Abstract
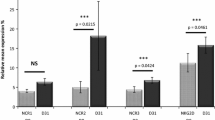
The function of natural killer (NK) cells after allogeneic hematopoietic stem cell transplantation (allo-HSCT) is regulated by the balance between inhibitory KIRs (iKIRs) and activating KIRs (aKIRs). However, few studies have examined the subsequent expression of KIR genes unique to the donor. We defined the set of KIR genes expressed only in the donor and designed a method for measuring the expression of these KIR genes by quantitative real-time polymerase chain reaction (RT-qPCR) based on genetic cloning techniques. In this study, we evaluated the recovery pattern of KIR genes in 252 donor-recipient pairs. The expression of each KIR unique to the donor was in line with that of KIR genes shared by the donor and recipient, such as KIR2DS1, KIR3DS1, KIR2DS4, or KIR2DS3. The timing of the peak mRNA expression of aKIRs unique to the donor was inconsistent but occurred within the first 3 months posttransplantation, whereas the peak mRNA expression of iKIRs was consistently observed in the third month after transplantation. The expression of KIR2DL2 in the third month posttransplantation was significantly higher in the transplant recipients than in the donors (p = 0.01). The KIR2DL1 and KIR3DL1 levels in the transplant recipients in the second and third months posttransplantation were also obviously higher than the donor levels (p < 0.0001). Thus, these observations should be considered when attempting to predict the correlation between mRNA expression and prognosis after allo-HSCT.
Similar content being viewed by others
References
Agresti A (2002) Categorical data analysis, vol 2002, 2nd edn. Wiley, New York, p 81
Anfossi N, André P, Guia S, Falk CS, Roetynck S, Stewart CA, Breso V, Frassati C, Reviron D, Middleton D, Romagné F, Ugolini S, Vivier E (2006) Human NK cell education by inhibitory receptors for MHC class I. Immunity 25:331–342. https://doi.org/10.1016/j.immuni.2006.06.013
Bao XJ, Wang M, Zhou HF, Zhang H, Wu X, Yuan X, Li Y, Wu D, He J (2015) Donor killer immunoglobulin-like receptor profile Bx1 imparts a negative effect and centromeric B-specific gene motifs render a positive effect on standard-risk acute myeloid leukemia/myelodysplastic syndrome patient survival after unrelated donor hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 22:232–239. https://doi.org/10.1016/j.bbmt.2015.09.007
Barten R, Torkar M, Haude A, Trowsdale J, Wilson MJ (2001) Divergent and convergent evolution of NK-cell receptors. Trends Immunol 22:52–57
Beillard E, Pallisgaard N, van der Velden VH, Bi W, Dee R, van der Schoot E, Delabesse E, Macintyre E, Gottardi E, Saglio G, Watzinger F, Lion T, van Dongen J, Hokland P, Gabert J (2003) Evaluation of candidate control genes for diagnosis and residual disease detection in leukemic patients using 'real-time' quantitative reverse-transcriptase polymerase chain reaction (RQ-PCR) - a Europe against cancer program. Leukemia 17:2474–2486. https://doi.org/10.1038/sj.leu.2403136
Boudreau JE, Liu XR, Zhao Z, Zhang A, Shultz LD, Greiner DL, Dupont B, Hsu KC (2016) Cell-extrinsic MHC class I molecule engagement augments human NK cell education programmed by cell-intrinsic MHC class I. Immunity 45:280–291. https://doi.org/10.1016/j.immuni.2016.07.005
Chaisri S, Traherne JA, Jayaraman J, Romphruk A, Trowsdale J, Leelayuwat C (2018) Novel KIR genotypes and gene copy number variations in northeastern Thais. Immunology 153:380–386. https://doi.org/10.1111/imm.12847
Chen X, Knowles J, Barfield RC, Kasow KA, Madden R, Woodard P, Srivastava DK, Horwitz EM, Handgretinger R, Hale GA (2009) A novel approach for quantification of KIR expression in healthy donors and pediatric recipients of hematopoietic SCTs. Bone Marrow Transplant 43:525–532. https://doi.org/10.1038/bmt.2008.352
Cooley S, Weisdorf DJ, Guethlein LA, Klein JP, Wang T, Marsh SG, Spellman S, Haagenson MD, Saeturn K, Ladner M, Trachtenberg E, Parham P, Miller JS (2014) Donor killer cell Ig-like receptor B haplotypes, recipient HLA-C1, and HLA-C mismatch enhance the clinical benefit of unrelated transplantation for acute myelogenous leukemia. J Immunol 192:4592–4600. https://doi.org/10.4049/jimmunol.1302517
Cooley S, Parham P, Miller JS (2018) Strategies to activate NK cells to prevent relapse and induce remission following hematopoietic stem cell transplantation. Blood:blood-2017-08-752170. https://doi.org/10.1182/blood-2017-08-752170
Denis L, Gagne K, Gueglio B, Kerdudou N, Milpied N, Simon P, Follea G, Bonneville M, Harousseau JL, Bignon JD (2005) NK-KIR transcript kinetics correlate with acute graft-versus-host disease occurrence after allogeneic bone marrow transplantation. Hum Immunol 66:447–459. https://doi.org/10.1016/j.humimm.2005.01.008
Elfishawi SM, Mossallam GI, El-Fattah RA, El-Haddad A, Kamel AM (2017) The effect of killer cell immunoglobulin-like receptor genotype on outcome of hematopoietic stem cell transplantation from matched sibling. Hum Immunol 78:684–691. https://doi.org/10.1016/j.humimm.2017.10.004
Farag SS, Fehniger TA, Ruggeri L, Velardi A, Caligiuri MA (2002) Natural killer cell receptors: new biology and insights into the graft-versus-leukemia effect. Blood 100:1935–1947. https://doi.org/10.1182/blood-2002-02-0350
Fernandez NC, Treiner E, Vance RE, Jamieson AM, Lemieux S, Raulet DH (2005) A subset of natural killer cells achieves self-tolerance without expressing inhibitory receptors specific for self-MHC molecules. Blood 105:4416–4423. https://doi.org/10.1182/blood-2004-08-3156
Gallez-Hawkins GM, Franck AE, Li X, Thao L, Oki A, Gendzekhadze K, Dagis A, Palmer J, Nakamura R, Forman SJ, Senitzer D, Zaia JA (2011) Expression of activating KIR2DS2 and KIR2DS4 genes after hematopoietic cell transplantation: relevance to cytomegalovirus infection. Biol Blood Marrow Transplant 17:1662–1672. https://doi.org/10.1016/j.bbmt.2011.04.008
Giebel S, Dziaczkowska J, Czerw T, Wojnar J, Krawczyk-Kulis M, Nowak I, Holowiecka A, Segatti A, Kyrcz-Krzemien S, Kusnierczyk P, Holowiecki J (2010) Sequential recovery of NK cell receptor repertoire after allogeneic hematopoietic SCT. Bone Marrow Transplant 45:1022–1030. https://doi.org/10.1038/bmt.2009.384
Gonzalez-Galarza FF, Takeshita LY, Santos EJ et al (2015) Allele frequency net 2015 update: new features for HLA epitopes, KIR and disease and HLA adverse drug reaction associations. Nucleic Acids Res 43:D784–D788. https://doi.org/10.1093/nar/gku1166
Heidenreich S, Kroger N (2017) Reduction of relapse after unrelated donor stem cell transplantation by KIR-based graft selection. Front Immunol 8:41. https://doi.org/10.3389/fimmu.2017.00041
Hsu KC, Chida S, Geraghty DE, Dupont B (2002) The killer cell immunoglobulin-like receptor (KIR) genomic region: gene-order, haplotypes and allelic polymorphism. Immunol Rev 190:40–52. https://doi.org/10.1034/j.1600-065x.2002.19004.x
Hu X, He J, Zhang HH et al (2017) Immune reconstruct regularity profile of KIR2DL1 and KIR3DL1 in unrelated-donor allogeneic hematopoietic stem cell transplantation. Chin J Hematol 38:667–672. https://doi.org/10.3760/cma.j.issn.0253-2727.2017.08.004
Kim S, Poursine-Laurent J, Truscott SM, Lybarger L, Song YJ, Yang L, French AR, Sunwoo JB, Lemieux S, Hansen TH, Yokoyama WM (2005) Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nature 436:709–713. https://doi.org/10.1038/nature03847
Kitpoka P, Tammakorn C, Chaisri S, Leelayuwat C, Mongkolsuk T, Thammanichanond D (2016) Genetic profiles of killer-cell immunoglobulin-like receptors and HLA ligands in Thai blood donors. Hum Immunol 77:470–475. https://doi.org/10.1016/j.humimm.2016.04.019
Martin AM, Freitas EM, Witt CS, Christiansen FT (2000) The genomic organization and evolution of the natural killer immunoglobulin-like receptor (KIR) gene cluster. Immunogenetics 51:268–280. https://doi.org/10.1007/s002510050620
Martin AM, Kulski JK, Gaudieri S, Witt CS, Freitas EM, Trowsdale J, Christiansen FT (2004) Comparative genomic analysis, diversity and evolution of two KIR haplotypes a and B. Gene 335:121–131. https://doi.org/10.1016/j.gene.2004.03.018
Mehta RS, Randolph B, Daher M, Rezvani K (2018) NK cell therapy for hematologic malignancies. Int J Hematol 107:262–270. https://doi.org/10.1007/s12185-018-2407-5
Neuchel C, Furst D, Niederwieser D et al (2017) Impact of donor activating KIR genes on HSCT outcome in C1-ligand negative myeloid disease patients transplanted with unrelated donors-a retrospective study. PLoS One 12:e0169512. https://doi.org/10.1371/journal.pone.0169512
Ruggeri L, Capanni M, Urbani E et al (2002) Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science 295:2097–2100. https://doi.org/10.1126/science.1068440
Stringaris K, Adams S, Uribe M, Eniafe R, Wu CO, Savani BN, Barrett AJ (2010) Donor KIR genes 2DL5A, 2DS1 and 3DS1 are associated with a reduced rate of leukemia relapse after HLA-identical sibling stem cell transplantation for acute myeloid leukemia but not other hematologic malignancies. Biol Blood Marrow Transplant 16:1257–1264. https://doi.org/10.1016/j.bbmt.2010.03.004
Symons HJ, Leffell MS, Rossiter ND, Zahurak M, Jones RJ, Fuchs EJ (2010) Improved survival with inhibitory killer immunoglobulin receptor (KIR) gene mismatches and KIR haplotype B donors after nonmyeloablative, HLA-haploidentical bone marrow transplantation. Biol Blood Marrow Transplant 16:533–542. https://doi.org/10.1016/j.bbmt.2009.11.022
Terszowski G, Passweg JR, Stern M (2012) Natural killer cell immunity after transplantation. Swiss Med Wkly 142:w13700. https://doi.org/10.4414/smw.2012.13700
Wang HD, Zhang FX, Shen CM, Wu YM, Lv YG, Xie ST, Yang G, Qin HX, Fan SL, Zhu BF (2012) The distribution of genetic diversity of KIR genes in the Chinese Mongolian population. Hum Immunol 73:1031–1038. https://doi.org/10.1016/j.humimm.2012.07.317
Yao Y, Shi L, Yu J, Liu S, Tao Y, Shi L (2019) Distribution of killer-cell immunoglobulin-like receptor genes and combinations of their human leucocyte antigen ligands in 11 ethnic populations in China. Cells 8. https://doi.org/10.3390/cells8070711
Yawata M, Yawata N, Draghi M, Partheniou F, Little AM, Parham P (2008) MHC class I-specific inhibitory receptors and their ligands structure diverse human NK-cell repertoires toward a balance of missing self-response. Blood 112:2369–2380. https://doi.org/10.1182/blood-2008-03-143727
Zhang HH, He J, Bao XJ et al (2017) Distribution of donor-specific aKIR after unrelated allogeneic hematopoietic stem cell transplantation. Chin J Hematol 38:421–426. https://doi.org/10.3760/cma.j.issn.0253-2727.2017.05.013
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 81671549), the Jiangsu Province Medical Innovation Team (CXTDB2017009), the Jiangsu Provincial Key Research and Development Program (BE2019656), and the Collaborative Innovation Center of Hematology (SX21100117).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This protocol was approved by the local ethics committee. In accordance with the ethical guidelines of the Declaration of Helsinki; written informed consent was obtained from all the subjects.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, Y., Wang, T., Hu, X. et al. Study of KIR gene expression at the mRNA level in specific donor-derived NK cells after allogeneic HSCT. Immunogenetics 72, 135–141 (2020). https://doi.org/10.1007/s00251-019-01153-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00251-019-01153-6