Abstract
Objective:
To quantify sepsis-induced alterations in changes in muscle tissue oxygenation (StO2) after an ischemic challenge using near-infrared spectroscopy (NIRS), and to test the hypothesis that these alterations are related to outcome.
Design
Prospective study.
Setting
Thirty-one-bed, university hospital Department of Intensive Care.
Patients
Seventy-two patients with severe sepsis or septic shock, 18 hemodynamically stable, acutely ill patients without infection, and 18 healthy volunteers.
Interventions
Three-minute occlusion of the brachial artery using a cuff inflated 50 mmHg above systolic arterial pressure.
Measurements and main results
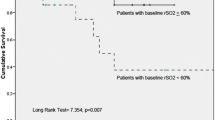
Thenar eminence StO2 was measured continuously by NIRS before (StO2baseline), during, and after the 3-min occlusion. Changes in StO2 were assessed by the slope of increase in StO2 during the first 14 s following the ischemic period and by the difference between the maximum StO2 and StO2baseline (Δ). The slope was lower in septic patients than in controls and volunteers [2.3 (1.3–3.6), 4.8 (3.5–6.0), and 4.7 (3.2–6.3) %/s, p < 0.001]. Δ was also significantly lower in septic patients than in the other groups. Slopes were lower in septic patients with than without shock [2.0 (1.2–2.9) vs 3.2 (1.8–4.5) %/s, p < 0.05]. In 52 septic patients, in whom the slope was obtained every 24 h for 48 h, slopes were higher in survivors than in non-survivors and tended to increase in survivors but not in non-survivors.
Conclusions
Altered recovery in StO2 after an ischemic challenge is frequent in septic patients and more pronounced in the presence of shock. The presence and persistence of these alterations in the first 24 h of sepsis are associated with worse outcome.




Similar content being viewed by others
References
Lam C, Tyml K, Martin C, Sibbald W (1994) Microvascular perfusion is impaired in a rat model of normotensive sepsis. J Clin Invest 94:2077–2083
De Backer D, Creteur J, Preiser JC, Dubois MJ, Vincent JL (2002) Microvascular blood flow is altered in patients with sepsis. Am J Respir Crit Care Med 166:98–104
Ellis CG, Bateman RM, Sharpe MD, Sibbald WJ, Gill R (2002) Effect of a maldistribution of microvascular blood flow on capillary O(2) extraction in sepsis. Am J Physiol Heart Circ Physiol 282:H156–H164
Astiz ME, DeGent GE, Lin RY, Rackow EC (1995) Microvascular function and rheologic changes in hyperdynamic sepsis. Crit Care Med 23:265–271
Croner RS, Hoerer E, Kulu Y, Hackert T, Gebhard MM, Herfarth C, Klar E (2006) Hepatic platelet and leukocyte adherence during endotoxemia. Crit Care 10:R15
Piagnerelli M, Boudjeltia KZ, Vanhaeverbeek M, Vincent JL (2003) Red blood cell rheology in sepsis. Intensive Care Med 29:1052–1061
Bateman RM, Jagger JE, Sharpe MD, Ellsworth ML, Mehta S, Ellis CG (2001) Erythrocyte deformability is a nitric oxide-mediated factor in decreased capillary density during sepsis. Am J Physiol Heart Circ Physiol 280:H2848–H2856
Piper RD, Pitt-Hyde M, Li F, Sibbald WJ, Potter RF (1996) Microcirculatory changes in rat skeletal muscle in sepsis. Am J Respir Crit Care Med 154:931–937
Tyml K, Yu J, McCormack DG (1998) Capillary and arteriolar responses to local vasodilators are impaired in a rat model of sepsis. J Appl Physiol 84:837–844
Gocan NC, Scott JA, Tyml K (2000) Nitric oxide produced via neuronal NOS may impair vasodilatation in septic rat skeletal muscle. Am J Physiol Heart Circ Physiol 278:H1480–H1489
Hollenberg SM, Broussard M, Osman J, Parrillo JE (2000) Increased microvascular reactivity and improved mortality in septic mice lacking inducible nitric oxide synthase. Circ Res 86:774–778
De Blasi RA, Palmisani S, Alampi D, Mercieri M, Romano R, Collini S, Pinto G (2005) Microvascular dysfunction and skeletal muscle oxygenation assessed by phase-modulation near-infrared spectroscopy in patients with septic shock. Intensive Care Med 31:1661–1668
Mancini DM, Bolinger L, Li H, Kendrick K, Chance B, Wilson JR (1994) Validation of near-infrared spectroscopy in humans. J Appl Physiol 77:2740–2747
Vallet B (1998) Vascular reactivity and tissue oxygenation. Intensive Care Med 24:3–11
Young JD, Cameron EM (1995) Dynamics of skin blood flow in human sepsis. Intensive Care Med 21:669–674
Neviere R, Mathieu D, Chagnon JL, Lebleu N, Millien JP, Wattel F (1996) Skeletal muscle microvascular blood flow and oxygen transport in patients with severe sepsis. Am J Respir Crit Care Med 153:191–195
Knotzer H, Pajk W, Dunser MW, Maier S, Mayr AJ, Ritsch N, Friesenecker B, Hasibeder WR (2006) Regional microvascular function and vascular reactivity in patients with different degrees of multiple organ dysfunction syndrome. Anesth Analg 102:1187–1193
Haisjackl M, Hasibeder W, Klaunzer S, Altenberger H, Koller W (1990) Diminished reactive hyperemia in the skin of critically ill patients. Crit Care Med 18:813–818
Carollo T, Creteur J, De Backer D, Vincent JL (2004) Persistent altered microvascular reactivity is predictive of mortality in septic patients. Intensive Care Med 30 (Suppl 1):S163 (abstract)
Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G (2003) 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Intensive Care Med 29:530–538
Cui W, Kumar C, Chance B (1991) Experimental study of migration depth for the photons measured at sample surface. Proc SPIE 1431:180–191
Knaus WA, Draper EA, Wagner DP, Zimmerman JE (1985) APACHE II: a severity of disease classification system. Crit Care Med 13:818–829
Vincent JL, Moreno R, Takala J, Willatts S, Mendonça A de, Bruining H, Reinhart CK, Suter PM, Thijs LG (1996) The SOFA (Sepsis-Related Organ Failure Assessment) score to describe organ dysfunction/failure. Intensive Care Med 22:707–710
Wariar R, Gaffke JN, Haller RG, Bertocci LA (2000) A modular NIRS system for clinical measurement of impaired skeletal muscle oxygenation. J Appl Physiol 88:315–325
Pareznik R, Knezevic R, Voga G, Podbregar M (2006) Changes in muscle tissue oxygenation during stagnant ischemia in septic patients. Intensive Care Med 32:87–92
Boushel R, Langberg H, Olesen J, Gonzales-Alonzo J, Bulow J, Kjaer M (2001) Monitoring tissue oxygen availability with near infrared spectroscopy (NIRS) in health and disease. Scand J Med Sci Sports 11:213–222
Pohl U, Busse R (1989) Hypoxia stimulates release of endothelium-derived relaxant factor. Am J Physiol 256:H1595–H1600
Curtis SE, Vallet B, Winn MJ, Caufield JB, King CE, Chapler CK, Cain SM (1995) Role of the vascular endothelium in O2 extraction during progressive ischemia in canine skeletal muscle. J Appl Physiol 79:1351–1360
Greenberg B, Kishiyama S (1993) Endothelium-dependent and -independent responses to severe hypoxia in rat pulmonary artery. Am J Physiol 265:H1712–H1720
Vallet B, Curtis SE, Winn MJ, King CE, Chapler CK, Cain SM (1994) Hypoxic vasodilation does not require nitric oxide (EDRF/NO) synthesis. J Appl Physiol 76:1256–1261
Michiels C, Arnould T, Knott I, Dieu M, Remacle J (1993) Stimulation of prostaglandin synthesis by human endothelial cells exposed to hypoxia. Am J Physiol 264:C866–C874
Vallet B, Curtis SE, Guery B, Mangalaboyi J, Menager P, Cain SM, Chopin C, Dupuis BA (1995) ATP-sensitive K+ channel blockade impairs O2 extraction during progressive ischemia in pig hindlimb. J Appl Physiol 79:2035–2042
Davies NW (1990) Modulation of ATP-sensitive K+ channels in skeletal muscle by intracellular protons. Nature 343:375–377
Keung EC, Li Q (1991) Lactate activates ATP-sensitive potassium channels in guinea pig ventricular myocytes. J Clin Invest 88:1772–1777
Landry DW, Oliver JA (2001) The pathogenesis of vasodilatory shock. N Engl J Med 345:588–595
Vallet B (2002) Endothelial cell dysfunction and abnormal tissue perfusion. Crit Care Med 30:S229–S234
Gustafsson F, Holstein-Rathlou N (1999) Conducted vasomotor responses in arterioles: characteristics, mechanisms and physiological significance. Acta Physiol Scand 167:11–21
Tyml K, Wang X, Lidington D, Ouellette Y (2001) Lipopolysaccharide reduces intercellular coupling in vitro and arteriolar conducted response in vivo. Am J Physiol Heart Circ Physiol 281:H1397–H1406
Vallet B, Wiel E (2001) Endothelial cell dysfunction and coagulation. Crit Care Med 29:S36–S41
Kubli S, Boegli Y, Ave AD, Liaudet L, Revelly JP, Golay S, Broccard A, Waeber B, Schaller MD, Feihl F (2003) Endothelium-dependent vasodilation in the skin microcirculation of patients with septic shock. Shock 19:274–280
Bateman RM, Sharpe MD, Ellis CG (2003) Bench-to-bedside review: microvascular dysfunction in sepsis: hemodynamics, oxygen transport, and nitric oxide. Crit Care 7:359–373
Sakr Y, Dubois MJ, De Backer D, Creteur J, Vincent JL (2004) Persistent microcirculatory alterations are associated with organ failure and death in patients with septic shock. Crit Care Med 32:1825–1831
van Beekvelt MC, Borghuis MS, van Engelen BG, Wevers RA, Colier WN (2001) Adipose tissue thickness affects in vivo quantitative near-IR spectroscopy in human skeletal muscle. Clin Sci (Lond) 101:21–28
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Creteur, J., Carollo, T., Soldati, G. et al. The prognostic value of muscle StO2 in septic patients. Intensive Care Med 33, 1549–1556 (2007). https://doi.org/10.1007/s00134-007-0739-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-007-0739-3