017;42:e183–e7.
Chalaye J, Costentin CE, Luciani A, Amaddeo G, Ganne-Carrié N, Baranes L, et al. Positron emission tomography/computed tomography with 18F-fluorocholine improve tumor staging and treatment allocation in patients with hepatocellular carcinoma. J Hepatol. 2018;69:336–44.
Lee M, Jeon JY, Neugent ML, Kim J-W, Yun M. 18F-Fluorodeoxyglucose uptake on positron emission tomography/computed tomography is associated with metastasis and epithelial-mesenchymal transition in hepatocellular carcinoma. Clinical & Experimental Metastasis. 2017;34:251–60.
Fleming IN, Manavaki R, Blower PJ, West C, Williams KJ, Harris AL, et al. Imaging tumour hypoxia with positron emission tomography. Br J Cancer. 2015;112:238.
Zhang J-Z, Behrooz A, Ismail-Beigi F. Regulation of glucose transport by hypoxia. Am J Kidney Dis. 1999;34:189–202.
Li X-F, Ma Y, Sun X, Humm JL, Ling CC, O'Donoghue JA. High 18F-FDG uptake in microscopic peritoneal tumors requires physiologic hypoxia. J Nucl Med. 2010;51:632–8.
Lee JH, Park JY, Kim DY, Ahn SH, Han KH, Seo HJ, et al. Prognostic value of 18F-FDG PET for hepatocellular carcinoma patients treated with sorafenib. Liver Int. 2011;31:1144–9.
Cho KJ, Choi NK, Shin MH, Chong AR. Clinical usefulness of FDG-PET in patients with hepatocellular carcinoma undergoing surgical resection. Annals of Hepato-Biliary-Pancreatic Surgery. 2017;21:194–8.
Wu B, Zhang Y, Tan H, Shi H. Value of 18 F-FDG PET/CT in the diagnosis of portal vein tumor thrombus in patients with hepatocellular carcinoma. Abdominal Radiology. 2019:1–6.
Bains S, Behr S, Corvera C, Khan S, Win A, Seo Y, et al. SUVmax values in FDG-PET/CT scans of patients with HCC: a possible new prognostic factor. Journal of Nuclear Medicine. 2012;53:1308.
Soukupova J, Malfettone A, Hyroššová P, Hernández-Alvarez M-I, Peñuelas-Haro I, Bertran E, et al. Role of the Transforming Growth Factor-β in regulating hepatocellular carcinoma oxidative metabolism. Sci Rep. 2017;7:12486.
Thorens B, Mueckler M. Glucose transporters in the 21st century. American Journal of Physiology-Endocrinology and Metabolism. 2009;298:E141–E5.
Seo S, Hatano E, Higashi T, Hara T, Tada M, Tamaki N, et al. Fluorine-18 fluorodeoxyglucose positron emission tomography predicts tumor differentiation, P-glycoprotein expression, and outcome after resection in hepatocellular carcinoma. Clin Cancer Res. 2007;13:427–33.
Chen R, Li J, Zhou X, Liu J, Huang G. Fructose-1, 6-bisphosphatase 1 reduces 18F FDG uptake in hepatocellular carcinoma. Radiology. 2017;284:844–53.
Trojan J, Schroeder O, Raedle J, Baum RP, Herrmann G, Jacobi V, et al. Fluorine-18 FDG positron emission tomography for imaging of hepatocellular carcinoma. Am J Gastroenterol. 1999;94:3314–9.
Khan MA, Combs CS, Brunt EM, Lowe VJ, Wolverson MK, Solomon H, et al. Positron emission tomography scanning in the evaluation of hepatocellular carcinoma. J Hepatol. 2000;32:792–7.
Bieze M, Klumpen H, Verheij J, Beuers U, Phoa S, van Gulik T. Diagnostic accuracy of 18F-methyl-choline PET/CT for intra-and extrahepatic hepatocellular carcinoma. Hepatology. 2014;59:996–1006.
Kuyumcu S, Has-Simsek D, Iliaz R, Sanli Y, Buyukkaya F, Akyuz F, et al. Evidence of prostate-specific membrane antigen expression in hepatocellular carcinoma using 68Ga-PSMA PET/CT. Clin Nucl Med. 2019;44:702–6.
Sasikumar A, Joy A, Nanabala R, Pillai M, Thomas B, Vikraman K. 68Ga-PSMA PET/CT imaging in primary hepatocellular carcinoma. Eur J Nucl Med Mol Imaging. 2016;43:795–6.
Taneja S, Taneja R, Kashyap V, Jha A, Jena A. 68Ga-PSMA uptake in hepatocellular carcinoma. Clin Nucl Med. 2017;42:e69–70.
Koh YW, Park SY, Hyun SH, Lee SJ. Associations between PET textural features and GLUT1 expression, and the prognostic significance of textural features in lung adenocarcinoma. Anticancer Res. 2018;38:1067–71.
Kawai T, Yasuchika K, Seo S, Higashi T, Ishii T, Miyauchi Y, et al. Identification of keratin 19–positive cancer stem cells associating human hepatocellular carcinoma using 18F-Fluorodeoxyglucose positron emission tomography. Clin Cancer Res. 2017;23:1450–60.
Patel D, Gara SK, Ellis RJ, Boufraqech M, Nilubol N, Millo C, et al. FDG PET/CT scan and functional adrenal tumors: a pilot study for lateralization. World J Surg. 2016;40:683–9.
Yoon M, Jung SJ, Kim TH, Ha TK, Urm S-H, Park JS, et al. Relationships between transporter expression and the status of BRAF V600E mutation and F-18 FDG uptake in papillary thyroid carcinomas. Endocr Res. 2016;41:64–9.
Zhuang H, Pourdehnad M, Lambright ES, Yamamoto AJ, Lanuti M, Li P, et al. Dual time point 18F-FDG PET imaging for differentiating malignant from inflammatory processes. J Nucl Med. 2001;42:1412–7.
Iyer NV, Kotch LE, Agani F, Leung SW, Laughner E, Wenger RH, et al. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1α. Genes Dev. 1998;12:149–62.
Ci-Ai Lin, Lin-Lin Chang, Hong Zhu, Qiao-Jun He, Bo Yang, (2018) Hypoxic microenvironment and hepatocellular carcinoma treatment. Hepatoma Research 4 (6):26
Acknowledgments
We thank Dr. Sidney Yu from the Department of Nuclear Medicine and Molecular Imaging Singapore General Hospital for advising the PET-CT imaging.
Funding
This study was funded by the grants from the National Medical Research Council of Singapore, National Young 1000 Talents Program of China and Jiangsu Province Education Department, Jiangsu Province “Innovative and Entrepreneurial Team” and “Innovative and Entrepreneurial Talent.”, Key Project of National Natural Science Foundation of China (81730108), Key Project of Zhejiang Province Ministry of Science and Technology (2015C03055), Key Project of Hangzhou Ministry of Science and Technology (20162013A07) and Start-up Grant of HZNU, National Natural Science Foundation of China (81802328).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study was approved by the Institutional Review Board (IRB) of Sun Yat-Sen University Cancer Center. All procedures performed in studies involving human participants were following the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The animal study was approved by the Institutional Animal Care and Use Committee (IACUC) of SingHealth. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Oncology – Digestive tract
Electronic supplementary material
ESM 1
(DOCX 23 kb)
Rights and permissions
About this article
Cite this article
Xia, H., Chen, J., Gao, H. et al. Hypoxia-induced modulation of glucose transporter expression impacts 18F-fluorodeoxyglucose PET-CT imaging in hepatocellular carcinoma. Eur J Nucl Med Mol Imaging 47, 787–797 (2020). https://doi.org/10.1007/s00259-019-04638-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-019-04638-4