Abstract
Aims/hypothesis
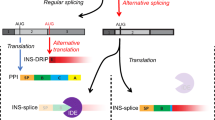
Thymic expression of self-antigens during T-lymphocyte development is believed to be crucial for preventing autoimmunity. It has been suggested that G6PC2, the gene encoding islet-specific glucose-6-phosphatase catalytic subunit-related protein (IGRP), is differentially spliced between pancreatic beta cells and the thymus. This may contribute to incomplete elimination of IGRP-specific T lymphocytes in the thymus, predisposing individuals to type 1 diabetes. We tested whether specific splice variation in islets vs thymus correlates with loss of tolerance to IGRP in type 1 diabetes.
Methods
Expression of G6PC2 splice variants was compared among thymus, purified medullary thymic epithelial cells and pancreatic islets by RT-PCR. Differential immunogenicity of IGRP splice variants was tested in patients and healthy individuals for autoantibodies and specific cytotoxic T lymphocytes using radiobinding assays and HLA class I multimers, respectively.
Results
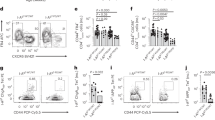
Previously reported G6PC2 splice variants, including full-length G6PC2, were confirmed, albeit that they occurred in both pancreas and thymus, rather than islets alone. Yet, their expression levels were profoundly greater in islets than in thymus. Moreover, three novel G6PC2 variants were discovered that occur in islets only, leading to protein truncations, frame shifts and neo-sequences prone to immunogenicity. However, autoantibodies to novel or known IGRP splice variants did not differ between patients and healthy individuals, and similar frequencies of IGRP-specific cytotoxic T lymphocytes could be detected in both patients with type 1 diabetes and healthy individuals.
Conclusions/interpretation
We propose that post-transcriptional variation of tissue-specific self-proteins may affect negative thymic selection, although this need not necessarily lead to disease.




Similar content being viewed by others
Abbreviations
- CTL:
-
Cytotoxic T lymphocyte
- IGRP:
-
Islet-specific glucose-6-phosphatase catalytic subunit-related protein
- mTEC:
-
Medullary thymic epithelial cells
- PBMC:
-
Peripheral blood mononuclear cell
References
Palmer E (2003) Negative selection: clearing out the bad apples from the T cell repertoire. Nat Rev Immunol 3:383–391
Yang J, Danke NA, Berger D et al (2006) Islet-specific glucose-6-phosphatase catalytic subunit-related protein-reactive CD4+ T cells in human subjects. J Immunol 176:2781–2789
Velthuis JH, Unger WW, Abreu JR et al (2010) Simultaneous detection of circulating autoreactive CD8+ T cells specific for different islet cell-associated epitopes using combinatorial MHC multimers. Diabetes 59:1721–1730
Unger WW, Velthuis J, Abreu JR et al (2011) Discovery of low-affinity preproinsulin epitopes and detection of autoreactive CD8 T cells using combinatorial MHC multimers. J Autoimmun 37:151–159
Anderson MS, Venanzi ES, Klein L et al (2002) Projection of an immunological self shadow within the thymus by the aire protein. Science 298:1395–1401
Derbinski J, Schulte A, Kyewski B, Klein L (2001) Promiscuous gene expression in medullary thymic epithelial cells mirrors the peripheral self. Nat Immunol 2:1032–1039
Abreu JR, Martina S, Verrijn Stuart AA et al (2012) CD8 T cell autoreactivity to preproinsulin epitopes with very low human leucocyte antigen class I binding affinity. Clin Exp Immunol 170:57–65
Zehn D, Bevan MJ (2006) T cells with low avidity for a tissue-restricted antigen routinely evade central and peripheral tolerance and cause autoimmunity. Immunity 25:261–270
Klein L, Klugmann M, Nave KA, Tuohy VK, Kyewski B (2000) Shaping of the autoreactive T cell repertoire by a splice variant of self protein expressed in thymic epithelial cells. Nat Med 6:56–61
Mukherjee R, Wagar D, Stephens TA, Lee-Chan E, Singh B (2005) Identification of CD4+ T cell-specific epitopes of islet-specific glucose-6-phosphatase catalytic subunit-related protein: a novel beta cell autoantigen in type 1 diabetes. J Immunol 174:5306–5315
Wong CP, Li L, Frelinger JA, Tisch R (2006) Early autoimmune destruction of islet grafts is associated with a restricted repertoire of IGRP-specific CD8(+) T cells in diabetic nonobese diabetic mice. J Immunol 176:1637–1644
Lieberman SM, Evans AM, Han B et al (2003) Identification of the beta cell antigen targeted by a prevalent population of pathogenic CD8+ T cells in autoimmune diabetes. Proc Natl Acad Sci U S A 100:8384–8388
Shieh JJ, Pan CJ, Mansfield BC, Chou JY (2004) The islet-specific glucose-6-phosphatase-related protein, implicated in diabetes, is a glycoprotein embedded in the endoplasmic reticulum membrane. Febs Letters 562:160–164
Petrolonis AJ, Yang Q, Tummino PJ et al (2004) Enzymatic characterization of the pancreatic islet-specific glucose-6-phosphatase-related protein (IGRP). J Biol Chem 279:13976–13983
Standifer NE, Ouyang Q, Panagiotopoulos C et al (2006) Identification of novel HLA-A*0201-restricted epitopes in recent-onset type 1 diabetic subjects and antibody-positive relatives. Diabetes 55:3061–3067
Dogra RS, Vaidyanathan P, Prabakar KR, Marshall KE, Hutton JC, Pugliese A (2006) Alternative splicing of G6PC2, the gene coding for the islet-specific glucose-6-phosphatase catalytic subunit-related protein (IGRP), results in differential expression in human thymus and spleen compared with pancreas. Diabetologia 49:953–957
Weerkamp F, de Haas EF, Naber BA et al (2005) Age-related changes in the cellular composition of the thymus in children. J Allergy Clin Immunol 115:834–840
Gorus FK, Goubert P, Semakula C et al (1997) IA-2-autoantibodies complement GAD65-autoantibodies in new-onset IDDM patients and help predict impending diabetes in their siblings. The Belgian Diabetes Registry. Diabetologia 40:95–99
Tan TL, Geluk A, Toebes M, Ottenhoff TH, Drijfhout JW (1997) A novel, highly efficient peptide-HLA class I binding assay using unfolded heavy chain molecules: identification of HIV-1 derived peptides that bind to HLA-A*0201 and HLA-A*0301. J Immunol Methods 205:201–209
Hadrup SR, Bakker AH, Shu CJ et al (2009) Parallel detection of antigen-specific T cell responses by multidimensional encoding of MHC multimers. Nat Methods 6:520–526
Pobezinsky LA, Angelov GS, Tai X et al (2012) Clonal deletion and the fate of autoreactive thymocytes that survive negative selection. Nat Immunol 13:569–578
Bulek AM, Cole DK, Skowera A et al (2012) Structural basis for the killing of human beta cells by CD8(+) T cells in type 1 diabetes. Nat Immunol 13:283–289
James EA, Kwok WW (2008) Low-affinity major histocompatibility complex-binding peptides in type 1 diabetes. Diabetes 57:1788–1789
Mueller DL (2010) Mechanisms maintaining peripheral tolerance. Nat Immunol 11:21–27
Gardner JM, Devoss JJ, Friedman RS et al (2008) Deletional tolerance mediated by extrathymic Aire-expressing cells. Science 321:843–847
Wang J, Tsai S, Han B, Tailor P, Santamaria P (2012) Autoantigen recognition is required for recruitment of IGRP(206-214)-autoreactive CD8+ T cells but is dispensable for tolerance. J Immunol 189:2975–2984
Mallone R, Martinuzzi E, Blancou P et al (2007) CD8+ T cell responses identify beta-cell autoimmunity in human type 1 diabetes. Diabetes 56:613–621
Krishnamurthy B, Dudek NL, McKenzie MD et al (2006) Responses against islet antigens in NOD mice are prevented by tolerance to proinsulin but not IGRP. J Clin Invest 116:3258–3265
Krishnamurthy B, Mariana L, Gellert SA et al (2008) Autoimmunity to both proinsulin and IGRP is required for diabetes in nonobese diabetic 8.3 TCR transgenic mice. J Immunol 180:4458–4464
Acknowledgements
We are grateful to P. Goubert and E. Quartier (Brussels Free University and University Hospital, Brussels, Belgium) for technical assistance in performing the radiobinding assays, and to the Belgian Diabetes Registry for providing sera from well-characterised patients with recent-onset type 1 diabetes and controls (list of active BDR members on www.bdronline.be).
Funding
This work was funded by grants from the Leiden University Medical Center, the Dutch Diabetes Research Foundation, The Juvenile Diabetes Research Foundation, the 7th Framework Program of the European Commission (FP-7 project no. 241833), the Belgian Fund for Scientific Research (FWO-Vlaanderen projects G.0311.07 and G.0868.11), the research council (OZR) of the Brussels Free University and the Willy Gepts Funds (University Hospital Brussels).
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Contribution statement
VMdJ and JRFA contributed to the conception and design, acquisition and interpretation of data and writing and revision of the manuscript. ARvdS, KV and FKG contributed to experimental design, acquisition and interpretation of data and revision of the manuscript. AAVS, MAE, BB and FJTS were responsible for data acquisition and revision of the manuscript. BOR was responsible for the conception of the study, analysis and interpretation of the data, and writing and revision of the manuscript. All authors approved the final version of the article.
Author information
Authors and Affiliations
Corresponding author
Additional information
V. M. de Jong and Joana R. F. Abreu contributed equally to this study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM Fig. 1
(PDF 260 kb)
ESM Fig. 2
(PDF 119 kb)
ESM Fig. 3
(PDF 351 kb)
ESM Table 1
(PDF 13 kb)
ESM Table 2
(PDF 26 kb)
Rights and permissions
About this article
Cite this article
de Jong, V.M., Abreu, J.R.F., Verrijn Stuart, A.A. et al. Alternative splicing and differential expression of the islet autoantigen IGRP between pancreas and thymus contributes to immunogenicity of pancreatic islets but not diabetogenicity in humans. Diabetologia 56, 2651–2658 (2013). https://doi.org/10.1007/s00125-013-3034-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-013-3034-6