Abstract
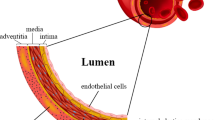
Tissue engineering holds great promise in regenerative medicine. However, the field of tissue engineering faces a myriad of difficulties. A major challenge is the necessity to integrate vascular networks into bioengineered constructs to enable physiological functions including adequate oxygenation, nutrient delivery, and removal of waste products. The last two decades have seen remarkable progress in our collective effort to bioengineer human-specific vascular networks. Studies have included both in vitro and in vivo investigations, and multiple methodologies have found varying degrees of success. What most approaches to bioengineer human vascular networks have in common, however, is the synergistic use of both (1) endothelial cells (ECs)—the cells used to line the lumen of the vascular structures and (2) perivascular cells—usually used to support EC function and provide perivascular stability to the networks. Here, we have highlighted trends in the use of various cellular sources over the last two decades of vascular network bioengineering research. To this end, we comprehensively reviewed all life science and biomedical publications available at the MEDLINE database up to 2018. Emphasis was put on selective studies that definitively used human ECs and were specifically related to bioengineering vascular networks. To facilitate this analysis, all papers were stratified by publication year and then analyzed according to their use of EC and perivascular cell types. This study provides an illustrating discussion on how each alternative source of cells has come to be used in the field. Our intention was to reveal trends and to provide new insights into the trajectory of vascular network bioengineering with regard to cellular sources.




Similar content being viewed by others
References
Atala A, Kasper FK, Mikos AG (2012) Engineering complex tissues. Sci Transl Med 4:160rv12. https://doi.org/10.1126/scitranslmed.3004890
Takeshita S, Zheng LP, Brogi E et al (1994) Therapeutic angiogenesis. A single intraarterial bolus of vascular endothelial growth factor augments revascularization in a rabbit ischemic hind limb model. J Clin Investig 93:662–670. https://doi.org/10.1172/JCI117018
Simons M, Ware JA (2003) Therapeutic angiogenesis in cardiovascular disease. Nat Rev Drug Discov 2:863–871. https://doi.org/10.1038/nrd1226
Phelps EA, García AJ (2009) Update on therapeutic vascularization strategies. Regen Med 4:65–80. https://doi.org/10.2217/17460751.4.1.65
Said SS, Pickering JG, Mequanint K (2013) Advances in growth factor delivery for therapeutic angiogenesis. J Vasc Res 50:35–51. https://doi.org/10.1159/000345108
Marino D, Luginbühl J, Scola S et al (2014) Bioengineering dermo-epidermal skin grafts with blood and lymphatic capillaries. Sci Transl Med 6:221ra14. https://doi.org/10.1126/scitranslmed.3006894
Maisel K, Sasso MS, Potin L, Swartz MA (2017) Exploiting lymphatic vessels for immunomodulation: rationale, opportunities, and challenges. Adv Drug Deliv Rev 114:43–59. https://doi.org/10.1016/j.addr.2017.07.005
Allen P, Melero Martin J, Bischoff J (2011) Type I collagen, fibrin and PuraMatrix matrices provide permissive environments for human endothelial and mesenchymal progenitor cells to form neovascular networks. J Tissue Eng Regen Med 5:e74–e86. https://doi.org/10.1002/term.389
Bae H, Puranik AS, Gauvin R et al (2012) Building vascular networks. Sci Transl Med 4:160ps23. https://doi.org/10.1126/scitranslmed.3003688
Chen Y-C, Chen Y-C, Lin R-Z et al (2012) Functional human vascular network generated in photocrosslinkable gelatin methacrylate hydrogels. Adv Funct Mater 22:2027–2039. https://doi.org/10.1002/adfm.201101662
Serbo JV, Gerecht S (2013) Vascular tissue engineering: biodegradable scaffold platforms to promote angiogenesis. Stem Cell Res Ther 4:8. https://doi.org/10.1186/scrt156
Chang WG, Niklason LE (2017) A short discourse on vascular tissue engineering. NPJ Regen Med. https://doi.org/10.1038/s41536-017-0011-6
Song H-HG, Rumma RT, Ozaki CK et al (2018) Vascular tissue engineering: progress, challenges, and clinical promise. Cell Stem Cell 22:340–354. https://doi.org/10.1016/j.stem.2018.02.009
Gimbrone MA, Cotran RS, Folkman MJ (1974) Human vascular endothelial cells in culture. Growth and DNA synthesis. J Cell Biol 60:673–684
Black AF, Berthod F, L’heureux N et al (1998) In vitro reconstruction of a human capillary-like network in a tissue-engineered skin equivalent. FASEB J 12:1331–1340
Schechner JS, Nath AK, Zheng L et al (2000) In vivo formation of complex microvessels lined by human endothelial cells in an immunodeficient mouse. Proc Natl Acad Sci USA 97:9191–9196. https://doi.org/10.1073/pnas.150242297
Koike N, Fukumura D, Gralla O et al (2004) Tissue engineering: creation of long-lasting blood vessels. Nature 428:138–139. https://doi.org/10.1038/428138a
Levenberg S, Rouwkema J, Macdonald M et al (2005) Engineering vascularized skeletal muscle tissue. Nat Biotechnol 23:879–884. https://doi.org/10.1038/nbt1109
Tsigkou O, Pomerantseva I, Spencer JA et al (2010) Engineered vascularized bone grafts. Proc Natl Acad Sci USA 107:3311–3316. https://doi.org/10.1073/pnas.0905445107
Caspi O, Lesman A, Basevitch Y et al (2007) Tissue engineering of vascularized cardiac muscle from human embryonic stem cells. Circ Res 100:263–272. https://doi.org/10.1161/01.RES.0000257776.05673.ff
Davison PM, Bensch K, Karasek MA (1980) Isolation and growth of endothelial cells from the microvessels of the newborn human foreskin in cell culture. J Investig Dermatol 75:316–321
Kern PA, Knedler A, Eckel RH (1983) Isolation and culture of microvascular endothelium from human adipose tissue. J Clin Investig 71:1822–1829
Nör JE, Peters MC, Christensen JB et al (2001) Engineering and characterization of functional human microvessels in immunodeficient mice. Lab Investig 81:453–463
Peters MC, Polverini PJ, Mooney DJ (2002) Engineering vascular networks in porous polymer matrices. J Biomed Mater Res 60:668–678
Szöke K, Beckstrøm KJ, Brinchmann JE (2012) Human adipose tissue as a source of cells with angiogenic potential. Cell Transplant 21:235–250. https://doi.org/10.3727/096368911X580518
Lin R-Z, Moreno-Luna R, Moreno-Luna R et al (2013) Human white adipose tissue vasculature contains endothelial colony-forming cells with robust in vivo vasculogenic potential. Angiogenesis 16:735–744. https://doi.org/10.1007/s10456-013-9350-0
Cerqueira MT, Pirraco RP, Martins AR et al (2014) Cell sheet technology-driven re-epithelialization and neovascularization of skin wounds. Acta Biomater 10:3145–3155. https://doi.org/10.1016/j.actbio.2014.03.006
Klar AS, Guven S, Zimoch J et al (2016) Characterization of vasculogenic potential of human adipose-derived endothelial cells in a three-dimensional vascularized skin substitute. Pediatr Surg Int 32:17–27. https://doi.org/10.1007/s00383-015-3808-7
Freiman A, Shandalov Y, Rozenfeld D et al (2016) Adipose-derived endothelial and mesenchymal stem cells enhance vascular network formation on three-dimensional constructs in vitro. Stem Cell Res Ther 7:5. https://doi.org/10.1186/s13287-015-0251-6
Yoshida T, Komaki M, Hattori H et al (2010) Therapeutic angiogenesis by implantation of a capillary structure constituted of human adipose tissue microvascular endothelial cells. Arterioscler Thromb Vasc Biol 30:1300–1306. https://doi.org/10.1161/atvbaha.109.198994
Sasagawa T, Shimizu T, Yamato M, Okano T (2014) Expression profiles of angiogenesis-related proteins in prevascular three-dimensional tissues using cell-sheet engineering. Biomaterials 35:206–213. https://doi.org/10.1016/j.biomaterials.2013.09.104
Bhattacharyya A, Lin S, Sandig M, Mequanint K (2014) Regulation of vascular smooth muscle cell phenotype in three-dimensional coculture system by Jagged1-selective Notch3 signaling. Tissue Eng Part A 20:1175–1187. https://doi.org/10.1089/ten.TEA.2013.0268
Herland A, van der Meer AD, FitzGerald EA et al (2016) Distinct contributions of astrocytes and pericytes to neuroinflammation identified in a 3D human blood–brain barrier on a chip. PLoS One 11:e0150360. https://doi.org/10.1371/journal.pone.0150360
Valarmathi MT, Fuseler JW, Davis JM, Price RL (2017) A novel human tissue-engineered 3-D functional vascularized cardiac muscle construct. Front Cell Dev Biol 5:2. https://doi.org/10.3389/fcell.2017.00002
Amano Y, Nishiguchi A, Matsusaki M et al (2016) Development of vascularized iPSC derived 3D-cardiomyocyte tissues by filtration layer-by-layer technique and their application for pharmaceutical assays. Acta Biomater 33:110–121. https://doi.org/10.1016/j.actbio.2016.01.033
Tan Q, Choi KM, Sicard D, Tschumperlin DJ (2017) Human airway organoid engineering as a step toward lung regeneration and disease modeling. Biomaterials 113:118–132. https://doi.org/10.1016/j.biomaterials.2016.10.046
Rafii S, Lyden D (2003) Therapeutic stem and progenitor cell transplantation for organ vascularization and regeneration. Nat Med 9:702–712. https://doi.org/10.1038/nm0603-702
Lin Y, Weisdorf DJ, Solovey A, Hebbel RP (2000) Origins of circulating endothelial cells and endothelial outgrowth from blood. J Clin Investig 105:71–77. https://doi.org/10.1172/JCI8071
Medina RJ, Barber CL, Sabatier F et al (2017) Endothelial progenitors: a consensus statement on nomenclature. Stem Cells Transl Med 229:10–1320. https://doi.org/10.1002/sctm.16-0360
Melero-Martin JM, Melero-Martin JM, Khan ZA et al (2007) In vivo vasculogenic potential of human blood-derived endothelial progenitor cells. Blood 109:4761–4768. https://doi.org/10.1182/blood-2006-12-062471
Yoder MC, Mead LE, Prater D et al (2007) Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood 109:1801–1809. https://doi.org/10.1182/blood-2006-08-043471
Sieminski AL, Hebbel RP, Gooch KJ (2005) Improved microvascular network in vitro by human blood outgrowth endothelial cells relative to vessel-derived endothelial cells. Tissue Eng 11:1332–1345. https://doi.org/10.1089/ten.2005.11.1332
Au P, Daheron LM, Duda DG et al (2008) Differential in vivo potential of endothelial progenitor cells from human umbilical cord blood and adult peripheral blood to form functional long-lasting vessels. Blood 111:1302–1305. https://doi.org/10.1182/blood-2007-06-094318
Wu X, Rabkin-Aikawa E, Guleserian KJ et al (2004) Tissue-engineered microvessels on three-dimensional biodegradable scaffolds using human endothelial progenitor cells. Am J Physiol Heart Circ Physiol 287:H480–H487. https://doi.org/10.1152/ajpheart.01232.2003
Fuchs S, Hofmann A, Kirkpatrick CJ (2007) Microvessel-like structures from outgrowth endothelial cells from human peripheral blood in 2-dimensional and 3-dimensional co-cultures with osteoblastic lineage cells. Tissue Eng 13:2577–2588. https://doi.org/10.1089/ten.2007.0022
Shepherd BR, Enis DR, Wang F et al (2006) Vascularization and engraftment of a human skin substitute using circulating progenitor cell-derived endothelial cells. FASEB J 20:1739–1741. https://doi.org/10.1096/fj.05-5682fje
Chen X, Aledia AS, Popson SA et al (2010) Rapid anastomosis of endothelial progenitor cell-derived vessels with host vasculature is promoted by a high density of cotransplanted fibroblasts. Tissue Eng Part A 16:585–594. https://doi.org/10.1089/ten.TEA.2009.0491
Melero-Martin JM, De Obaldia ME, Kang SY et al (2008) Engineering robust and functional vascular networks in vivo with human adult and cord blood-derived progenitor cells. Circ Res 103:194–202. https://doi.org/10.1161/CIRCRESAHA.108.178590
Traktuev DO, Prater DN, Merfeld-Clauss S et al (2009) Robust functional vascular network formation in vivo by cooperation of adipose progenitor and endothelial cells. Circ Res 104:1410–1420. https://doi.org/10.1161/CIRCRESAHA.108.190926
Fuchs S, Ghanaati S, Orth C et al (2009) Contribution of outgrowth endothelial cells from human peripheral blood on in vivo vascularization of bone tissue engineered constructs based on starch polycaprolactone scaffolds. Biomaterials 30:526–534. https://doi.org/10.1016/j.biomaterials.2008.09.058
Kang K-T, Allen P, Bischoff J (2011) Bioengineered human vascular networks transplanted into secondary mice reconnect with the host vasculature and re-establish perfusion. Blood 118:6718–6721. https://doi.org/10.1182/blood-2011-08-375188
Tasev D, Koolwijk P, Van Hinsbergh VWM (2016) Therapeutic potential of human-derived endothelial colony-forming cells in animal models. Tissue Eng Part B Rev 22:371–382. https://doi.org/10.1089/ten.TEB.2016.0050
Ingram DA, Mead LE, Tanaka H et al (2004) Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood 104:2752–2760. https://doi.org/10.1182/blood-2004-04-1396
Mund JA, Estes ML, Yoder MC et al (2012) Flow cytometric identification and functional characterization of immature and mature circulating endothelial cells. Arterioscler Thromb Vasc Biol 32:1045–1053. https://doi.org/10.1161/ATVBAHA.111.244210
Rignault-Clerc S, Bielmann C, Delodder F et al (2013) Functional late outgrowth endothelial progenitors isolated from peripheral blood of burned patients. Burns 39:694–704. https://doi.org/10.1016/j.burns.2012.09.027
Stroncek JD, Grant BS, Brown MA et al (2009) Comparison of endothelial cell phenotypic markers of late-outgrowth endothelial progenitor cells isolated from patients with coronary artery disease and healthy volunteers. Tissue Eng Part A 15:3473–3486. https://doi.org/10.1089/ten.TEA.2008.0673
Thill M, Strunnikova NV, Berna MJ et al (2008) Late outgrowth endothelial progenitor cells in patients with age-related macular degeneration. Investig Ophthalmol Vis Sci 49:2696–2708. https://doi.org/10.1167/iovs.07-0955
Thomson JA, Itskovitz-Eldor J, Shapiro SS et al (1998) Embryonic stem cell lines derived from human blastocysts. Science 282:1145–1147
Wilmut I, Beaujean N, de Sousa PA et al (2002) Somatic cell nuclear transfer: PubMed—NCBI. Nature 419:583–587
Levenberg S, Golub JS, Amit M et al (2002) Endothelial cells derived from human embryonic stem cells. Proc Natl Acad Sci USA 99:4391–4396. https://doi.org/10.1073/pnas.032074999
Wang ZZ, Au P, Chen T et al (2007) Endothelial cells derived from human embryonic stem cells form durable blood vessels in vivo. Nat Biotechnol 25:317–318. https://doi.org/10.1038/nbt1287
Nourse MB, Halpin DE, Scatena M et al (2010) VEGF induces differentiation of functional endothelium from human embryonic stem cells: implications for tissue engineering. Arterioscler Thromb Vasc Biol 30:80–89. https://doi.org/10.1161/ATVBAHA.109.194233
Kraehenbuehl TP, Ferreira LS, Hayward AM et al (2011) Human embryonic stem cell-derived microvascular grafts for cardiac tissue preservation after myocardial infarction. Biomaterials 32:1102–1109. https://doi.org/10.1016/j.biomaterials.2010.10.005
Takahashi K, Tanabe K, Ohnuki M et al (2007) Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131:861–872. https://doi.org/10.1016/j.cell.2007.11.019
Yu J, Vodyanik MA, Smuga-Otto K et al (2007) Induced pluripotent stem cell lines derived from human somatic cells. Science 318:1917–1920. https://doi.org/10.1126/science.1151526
Park I-H, Zhao R, West JA et al (2008) Reprogramming of human somatic cells to pluripotency with defined factors. Nature 451:141–146. https://doi.org/10.1038/nature06534
Wu SM, Hochedlinger K (2011) Harnessing the potential of induced pluripotent stem cells for regenerative medicine. Nat Cell Biol 13:497–505. https://doi.org/10.1038/ncb0511-497
Taura D, Sone M, Homma K et al (2009) Induction and isolation of vascular cells from human induced pluripotent stem cells—brief report. Arterioscler Thromb Vasc Biol 29:1100–1103. https://doi.org/10.1161/ATVBAHA.108.182162
Rufaihah AJ, Huang NF, Jamé S et al (2011) Endothelial cells derived from human iPSCS increase capillary density and improve perfusion in a mouse model of peripheral arterial disease. Arterioscler Thromb Vasc Biol 31:e72–e79. https://doi.org/10.1161/ATVBAHA.111.230938
Barrilleaux B, Knoepfler PS (2011) Inducing iPSCs to escape the dish. Cell Stem Cell 9:103–111. https://doi.org/10.1016/j.stem.2011.07.006
Samuel R, Daheron L, Liao S et al (2013) Generation of functionally competent and durable engineered blood vessels from human induced pluripotent stem cells. Proc Natl Acad Sci USA 110:12774–12779. https://doi.org/10.1073/pnas.1310675110
Kusuma S, Gerecht S (2016) Derivation of endothelial cells and pericytes from human pluripotent stem cells. Methods Mol Biol 1307:213–222. https://doi.org/10.1007/7651_2014_149
Cleaver O, Melton DA (2003) Endothelial signaling during development. Nat Med 9:661–668. https://doi.org/10.1038/nm0603-661
Cool J, DeFalco TJ, Capel B (2011) Vascular-mesenchymal cross-talk through Vegf and Pdgf drives organ patterning. Proc Natl Acad Sci USA 108:167–172. https://doi.org/10.1073/pnas.1010299108
Lammert E, Cleaver O, Melton D (2001) Induction of pancreatic differentiation by signals from blood vessels. Science 294:564–567. https://doi.org/10.1126/science.1064344
Matsumoto K, Yoshitomi H, Rossant J, Zaret KS (2001) Liver organogenesis promoted by endothelial cells prior to vascular function. Science 294:559–563. https://doi.org/10.1126/science.1063889
Nolan DJ, Ginsberg M, Israely E et al (2013) Molecular signatures of tissue-specific microvascular endothelial cell heterogeneity in organ maintenance and regeneration. Dev Cell 26:204–219. https://doi.org/10.1016/j.devcel.2013.06.017
White MP, Rufaihah AJ, Liu L et al (2013) Limited gene expression variation in human embryonic stem cell and induced pluripotent stem cell-derived endothelial cells. Stem Cells 31:92–103. https://doi.org/10.1002/stem.1267
Reed DM, Foldes G, Kirkby NS et al (2014) Morphology and vasoactive hormone profiles from endothelial cells derived from stem cells of different sources. Biochem Biophys Res Commun 455:172–177. https://doi.org/10.1016/j.bbrc.2014.10.140
Yoder M (2015) Differentiation of pluripotent stem cells into endothelial cells. Curr Opin Hematol 22:252–257. https://doi.org/10.1097/MOH.0000000000000140
Lippmann ES, Azarin SM, Kay JE et al (2012) Derivation of blood–brain barrier endothelial cells from human pluripotent stem cells. Nat Biotechnol 30:783–791. https://doi.org/10.1038/nbt.2247
Stevens KR, Murry CE (2018) Human pluripotent stem cell-derived engineered tissues: clinical considerations. Cell Stem Cell 22:294–297. https://doi.org/10.1016/j.stem.2018.01.015
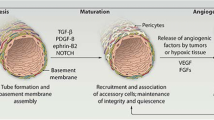
Jain RK (2003) Molecular regulation of vessel maturation. Nat Med 9:685–693. https://doi.org/10.1038/nm0603-685
Darland DC, D’Amore P (1999) Blood vessel maturation: vascular development comes of age. J Clin Investig 103:157–158. https://doi.org/10.1172/JCI6127
Folkman MJ, D’Amore P (1996) Blood vessel formation: what is its molecular basis? Cell 87:1153–1155
Hellström M, Kalén M, Lindahl P et al (1999) Role of PDGF-B and PDGFR-beta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development 126:3047–3055
Armulik A, Abramsson A, Betsholtz C (2005) Endothelial/pericyte interactions. Circ Res 97:512–523. https://doi.org/10.1161/01.RES.0000182903.16652.d7
Shepherd BR, Jay SM, Saltzman WM et al (2009) Human aortic smooth muscle cells promote arteriole formation by coengrafted endothelial cells. Tissue Eng Part A 15:165–173. https://doi.org/10.1089/ten.tea.2008.0010
Maier CL, Shepherd BR, Yi T, Pober JS (2010) Explant outgrowth, propagation and characterization of human pericytes. Microcirculation 17:367–380. https://doi.org/10.1111/j.1549-8719.2010.00038.x
Lynch MD, Watt FM (2018) Fibroblast heterogeneity: implications for human disease. J Clin Investig 128:26–35. https://doi.org/10.1172/JCI93555
Sato G (2008) Tissue culture: the unrealized potential. Cytotechnology 57:111–114. https://doi.org/10.1007/s10616-007-9109-9
Wilmut I (2007) The first direct reprogramming of adult human fibroblasts. Cell Stem Cell 1:593–594. https://doi.org/10.1016/j.stem.2007.11.013
Shandalov Y, Egozi D, Koffler J et al (2014) An engineered muscle flap for reconstruction of large soft tissue defects. Proc Natl Acad Sci USA 111:6010–6015. https://doi.org/10.1073/pnas.1402679111
Tonello C, Vindigni V, Zavan B et al (2005) In vitro reconstruction of an endothelialized skin substitute provided with a microcapillary network using biopolymer scaffolds. FASEB J 19:1546–1548. https://doi.org/10.1096/fj.05-3804fje
Hendrickx B, Verdonck K, Van den Berge S et al (2010) Integration of blood outgrowth endothelial cells in dermal fibroblast sheets promotes full thickness wound healing. Stem Cells 28:1165–1177. https://doi.org/10.1002/stem.445
Sorrell JM, Caplan AI (2004) Fibroblast heterogeneity: more than skin deep. J Cell Sci 117:667–675. https://doi.org/10.1242/jcs.01005
Driskell RR, Watt FM (2015) Understanding fibroblast heterogeneity in the skin. Trends Cell Biol 25:92–99. https://doi.org/10.1016/j.tcb.2014.10.001
Driskell RR, Lichtenberger BM, Hoste E et al (2013) Distinct fibroblast lineages determine dermal architecture in skin development and repair. Nature 504:277–281. https://doi.org/10.1038/nature12783
Alt E, Yan Y, Gehmert S et al (2011) Fibroblasts share mesenchymal phenotypes with stem cells, but lack their differentiation and colony-forming potential. Biol Cell 103:197–208. https://doi.org/10.1042/BC20100117
Denu RA, Nemcek S, Bloom DD et al (2016) Fibroblasts and mesenchymal stromal/stem cells are phenotypically indistinguishable. Acta Haematol 136:85–97. https://doi.org/10.1159/000445096
Ankrum JA, Ong JF, Karp JM (2014) Mesenchymal stem cells: immune evasive, not immune privileged: PubMed—NCBI. Nat Biotechnol 32:252–260. https://doi.org/10.1038/nbt.2816
Pittenger MF, Mackay AM, Beck SC et al (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284:143–147
Ankrum J, Karp JM (2010) Mesenchymal stem cell therapy: two steps forward, one step back. Trends Mol Med 16:203–209. https://doi.org/10.1016/j.molmed.2010.02.005
Dominici M, Le Blanc K, Mueller I et al (2006) Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy 8:315–317. https://doi.org/10.1080/14653240600855905
Karp JM, Leng Teo GS (2009) Mesenchymal stem cell homing: the devil is in the details. Cell Stem Cell 4:206–216. https://doi.org/10.1016/j.stem.2009.02.001
Bianco P, Robey PG, Simmons PJ (2008) Mesenchymal stem cells: revisiting history, concepts, and assays. Cell Stem Cell 2:313–319. https://doi.org/10.1016/j.stem.2008.03.002
Crisan M, Yap S, Casteilla L et al (2008) A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 3:301–313. https://doi.org/10.1016/j.stem.2008.07.003
Prockop DJ (2009) Repair of tissues by adult stem/progenitor cells (MSCs): controversies, myths, and changing paradigms. Mol Ther 17:939–946. https://doi.org/10.1038/mt.2009.62
Pill K, Hofmann S, Redl H, Holnthoner W (2015) Vascularization mediated by mesenchymal stem cells from bone marrow and adipose tissue: a comparison. Cell Regen (Lond) 4:8. https://doi.org/10.1186/s13619-015-0025-8
Liang X, Ding Y, Zhang Y et al (2014) Paracrine mechanisms of mesenchymal stem cell-based therapy: current status and perspectives. Cell Transplant 23:1045–1059. https://doi.org/10.3727/096368913X667709
Ranganath SH, Levy O, Inamdar MS, Karp JM (2012) Harnessing the mesenchymal stem cell secretome for the treatment of cardiovascular disease. Cell Stem Cell 10:244–258. https://doi.org/10.1016/j.stem.2012.02.005
Borges J, Mueller MC, Padron NT et al (2003) Engineered adipose tissue supplied by functional microvessels. Tissue Eng 9:1263–1270. https://doi.org/10.1089/10763270360728170
Wenger A, Stahl A, Weber H et al (2004) Modulation of in vitro angiogenesis in a three-dimensional spheroidal coculture model for bone tissue engineering. Tissue Eng 10:1536–1547. https://doi.org/10.1089/ten.2004.10.1536
Ghajar CM, Blevins KS, Hughes CCW et al (2006) Mesenchymal stem cells enhance angiogenesis in mechanically viable prevascularized tissues via early matrix metalloproteinase upregulation. Tissue Eng 12:2875–2888. https://doi.org/10.1089/ten.2006.12.2875
Rohringer S, Hofbauer P, Schneider KH et al (2014) Mechanisms of vasculogenesis in 3D fibrin matrices mediated by the interaction of adipose-derived stem cells and endothelial cells. Angiogenesis 17:921–933. https://doi.org/10.1007/s10456-014-9439-0
Ghanaati S, Fuchs S, Webber MJ et al (2011) Rapid vascularization of starch-poly(caprolactone) in vivo by outgrowth endothelial cells in co-culture with primary osteoblasts. J Tissue Eng Regen Med 5:e136–e143. https://doi.org/10.1002/term.373
Holnthoner W, Hohenegger K, Husa A-M et al (2015) Adipose-derived stem cells induce vascular tube formation of outgrowth endothelial cells in a fibrin matrix. J Tissue Eng Regen Med 9:127–136. https://doi.org/10.1002/term.1620
Crisan M, Corselli M, Chen WCW, Péault B (2012) Perivascular cells for regenerative medicine. J Cell Mol Med 16:2851–2860. https://doi.org/10.1111/j.1582-4934.2012.01617.x
Au P, Tam J, Fukumura D, Jain RK (2008) Bone marrow-derived mesenchymal stem cells facilitate engineering of long-lasting functional vasculature. Blood 111:4551–4558. https://doi.org/10.1182/blood-2007-10-118273
Lin R-Z, Moreno-Luna R, Moreno-Luna R et al (2012) Equal modulation of endothelial cell function by four distinct tissue-specific mesenchymal stem cells. Angiogenesis 15:443–455. https://doi.org/10.1007/s10456-012-9272-2
Lin R-Z, Moreno-Luna R, Li D et al (2014) Human endothelial colony-forming cells serve as trophic mediators for mesenchymal stem cell engraftment via paracrine signaling. Proc Natl Acad Sci USA 111:10137–10142. https://doi.org/10.1073/pnas.1405388111
Xie C, Ritchie RP, Huang H et al (2011) Smooth muscle cell differentiation in vitro: models and underlying molecular mechanisms. Arterioscler Thromb Vasc Biol 31:1485–1494. https://doi.org/10.1161/ATVBAHA.110.221101
Kattman SJ, Huber TL, Keller GM (2006) Multipotent flk-1 + cardiovascular progenitor cells give rise to the cardiomyocyte, endothelial, and vascular smooth muscle lineages. Dev Cell 11:723–732. https://doi.org/10.1016/j.devcel.2006.10.002
Bajpai VK, Mistriotis P, Loh Y-H et al (2012) Functional vascular smooth muscle cells derived from human induced pluripotent stem cells via mesenchymal stem cell intermediates. Cardiovasc Res 96:391–400. https://doi.org/10.1093/cvr/cvs253
Ferreira LS, Gerecht S, Shieh HF et al (2007) Vascular progenitor cells isolated from human embryonic stem cells give rise to endothelial and smooth muscle like cells and form vascular networks in vivo. Circ Res 101:286–294. https://doi.org/10.1161/CIRCRESAHA.107.150201
Reznikoff CA, Bertram JS, Brankow DW, Heidelberger C (1973) Quantitative and qualitative studies of chemical transformation of cloned C3H mouse embryo cells sensitive to postconfluence inhibition of cell division. Can Res 33:3239–3249
Hirschi KK, Rohovsky SA, D’Amore P (1998) PDGF, TGF-beta, and heterotypic cell-cell interactions mediate endothelial cell-induced recruitment of 10T1/2 cells and their differentiation to a smooth muscle fate. J Cell Biol 141:805–814
Hirschi KK, Rohovsky SA, Beck LH et al (1999) Endothelial cells modulate the proliferation of mural cell precursors via platelet-derived growth factor-BB and heterotypic cell contact. Circ Res 84:298–305
Cheng G, Liao S, Wong HK et al (2011) Engineered blood vessel networks connect to host vasculature via wrapping-and-tapping anastomosis. Blood. https://doi.org/10.1182/blood-2011-02-338426
Acknowledgements
This work was supported by National Institutes of Health Grants R01AR069038, R01HL128452, and R21AI123883 to J. M.-M.
Author information
Authors and Affiliations
Contributions
KW, R-ZL, and JMM-M conceived and designed the project, analyzed the data, discussed and edited the results and wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Wang, K., Lin, RZ. & Melero-Martin, J.M. Bioengineering human vascular networks: trends and directions in endothelial and perivascular cell sources. Cell. Mol. Life Sci. 76, 421–439 (2019). https://doi.org/10.1007/s00018-018-2939-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-018-2939-0