Abstract
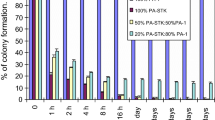
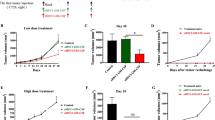
Suicide gene therapy is a promising concept in oncology. We have recently introduced a novel suicide gene, TK.007, which was shown to excel established herpes simplex virus thymidine kinase (HSVtk) variants when used for donor-lymphocyte modification in adoptive immunotherapy models. Here, the potential of TK.007 in killing cancer cells was studied. Initially, we transduced tumour cell lines derived from different neoplasias (glioblastoma, melanoma, lung cancer, colon cancer) with lentiviral LeGO vectors encoding TK.007 or the splice-corrected (sc)HSVtk together with an eGFP/Neo-marker. Based on direct in vitro comparison, we found that TK.007 facilitates more efficient tumour cell killing at significantly lower ganciclovir doses in all tumour cell lines tested. Also, using different readout systems, we found a significantly stronger bystander effect of TK.007 as compared to scHSVtk. Importantly, in vitro data were confirmed in vivo using a subcutaneous G62 glioblastoma model in NOD/SCID mice. In mice transplanted with scHSVtk-positive tumours, treatment with low (10 mg/kg) or standard (50 mg/kg) ganciclovir doses resulted only in short-term growth inhibition or transient tumour remission, respectively. In striking contrast, in the TK.007 group, all animals achieved continuous complete remission after both standard and low-dose ganciclovir. Finally, a substantial bystander effect for TK.007 was also confirmed with the G62 model in vivo, where significantly prolonged survival for mice bearing tumours containing only 10% or 50% TK.007-expressing cells was observed. In summary, our data indicate strongly improved anti-tumour activity of TK.007 as compared to conventional HSVtk. We therefore suppose that TK.007 is an excellent candidate for cancer suicide gene therapy.






Similar content being viewed by others
References
Portsmouth D, Hlavaty J, Renner M (2007) Suicide genes for cancer therapy. Mol Aspects Med 28:4–41
Ostermann N, Lavie A, Padiyar S, Brundiers R, Veit T, Reinstein J, Goody RS, Konrad M, Schlichting I (2000) Potentiating AZT activation: structures of wild-type and mutant human thymidylate kinase suggest reasons for the mutants' improved kinetics with the HIV prodrug metabolite AZTMP. J Mol Biol 304:43–53
Thomis DC, Marktel S, Bonini C, Traversari C, Gilman M, Bordignon C, Clackson T (2001) A Fas-based suicide switch in human T cells for the treatment of graft-versus-host disease. Blood 97:1249–1257
Straathof KC, Pule MA, Yotnda P, Dotti G, Vanin EF, Brenner MK, Heslop HE, Spencer DM, Rooney CM (2005) An inducible caspase 9 safety switch for T-cell therapy. Blood 105:4247–4254
Introna M, Barbui AM, Bambacioni F, Casati C, Gaipa G, Borleri G, Bernasconi S, Barbui T, Golay J, Biondi A, Rambaldi A (2000) Genetic modification of human T cells with CD20: a strategy to purify and lyse transduced cells with anti-CD20 antibodies. Hum Gene Ther 11:611–620
Bonini C, Bondanza A, Perna SK, Kaneko S, Traversari C, Ciceri F, Bordignon C (2007) The suicide gene therapy challenge: how to improve a successful gene therapy approach. Mol Ther 15:1248–1252. doi:10.1038/sj.mt.6300190
Georgoudaki AM, Sutlu T, Alici E (2010) Suicide gene therapy for graft-versus-host disease. Immunotherapy 2:521–537
Lupo-Stanghellini MT, Provasi E, Bondanza A, Ciceri F, Bordignon C, Bonini C (2010) Clinical impact of suicide gene therapy in allogeneic hematopoietic stem cell transplantation. Hum Gene Ther 21:241–250
Fillat C, Carrio M, Cascante A, Sangro B (2003) Suicide gene therapy mediated by the herpes simplex virus thymidine kinase gene/ganciclovir system: fifteen years of application. Curr Gene Ther 3:13–26
Huszthy PC, Giroglou T, Tsinkalovsky O, Euskirchen P, Skaftnesmo KO, Bjerkvig R, von Laer D, Miletic H (2009) Remission of invasive, cancer stem-like glioblastoma xenografts using lentiviral vector-mediated suicide gene therapy. PLoS One 4:e6314
Moolten FL (1986) Tumor chemosensitivity conferred by inserted herpes thymidine kinase genes: paradigm for a prospective cancer control strategy. Cancer Res 46:5276–5281
Dilber MS, Abedi MR, Christensson B, Bjorkstrand B, Kidder GM, Naus CC, Gahrton G, Smith CI (1997) Gap junctions promote the bystander effect of herpes simplex virus thymidine kinase in vivo. Cancer Res 57:1523–1528
Preuss E, Treschow A, Newrzela S, Brucher D, Weber K, Felldin U, Alici E, Gahrton G, von Laer D, Dilber MS, Fehse B (2010) TK.007: a novel, codon-optimized HSVtk(A168H) mutant for suicide gene therapy. Hum Gene Ther 21:929–941
Kuriyama S, Mitoro A, Yamazaki M, Tsujinoue H, Nakatani T, Akahane T, Toyokawa Y, Kojima H, Okamoto S, Fukui H (1999) Comparison of gene therapy with the herpes simplex virus thymidine kinase gene and the bacterial cytosine deaminase gene for the treatment of hepatocellular carcinoma. Scand J Gastroenterol 34:1033–1041
Balzarini J, Liekens S, Solaroli N, El Omari K, Stammers DK, Karlsson A (2006) Engineering of a single conserved amino acid residue of herpes simplex virus type 1 thymidine kinase allows a predominant shift from pyrimidine to purine nucleoside phosphorylation. J Biol Chem 281:19273–19279
Chalmers D, Ferrand C, Apperley JF, Melo JV, Ebeling S, Newton I, Duperrier A, Hagenbeek A, Garrett E, Tiberghien P, Garin M (2001) Elimination of the truncated message from the herpes simplex virus thymidine kinase suicide gene. Mol Ther 4:146–148
Weber K, Bartsch U, Stocking C, Fehse B (2008) A multicolor panel of novel lentiviral "gene ontology" (LeGO) vectors for functional gene analysis. Mol Ther 16:698–706
Kustikova OS, Wahlers A, Kuhlcke K, Stahle B, Zander AR, Baum C, Fehse B (2003) Dose finding with retroviral vectors: correlation of retroviral vector copy numbers in single cells with gene transfer efficiency in a cell population. Blood 102:3934–3937
Miletic H, Fischer YH, Neumann H, Hans V, Stenzel W, Giroglou T, Hermann M, Deckert M, Von Laer D (2004) Selective transduction of malignant glioma by lentiviral vectors pseudotyped with lymphocytic choriomeningitis virus glycoproteins. Hum Gene Ther 15:1091–1100
Yu P, Rowley DA, Fu YX, Schreiber H (2006) The role of stroma in immune recognition and destruction of well-established solid tumors. Curr Opin Immunol 18:226–231
Attia MA, Weiss DW (1966) Immunology of spontaneous mammary carcinomas in mice. V. Acquired tumor resistance and enhancement in strain A mice infected with mammary tumor virus. Cancer Res 26:1787–1800
Tomayko MM, Reynolds CP (1989) Determination of subcutaneous tumor size in athymic (nude) mice. Cancer Chemother Pharmacol 24:148–154
Weber K, Mock U, Petrowitz B, Bartsch U, Fehse B (2010) Lentiviral gene ontology (LeGO) vectors equipped with novel drug-selectable fluorescent proteins: new building blocks for cell marking and multi-gene analysis. Gene Ther 17:511–520
Fehse B, Kustikova OS, Bubenheim M, Baum C (2004) Pois(s)on—it's a question of dose. Gene Ther 11:879–881
Immonen A, Vapalahti M, Tyynela K, Hurskainen H, Sandmair A, Vanninen R, Langford G, Murray N, Yla-Herttuala S (2004) AdvHSV-tk gene therapy with intravenous ganciclovir improves survival in human malignant glioma: a randomised, controlled study. Mol Ther 10:967–972
Zischek C, Niess H, Ischenko I, Conrad C, Huss R, Jauch KW, Nelson PJ, Bruns C (2009) Targeting tumor stroma using engineered mesenchymal stem cells reduces the growth of pancreatic carcinoma. Ann Surg 250:747–753
Tiberghien P, Ferrand C, Lioure B, Milpied N, Angonin R, Deconinck E, Certoux JM, Robinet E, Saas P, Petracca B, Juttner C, Reynolds CW, Longo DL, Herve P, Cahn JY (2001) Administration of herpes simplex-thymidine kinase-expressing donor T cells with a T-cell-depleted allogeneic marrow graft. Blood 97:63–72
Garin MI, Garrett E, Tiberghien P, Apperley JF, Chalmers D, Melo JV, Ferrand C (2001) Molecular mechanism for ganciclovir resistance in human T lymphocytes transduced with retroviral vectors carrying the herpes simplex virus thymidine kinase gene. Blood 97:122–129
Salomon B, Maury S, Loubiere L, Caruso M, Onclercq R, Klatzmann D (1995) A truncated herpes simplex virus thymidine kinase phosphorylates thymidine and nucleoside analogs and does not cause sterility in transgenic mice. Mol Cell Biol 15:5322–5328
Biron KK (2006) Antiviral drugs for cytomegalovirus diseases. Antiviral Res 71:154–163
Rainov NG (2000) A phase III clinical evaluation of herpes simplex virus type 1 thymidine kinase and ganciclovir gene therapy as an adjuvant to surgical resection and radiation in adults with previously untreated glioblastoma multiforme. Hum Gene Ther 11:2389–2401
Beck C, Cayeux S, Lupton SD, Dorken B, Blankenstein T (1995) The thymidine kinase/ganciclovir-mediated "suicide" effect is variable in different tumor cells. Hum Gene Ther 6:1525–1530
Frank O, Rudolph C, Heberlein C, von Neuhoff N, Schrock E, Schambach A, Schlegelberger B, Fehse B, Ostertag W, Stocking C, Baum C (2004) Tumor cells escape suicide gene therapy by genetic and epigenetic instability. Blood 104:3543–3549
Uckert W, Kammertons T, Haack K, Qin Z, Gebert J, Schendel DJ, Blankenstein T (1998) Double suicide gene (cytosine deaminase and herpes simplex virus thymidine kinase) but not single gene transfer allows reliable elimination of tumor cells in vivo. Hum Gene Ther 9:855–865
Pulkkanen KJ, Yla-Herttuala S (2005) Gene therapy for malignant glioma: current clinical status. Mol Ther 12:585–598
Sandmair AM, Loimas S, Puranen P, Immonen A, Kossila M, Puranen M, Hurskainen H, Tyynela K, Turunen M, Vanninen R, Lehtolainen P, Paljarvi L, Johansson R, Vapalahti M, Yla-Herttuala S (2000) Thymidine kinase gene therapy for human malignant glioma, using replication-deficient retroviruses or adenoviruses. Hum Gene Ther 11:2197–2205
Muul LM, Candotti F (2007) Immune responses to gene-modified T cells. Curr Gene Ther 7:361–368
Kammertoens T, Schuler T, Blankenstein T (2005) Immunotherapy: target the stroma to hit the tumor. Trends Mol Med 11:225–231
Acknowledgements
The authors wish to thank Daniela Brücher for expert technical assistance and Dr. Sebastian Newrzela for helpful support. We are indebted to Dr. Boris Brill and Ute Burkhardt for assistance with animal experiments. dsRed-positive G62 cells were kindly provided by Yvonne Heidemarie Fischer (Georg-Speyer-Haus Frankfurt am Main, Germany). This work was partially supported by the DFG (FE568/11-1) and the Frankfurter Stiftung für krebskranke Kinder.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Preuß, E., Muik, A., Weber, K. et al. Cancer suicide gene therapy with TK.007: superior killing efficiency and bystander effect. J Mol Med 89, 1113–1124 (2011). https://doi.org/10.1007/s00109-011-0777-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-011-0777-8